Abstract
The members of the organic anion transporting polypeptide superfamily (OATPs) are classified within the SLCO solute carrier family. All functionally well characterized members are predicted to have 12 transmembrane domains and are sodium-independent transport systems that mediate the transport of a broad range of endo- as well as xenobiotics. Substrates are mainly amphipathic organic anions with a molecular weight of more than 300 Da, but some of the known transported substrates are also neutral or even positively charged. Among the well characterized substrates are numerous drugs including statins, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, antibiotics, antihistaminics, antihypertensives and anticancer drugs. Based on their amino acid sequence identities, the different OATPs cluster into families (in general with more than 40% amino acid sequence identity) and subfamilies (more than 60% amino acid identity). With the sequencing of genomes from different species and the computerized prediction of encoded proteins more than 300 OATPs can be found in the databases, however only a fraction of them have been identified in humans, rodents, and some additional species important for pharmaceutical research like the rhesus monkey (Macaca mulatta), the dog (Canis lupus familiaris) and the pig (Sus scrofa). These OATPs form 6 families (OATP1–OATP6) and 13 subfamilies. In this review we try to summarize what is currently known about OATPs with respect to endogenous substrates, tissue distribution, transport mechanisms, regulation of expression, structure-function relationship and mutations and polymorphisms.
Keywords: SLCO, SLC21, OATP, bile acid, bilirubin, steroid conjugates
1. Introduction
Cells continuously need to take up nutrients as well as signaling molecules and to release metabolic endproducts for disposal. Most such substances, even if very lipopophilic, are not able to diffuse across plasma membranes and consequently need transport proteins to cross the cell boundaries. This is exemplified by cholesterol, which takes advantage of (transport) proteins to cross plasma membranes. For example, cholesterol absorption in the small intestine is mediated, at least in part, by the Niemann-Pick 1-like 1 (NPC1L1) protein (Lecerf and de Lorgeril, 2011), which is susceptible to inhibition by the drug ezetimibe. Else, release of cholesterol from the canalicular membrane of hepatocytes into bile is facilitated by the heterodimeric ATP-binding-cassette (ABC) transporter ABCG5/ABCG8 (Hazard and Patel, 2007). Bilirubin, the metabolic endproduct of the breakdown of heme, is practically water insoluble and has for a long time been assumed to enter hepatocytes by simple diffusion. While the issue of transmembrane movement of bilirubin was controversially discussed (diffusion across lipid bilayer versus involvement of protein(s) (Ostrow et al., 1994)), several groups have now provided solid evidence for the involvement of organic anion transporting polypeptides (OATPs) in bilirubin uptake into hepatocytes (see below). From these prototypic results, it is safe to extrapolate that most substances and even highly non-polar or lipophilic compounds require transmembrane transport proteins to be moved between the extracellular space and the cytoplasm. The importance of transport proteins in the disposition of drugs is also gaining wide acceptance (Dobson et al., 2009; Fenner et al., 2012). The solute carrier superfamily (SLC) covers hundreds of proteins mediating the plasma membrane crossing of small molecules or solutes of various degrees of hydrophilicity and lipophilicity (Hediger et al., 2004). Among the SLC superfamily members, OATPs play a prominent role in transporting endo- as well as xenobiotics including numerous drugs across plasma membranes.
In recent years, considerable progress has been made in identifying endogenous substrates of OATPs, in elucidating the roles OATPs play in drug disposition and transport of toxins, as well as in the characterization of genetic variants. In this overview on the current status of OATP research, we do not attempt to summarize the so far characterized drugs and xenobiotics, which have been identified as OATP substrates as this information can be found in several recent reviews (Fahrmayr et al., 2010; Giacomini et al., 2010; Hagenbuch and Gui, 2008; Kalliokoski and Niemi, 2009; Konig, 2011; Kusuhara and Sugiyama, 2009; Roth et al., 2012).
2. Phylogenesis of OATPs
The first OATP, rat OATP1A1 (originally called Oatp) was isolated in 1994 using expression cloning (Jacquemin et al., 1994) and the first human OATP, OATP1A2 (originally called OATP) was isolated a year later by hybridization screening (Kullak-Ublick et al., 1995). In the following years several additional OATPs from humans and rodents were identified and characterized and we know today that there are eleven OATPs in humans. In 2004, an amino acid sequence based classification and nomenclature system was introduced and approved by the HUGO Gene Nomenclature Committee (Hagenbuch and Meier, 2004). This classification system allows us to name any newly identified OATP with a unique name if it is a unique member of the family or with the name of its already known orthologue. The general rules for this classification system are that proteins with more than 40 % identity belong to the same family while proteins with more than 60 % identity belong to the same subfamily (Hagenbuch and Meier, 2004). On this basis, the human and rodent OATPs form 6 families (OATP1, OATP2, OATP3, OATP4, OATP5, and OATP6) and each family can have subfamilies (e.g. OATP1A, OATP1B, OATP1C). Within these subfamilies the individual OATPs are numbered according to the chronology of their identification and if there is already an orthologue known they are given the same number. The symbols for human and rodent proteins are always given in capitals (e.g. OATP1A2). The corresponding gene symbols begin with SLCO for human and Slco for rodents and have the same family number, subfamily letter and chronological number as the protein symbol (e.g. SLCO1A2 for OATP1A2, Slco1a1 for mouse OATP1A1). In contrast to protein symbols, gene symbols are always given in italics. However, it turned out that the 40% and 60% are not absolute numbers because e.g. X. laevis oatp1a2 has only 48% amino acid sequence identity to human OATP1A2 but based on phylogenetic analysis clearly is an orthologue of human OATP1A2 and does not belong to the 1B or 1C subfamily. Thus, newly identified OATPs should be carefully classified before a new name is assigned. The currently approved human members of the SLCO superfamily are summarized in Table 1. In the transporter classification database maintained by Milton Saier OATPs are found in the “The Organo Anion Transporter (OAT) Family” 2.A.60 (Saier et al., 1999).
Table 1.
The human members of the organic anion transporting superfamily
| New gene symbol | New protein name | Predominant substrates | Tissue distribution/subcellular expression | Link to disease | Human gene locus | Sequence Accession ID | Splice variants |
|---|---|---|---|---|---|---|---|
| SLCO1A2 | OATP1A2 | Bile salts, organic anions and cations | Brain (endothelial cells), kidney (apical), intestine (apical), liver (cholangiocytes), eye (ciliary body) | 12p12 |
NM_021094 NM_134431 |
2 | |
| SLCO1B1 | OATP1B1 | Bile salts, organic anions | Liver (hepatocytes) | (Statin-induced myopathy, Rotor Syndrome) | 12p | NM_6446 | |
| SLCO1B3 | OATP1B3 | Bile salts, organic anions | Liver (hepatocytes) | (Unconjugated hyperbilirubinemia, Rotor syndrome) | 12p12 | NM_019844 | |
| SLCO1C1 | OATP1C1 | T4,T3, rT3, BSP | Brain (blood-brain barrier), testis (Leydig cells) | (Hyperthyroidism) | 12p12.2 |
NM_017435 NM_001145944 NM_001145945 NM_001145946 |
4 |
| SLCO2A1 | OATP2A1 | Prostaglandins | Ubiquitous | 3q21 | NM_005630 | ||
| SLCO2B1 | OATP2B1 | E-3-S, DHEAS, BSP | Liver (hepatocytes), placenta, intestine (apical), eye (ciliary body) | 11q13 |
NM_007256 NM_001145211 NM_001145212 |
3 | |
| SLCO3A1 | OATP3A1 | E-3-S, prostaglandin | Testis, heart, brain, ovary | 15q26 |
NM_013272 NM_001145044 |
2 | |
| SLCO4A1 | OATP4A1 | Taurocholate, T3, prostaglandin | Ubiquitous | 20q13.33 | NM_016354 | ||
| SLCO4C1 | OATP4C1 | Digoxin, ouabain, thyroid hormones, methotrexate | Kidney (basolateral) | 5q21.2 | NM_180991 | ||
| SLCO5A1 | OATP5A1 | 8q13.3 |
NM_030958 NM_001146008 NM_001146009 |
3 | |||
| SLCO6A1 | OATP6A1 | Testis | 5q21.1 | NM_173488 |
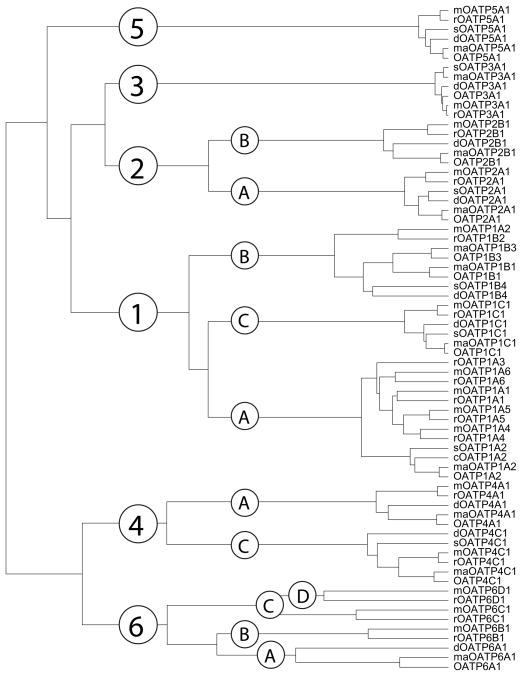
Compared to the 52 members of the OATP superfamily reported in 2004, today more than 300 members have been identified and/or predicted from over 40 species. Figure 1 shows the phylogenetic tree of 70 OATPs from human, monkey, dog, pig, rat and mouse. The OATP1 family is the largest with 27 members, followed by the OATP6 family. In both families gene duplications in rodents resulted in several genes/proteins for rats and mice as compared to humans, while in subfamily OATP1B a gene duplication resulted in two genes/proteins for humans and monkeys (OATP1B1 and OATP1B3 as compared to rodent OATP1B2 or OATP1B4). The OATP3 family has the most conserved members with amino acid sequence identities between 94 and 99 % while the OATP6 family is the most diverged. It is interesting to note that no OATP homologues have been found in bacteria or yeast suggesting that OATPs are specific to the animal kingdom.
Figure 1.
Phylogenetic tree and classification of 70 members of the OATP/SLCO superfamily of transporters. Human (all capitalized OATP), monkey (maOATP), dog (dOATP), pig (sOATP), rat (rOATP) and mouse (mOATP) proteins are grouped into families (with more than 40% amino acid sequence identity) and subfamilies (with more than 60% amino acid sequence identity).
3. Endogenous substrates of OATPs
Rat OATP1A1, the founding member of the SLCO superfamily of organic anion transporters, was isolated with an expression-cloning approach using the anion bromosulphophthalein as substrate (Hagenbuch and Meier, 2004; Jacquemin et al., 1994). Functional characterization of rat OATP1A1 in heterologous expression systems revealed that it can transport bile acids (e.g. cholate) and bile acid conjugates (e.g. taurocholate) (Eckhardt et al., 1999; Jacquemin et al., 1994) in a sodium-independent way with a preference for unconjugated over conjugated bile acids (Meier et al., 1997). Hence, bile salts can be considered the first identified endogenous OATP substrates. OATP1A2 can also transport unconjugated and conjugated bile acids (Table 2) (Kullak-Ublick et al., 1995). In addition, OATP1A2 can also transport dehydroepiandrosterone sulfate, a precursor for the synthesis of steroid hormones in many organs (Kullak-Ublick et al., 1998). Later, OATP1B1 (Abe et al., 1999), OATP1A2 and OATP4A1 (Fujiwara et al., 2001), OATP1C1 (Pizzagalli et al., 2002) and OATP3A1_v1 (Huber et al., 2007) were also found to transport thyroid hormones (Jansen et al., 2005). Additional endogenous OATP substrates are listed in Table 2.
Table 2. Endogenous substrates of organic anion transporting polypeptides.
If Km-values are available, data demonstrating transport are omitted. Endogenous concentrations are given for systemic concentration and are, due to space reasons, not necessarily found in the references given in this table. The concentrations found for many substances in plasma vary widely (in some instances more than factor of 10). In addition, many of the listed substance have a considerable binding to plasma proteins. Consequently, the values given should only be taken as approximate.
| Transporter | Substrate | Affinity | Plasma concentration | Reference |
|---|---|---|---|---|
| Rat | ||||
| OATP1A1 | cholate | 54 μM | ~ 3 μM | (Eckhardt et al., 1999) |
| taurocholate | 32 – 50 μM | ~ 1 μM | (Eckhardt et al., 1999; Kullak-Ublick et al., 1994; Satlin et al., 1997) | |
| glycocholate | 54 μM | ~ 0.1 μM | (Eckhardt et al., 1999) | |
| taurochenodeoxycholate | 7 μM | ~ 0.1 μM | (Eckhardt et al., 1999) | |
| tauroursodeoxycholate | 13 μM | ~ 0.01 μM | (Eckhardt et al., 1999) | |
| bilirubinmonoglucuronide | ~ 1 μM | (Reichel et al., 1999) | ||
| dehydroepiandrostenone sulfate | 5 μM | ~ 0.1 nM | (Eckhardt et al., 1999) | |
| aldosterone | 15 nM | ~ 0.1 nM | (Bossuyt et al., 1996a) | |
| cortisol | 13 μM | ~ 0.1 μM | (Bossuyt et al., 1996a) | |
| estradiol-17β-glucuronide | 3 – 20 μM | (Bossuyt et al., 1996a; Eckhardt et al., 1999; Ishizuka et al., 1998; Kouzuki et al., 1999) | ||
| Estrone-3-sulfate | 5 – 12 μM | (Bossuyt et al., 1996a; Eckhardt et al., 1999) | ||
| Leukotriene C4 | ~ 1 pM | (Li et al., 1998) | ||
| diiodothyronine (T2) | (Friesema et al., 1999) | |||
| diiodothronine sulfate (T2S) | (Friesema et al., 1999) | |||
| prostaglandine E2 | pH 6.5 | ~ 1 nM | (Leuthold et al., 2009) | |
| triiodothyronine (T3) | ~ 1 nM | (Friesema et al., 1999) | ||
| triiodothyronine sulfate (T3S) | ~ 0.1 nM | (Friesema et al., 1999) | ||
| reverse triiodothyronine (rT3) | ~ 0.01 nM | (Friesema et al., 1999) | ||
| reverse triiodothyronine sulfate (rT3S) | ~ 0.1 nM | (Friesema et al., 1999) | ||
| thyroxine (T4) | ~ 50 nM | (Friesema et al., 1999) | ||
| thyroxine sulphate (T4S) | ~ 0.05 nM | (Friesema et al., 1999) | ||
| OATP1A3 | taurocholate | 10–31 μM | ~ 1 μM | (Masuda et al., 1999; Takeuchi et al., 2001) |
| dehydroepiandrostenone sulfate | 9 – 17 μM | ~ 0.1 nM | (Reichel et al., 1999; Takeuchi et al., 2001) | |
| estradiol-17β-glucuronide | 3–35 μM | (Noe et al., 1997; Takeuchi et al., 2001) | ||
| estrone-3-sulfate | 11–15 μM | Noe, 1997 #333}(Takeuchi et al., 2001) | ||
| thyroxine (T4) | 7–20 μM | ~ 50 nM | Abe, 1999 #670}(Takeuchi et al., 2001) | |
| triiodothyronine (T3) | 6–44 μM | ~ 1 nM | (Abe et al., 1999; Takeuchi et al., 2001) | |
| OATP1A4 | cholate | 46 μM | ~ 3 μM | (Noe et al., 1997) |
| taurocholate | 35 – 36 μM | ~ 1 μM | (Abe et al., 1999; Noe et al., 1997) | |
| glycocholate | 40 μM | ~ 0.1 μM | (Reichel et al., 1999) | |
| taurochenodeoxycholate | 12 μM | ~ 0.1 μM | (Reichel et al., 1999) | |
| tauroursodeoxycholate | 17 μM | ~ 0.01 μM | (Reichel et al., 1999) | |
| dehydroepiadrostenone sulfate | 17 μM | ~ 0.1 nM | (Reichel et al., 1999) | |
| estradiol-17β-glucuronide | 3 μM | (Noe et al., 1997) | ||
| estrone-3-sulfate | 11 μM | (Noe et al., 1997) | ||
| prostaglandine E2 | pH 6.5 | ~ 5 nM | (Leuthold et al., 2009) | |
| triiodothyronine (T3) | 6 μM | ~ 1 nM | (Abe et al., 1999) | |
| thyroxine (T4) | 7 μM | ~ 50 nM | (Abe et al., 1999) | |
| OATP1A5 | cholate | 9 μM | ~ 3 μM | (Walters et al., 2000) |
| taurocholate | 18 – 30 μM | ~ 1 μM | (Abe et al., 1999; Walters et al., 2000) | |
| glycocholate | 15 μM | ~ 0.1 μM | (Walters et al., 2000) | |
| taurochenodeoxycholate | 7 μM | ~ 0.1 μM | (Walters et al., 2000) | |
| glycochenodeoxycholate | 6 μM | (Walters et al., 2000) | ||
| tauroursodeoxycholate | 7 μM | ~ 0.01 μM | (Walters et al., 2000) | |
| glycoursodeoxycholate | 5 μM | (Walters et al., 2000) | ||
| taurodeoxycholate | 6 μM | ~ 0.1 μM | (Walters et al., 2000) | |
| glycodeoxycholate | 4 μM | (Walters et al., 2000) | ||
| dehydroepiandrostenone sulfate | 162 μM | ~ 0.1 nM | (Cattori et al., 2001) | |
| estradiol-17β-glucuronide | 39 μM | (Cattori et al., 2001) | ||
| estrone-3-sulfate | 268 μM | (Cattori et al., 2001) | ||
| leukotriene C4 | ~ 1 pM | (Cattori et al., 2001) | ||
| prostaglandine E2 | 35 μM | ~ 5 nM | (Cattori et al., 2001) | |
| thyroxine (T4) | 5 μM | ~ 50 nM | (Abe et al., 1999) | |
| triiodothyronine (T3) | 7 μM | ~ 1 nM | (Abe et al., 1999) | |
| OATP1B2 | taurocholate | 9 – 27 μM | ~ 1 μM | (Cattori et al., 2000; Kakyo et al., 1999) |
| dehydroepiadrostenone sulfate | 5 μM | ~ 0.1 nM | (Cattori et al., 2001) | |
| estradiol-17β-glucuronide | 32 μM | (Cattori et al., 2001) | ||
| estrone-3-sulfate | 37 μM | (Cattori et al., 2001) | ||
| arachidonate | 96 μM | ~ 10 μM | (Kanai et al., 1995) | |
| CCK8 | 15 μM | ~ 1 pM | (Ismair et al., 2001) | |
| leukotriene C4 | 7 μM | ~ 1 pM | (Cattori et al., 2001) | |
| prostaglandine E2 | 13 μM | ~ 5 nM | (Cattori et al., 2001) | |
| triiodothyronine (T3) | ~ 1 nM | (Cattori et al., 2000) | ||
| thyroxine (T4) | ~ 50 nM | (Cattori et al., 2000) | ||
| OATP1C1 | taurocholate | ~ 1 μM | (Sugiyama et al., 2003) | |
| CCK-8 | ~ 1 pM | (Sugiyama et al., 2003) | ||
| estradiol-17β-glucuronide | 11 μM | (Sugiyama et al., 2003) | ||
| estrone | ~ 0.1 nM | (Sugiyama et al., 2003) | ||
| estrone-3-sulfate | (Sugiyama et al., 2003) | |||
| dehydroepiandrostenone sulfate | ~ 0.1 nM | (Sugiyama et al., 2003) | ||
| dihydrotestosterone | ~ 1 nM | (Sugiyama et al., 2003) | ||
| leukotriene C4 | ~ 1 pM | (Sugiyama et al., 2003) | ||
| leukotriene E4 | (Sugiyama et al., 2003) | |||
| testosterone | ~ 10 nM | (Sugiyama et al., 2003) | ||
| thyroxine (T4) | 180 nM | ~ 50 nM | (Sugiyama et al., 2003) | |
| reverse triiodothyronine (rT3) | ~ 0.01 nM | (Sugiyama et al., 2003) | ||
| triiodothyronine (T3) | ~ 1 nM | (Sugiyama et al., 2003) | ||
| OATP2A1 | arachidonate | 96 μM | ~ 10 μM | (Kanai et al., 1995) |
| 6-keto prostaglandine F1α | 8 μM | ~ 1 nM | (Kanai et al., 1995) | |
| prostaglandine E1 | 70 nM | (Kanai et al., 1995) | ||
| prostaglandine E2 | 94 nM | ~ 0.1 nM | (Kanai et al., 1995) | |
| prostaglandine E2α | 104 nM | (Kanai et al., 1995) | ||
| thromboxane B2 | 423 nM | ~ 1 nM | (Kanai et al., 1995) | |
| OATP2B1 | taurocholate | 18 μM | ~1 μM | (Nishio et al., 2000) |
| leukotriene C4 | 3 μM | ~ 1 pM | (Nishio et al., 2000) | |
| prostaglandin D2 | 18 μM | ~ 10 nM | (Nishio et al., 2000) | |
| prostaglandin E1 | (Nishio et al., 2000) | |||
| prostaglandin E2 | ~ 0.1 nM | (Nishio et al., 2000) | ||
| thromboxane B2 | ~ 1 nM | (Nishio et al., 2000) | ||
| OATP4A1 | taurocholate | ~ 1 μM | (Fujiwara et al., 2001) | |
| triiodothyronine (T3) | ~ 1 nM | (Fujiwara et al., 2001) | ||
| OATP4C1 | triiodothyronine/T3) | 2 μM | ~ 1 nM | (Mikkaichi et al., 2004) |
| OATP6B1 | taurocholate | 9 μM | ~ 1 μM | (Suzuki et al., 2003) |
| dehydroepiandrostenone sulfate | 26 μM | ~ 0.1 nM | (Suzuki et al., 2003) | |
| thyroxine (T4) | 6 μM | ~ 50 nM+ | (Suzuki et al., 2003) | |
| triiodothyronine (T3) | ~ 1 nM | (Suzuki et al., 2003) | ||
| OATP6C1 | taurocholate | 3 μM | ~ 1 μM | (Suzuki et al., 2003) |
| dehydroepiandrostenone sulfate | 22 μM | ~ 0.1 nM | (Suzuki et al., 2003) | |
| thyroxine (T4) | 6 μM | ~ 50 nM | (Suzuki et al., 2003) | |
| triiodothyronine (T3) | ~ 1 nM | (Suzuki et al., 2003) | ||
| human | ||||
| OATP1A2 | cholate | 93 μM | ~ 1 μM | (Kullak-Ublick et al., 1995) |
| taurocholate | 60 μM | ~ 0.1 μM | (Kullak-Ublick et al., 1995) | |
| glycocholate | ~ 0.5 μM | (Kullak-Ublick et al., 1995; Kullak-Ublick et al., 2001) | ||
| taurochenodeoxycholate | ~ 0.5 μM | (Kullak-Ublick et al., 1995) | ||
| tauroursodeoxycholate | 19 | ~ 0.001 μM | (Kullak-Ublick et al., 1995) | |
| bilirubin | ~ 10 μM | (Briz et al., 2003) | ||
| estradiol-17β-glucuronide | (Briz et al., 2003; Kullak-Ublick et al., 2001) | |||
| estrone-3-sulfate | 16 – 59 | ~ 2 nM | (Bossuyt et al., 1996b; Lee et al., 2005) | |
| dehydroepiandrostenone sulfate | 7 μM | ~ 5 μM | (Kullak-Ublick et al., 1998) | |
| prostaglandin E2 | ~ 1 nM | (Kullak-Ublick et al., 2001) | ||
| reverse triiodothyronine (rT3) | ~ 0.1 nM | (Fujiwara et al., 2001) | ||
| triiodothyronine (T3) | 7 μM | ~ 1 nM | (Fujiwara et al., 2001) | |
| triiodothyronine sulfate (T3S) | ~ 0.1 nM | (van der Deure et al., 2010) | ||
| thyroxine (T4) | 8 μM | ~ 100 nM | (Fujiwara et al., 2001) | |
| thyroxine sulfate (T4S) | ~ 10 pM | (van der Deure et al., 2010) | ||
| OATP1B1 | cholate | 11 μM | ~ 1μM | (Cui et al., 2001) |
| taurocholate | 10 – 34 μM | ~ 0.1 μM | (Abe et al., 1999; Cui et al., 2001; Hsiang et al., 1999) | |
| glycocholate | ~ 0.5 μM | (Kullak-Ublick et al., 2001) | ||
| tauroursodeoxycholate | 7 μM | ~ 0.001 μM | (Maeda et al., 2006) | |
| glycoursodeoxycholate | 5 μM | ~ 0.1 μM | (Maeda et al., 2006) | |
| bilirubin | 8 – 160 nM | ~ 10 μM | (Briz et al., 2003; Cui et al., 2001) | |
| bilirubinmonoglucuronide | 100 nM | ~ 1 μM | (Cui et al., 2001) | |
| bilirubindiglucuronide | 280 nM | ~ 1 μM | (Cui et al., 2001) | |
| estradiol-17β-glucuronide | 4–14 μM | (Cui et al., 2001; Hirano et al., 2004; Konig et al., 2000b; Nakai et al., 2001; Tamai et al., 2001) | ||
| estrone-3-sulfate | 458 nM – 13 μM 68 – 94 nM and 5 – 7μM |
~ 2 nM | (Cui et al., 2001; Hirano et al., 2004) (Tamai et al., 2001) |
|
| dehydroepianstrostenone sulfate | 22 μM | ~ 5 μM | (Cui et al., 2001) | |
| leukotriene C4 | ~ 10 pM | (Abe et al., 1999; Kullak-Ublick et al., 2001) | ||
| leukotriene E4 | ~ 0.1 nM | (Abe et al., 1999) | ||
| prostaglandine E2 | ~ 1 nM | (Abe et al., 1999; Kullak-Ublick et al., 2001; Tamai et al., 2000) | ||
| reverse triiodothyronine sulphate (rT3S) | ~ 10 nM | (van der Deure et al., 2008a) | ||
| triiodothyronine (T3) | 3 μM | ~ 1 nM | (Abe et al., 1999) | |
| triiodothyronine sulphate (T3S) | ~ 0.1 nM | (van der Deure et al., 2008a) | ||
| thyroxine (T4) | 3 μM | ~ 100 nM | (Abe et al., 1999) | |
| thyroxine sulphate (T4S) | ~ 10 pM | (van der Deure et al., 2008a) | ||
| thromboxane B2 | ~ 0.1 nM | (Abe et al., 1999) | ||
| OATP1B3 | cholate | 42 μM | ~ 1 μM | (Briz et al., 2006) |
| taurocholate | 6 – 42 μM | ~ 0.1 μM | (Abe et al., 2001; Briz et al., 2006) | |
| glycocholate | 43 μM | ~ 0.5 μM | (Briz et al., 2006) | |
| taurochenodeoxycholate | ~ 0.5 μM | (Briz et al., 2006) | ||
| tauroursodeoxycholate | 16 μM | ~ 0.001 μM | (Maeda et al., 2006) | |
| glycoursodeoxycholate | 25 μM | ~ 0.1 μM | (Maeda et al., 2006) | |
| taurodeoxycholate | ~ 0.5 μM | (Briz et al., 2006) | ||
| bilirubin | 39 nM | ~ 10 μM | (Briz et al., 2003) | |
| bilirubinmonoglucuronide | 500 nM | ~ 1 μM | (Cui et al., 2001) | |
| CCK8 | 4 – 11 μM | ~ 1 pM | (Hirano et al., 2004; Ismair et al., 2001) | |
| dehydroepiandrostenone sulfate | ~ 5 μM | (Konig et al., 2000a) | ||
| estradiol-17β-glucuronide | 5 – 25 μM | (Cui et al., 2001; Hirano et al., 2004; Konig et al., 2000a) | ||
| estrone-3-sulfate | 73μM (pH 8.0) 55μM (pH 6.5) |
~ 2 nM | (Leuthold et al., 2009) | |
| dehydroepiandrostenone sulfate | > 30 μM | ~ 5 μM | (Cui et al., 2001) | |
| glutathione | 5 μM | (Briz et al., 2006) | ||
| leukotriene C4 | ~ 10 pM | (Konig et al., 2000a; Kullak-Ublick et al., 2001) | ||
| prostaglandine E2 | ~ 1 nM | (Kullak-Ublick et al., 2001; Tamai et al., 2000) | ||
| triiodothyronine (T3) | 6 μM | ~ 1 nM | (Abe et al., 2001) | |
| thyroxine (T4) | ~ 100 nM | (Kullak-Ublick et al., 2001) | ||
| OATP1C1 | taurocholate | ~ 0.1 μM | (Leuthold et al., 2009) | |
| estradiol-17β-glucuronide | (Pizzagalli et al., 2002) | |||
| estrone-3-sulfate | ~ 2 nM | (Pizzagalli et al., 2002) | ||
| reverse triiodothyronine (rT3) | 128 nM | ~ 0.1 nM | (Pizzagalli et al., 2002) | |
| thyroxine (T4) | 90 – 120 nM | ~ 100 nM | (Pizzagalli et al., 2002; van der Deure et al., 2008b) | |
| thyroxine sulfate (T4S) | 3 μM | ~ 10 pM | (van der Deure et al., 2008b) | |
| triiodothyronine (T3) | ~ 1 nM | (Pizzagalli et al., 2002) | ||
| OATP2B1 | taurocholate | 72μM (pH 5.0) | ~ 0.1 μM | (Nozawa et al., 2004) |
| estrone-3-sulfate | 5 – 21 μM | ~ 2 nM | (Kullak-Ublick et al., 2001; Tamai et al., 2001) (Grube et al., 2006a; Hirano et al., 2006; Nozawa et al., 2004; Pizzagalli et al., 2003) | |
| dehydroepadrostenone sulfate | 9 μM | ~ 5 μM | (Pizzagalli et al., 2003) | |
| prostaglandine E2 | ~ 1 nM | (Tamai et al., 2000) | ||
| tyroxine (T4) | 770 nM (pH 8.0) 310 nM (pH 6.5) |
~ 100 nM | (Leuthold et al., 2009) | |
| OATP2A1 | prostaglandin D2 | (Lu et al., 1996) | ||
| prostaglandin E1 | (Lu et al., 1996) | |||
| prostaglandin E2 | ~ 1 nM | (Kraft et al., 2010; Lu et al., 1996) | ||
| prostaglandin F2α | ~ 10 pM | (Lu et al., 1996) | ||
| thromboxane B2 | ~ 0.1 nM | (Lu et al., 1996) | ||
| OATP3A1 | estrone-3-sulfate | ~ 2 nM | (Tamai et al., 2000) | |
| prostaglandine E1 | 49 nM | (Adachi et al., 2003) | ||
| prostaglandine E2 | 56 nM | ~ 1 nM | (Adachi et al., 2003) | |
| prostaglandine F2 | (Adachi et al., 2003) | |||
| OATP3A1_v1 | estrone-3-sulfate | pH 6.5 | ~ 2 nM | (Leuthold et al., 2009) |
| prostaglandine E1 | 101 nM | (Huber et al., 2007) | ||
| prostaglandine E2 | 218 nM | ~ 1 nM | (Huber et al., 2007) | |
| tyroxine (T4) | ~ 100 nM | |||
| vasopressin | ~ 1 pM | (Huber et al., 2007) | ||
| OATP3A1_v2 | arachidonate | ~ 0.1 nM | (Huber et al., 2007) | |
| estrone-3-sulfate | pH 6.5 | ~ 2 nM | (Leuthold et al., 2009) | |
| prostaglandine E1 | 218 nM | (Huber et al., 2007) | ||
| prostaglandine E2 | 371 nM | ~ 1 nM | (Huber et al., 2007) | |
| tyroxine (T4) | pH 6.5 | ~ 100 nM | (Leuthold et al., 2009) | |
| vasopressin | ~ 1 pM | (Huber et al., 2007) | ||
| OATP4A1 | taurocholate | 15 μM | ~ 0.1 μM | (Fujiwara et al., 2001) |
| estradiol-17β-glucuronide | (Tamai et al., 2000) | |||
| estrone-3-sulfate | ~ 2 nM | (Tamai et al., 2000) | ||
| prostaglandine E2 | ~ 1 nM | (Tamai et al., 2000) | ||
| triiodothyronine (T3) | 1 μM | ~ 1 nM | (Fujiwara et al., 2001) | |
| reverse triiodothyronine (rT3) | ~ 0.1 nM | (Fujiwara et al., 2001) | ||
| thyroxine (T4) | ~ 100 nM | (Fujiwara et al., 2001) | ||
| OATP4C1 | glycoycholate | ~ 0.5 μM | (Yamaguchi et al., 2010) | |
| chenodeoxycholate | ~ 0.5 μM | (Yamaguchi et al., 2010) | ||
| cAMP | ~ 10 nM | (Mikkaichi et al., 2004) | ||
| estrone-3-sulfate | 27 μM | ~ 2 nM | (Yamaguchi et al., 2010) | |
| triiodothyronine (T3) | 6 μM | ~ 1 nM | (Mikkaichi et al., 2004) | |
| tyroxine (T4) | ~ 100 nM | (Mikkaichi et al., 2004) |
To date, no severe human diseases related to bile salt homeostasis, to thyroid hormone biogenesis and metabolism or to steroid hormone synthesis have been linked to mutations in genes coding for various OATPs. Consequently, generation of Slco knockout mice was required to more directly prove and study the physiologic roles of OATPs in handling of endogenous substrates. Mammalian hepatocytes express two (humans) or three (rodents) different OATPs, which are members of the SLCO families 1A and 1B, respectively (Hagenbuch and Meier, 2004). The genes encoding these OATPs are clustered on human chromosome 12 and on mouse chromosome 6. Mice with a disrupted locus for Slco1a/1b subfamily members are vital and display no obvious disease phenotype, but have mildly elevated serum bile salt levels, supporting a role for OATPs in the hepatic uptake of bile salts (van de Steeg et al., 2010). Importantly, analysis of the bile salt pattern in the serum of these knock-out animals revealed unchanged levels of conjugated bile acids compared to the parent mouse strain but 13-fold elevated serum levels of unconjugated bile acids (van de Steeg et al., 2010). These findings were confirmed by another research group, which also reported elevated unconjugated bile acids but normal conjugated bile acids in mice with a disrupted Slco1b2 gene (Csanaky et al., 2011). This latter finding is remarkable, as in general OATPs have overlapping substrate specificities (Table 2) and all rat orthologues of the mouse liver OATPs have been shown to transport bile salts in heterologous expression systems (Leuthold et al., 2009). Hence, in vivo, despite the coexpression of several OATPs in the same cells such as hepatocytes, a specific OATP may be required for the transport of a given substrate. If such an OATP is nonfunctional like in a knock-out mouse model or a human disease, the other OATPs do not seem to be capable in all instances to fully compensate for the loss of such transport activity. After cloning of the human hepatocellular OATPs, two groups reported that OATP1B1 and OATP1B3 could mediate the transport of unconjugated bilirubin (Briz et al., 2003; Cui et al., 2001)(Table 2). Serum analysis of the knock-out mice lacking the OATP1A/1B family members revealed hyperbilirubinemia (van de Steeg et al., 2010). These animals display more than 40-fold elevated serum levels of total bilirubin compared to wild-type mice. About 95 % of the increased bilirubin was due to bilirubin mono- and bis-glucuronide, but also unconjugated bilirubin was elevated about 2.5-fold. Hence, this observation supports the concept that hepatocellular OATPs are involved in the transport of unconjugated and conjugated bilirubin into hepatocytes. Again, mice with an inactivated Slco1b2 gene also have a mild hyperbilirubinemia (Csanaky et al., 2011; Zaher et al., 2008) suggesting that in mice, OATP1B2 plays a major role in bilirubin handling. The impact of the thyroid hormone transporter OATP1C1 on the disposition of thyroid hormones has lately been studied in knock-out mice. These animals have no alterations in their serum levels of thyroid hormones and their metabolites, but display reduced thyroxin levels and altered expression of deiodinases in their brains (Mayerl et al., 2012). These examples illustrate that knock-out mice are a powerful tool in the identification of endogenous or physiologic substrates of OATPs as well as in the explanation of human diseases with unknown etiology.
4. Tissue distribution
The expression of OATPs has been studied both at the mRNA and the protein level. In general, OATPs have been detected in essentially every organ in epithelial or endothelial cells. Some OATPs have a restricted expression and are therefore assumed to be organ specific, while others are expressed ubiquitously. Examples for such a restricted expression are the two human transporters OATP1B1 and OATP1B3 that are considered to be liver-specific (Roth et al., 2012) or the brain-specific OATP1C1 characterized in the rat (Sugiyama et al., 2003). Examples of ubiquitously expressed OATPs are the human OATP2A1, OATP3A1 and OATP4A1 whose mRNA was found in essentially all the tissues analyzed (Roth et al., 2012).
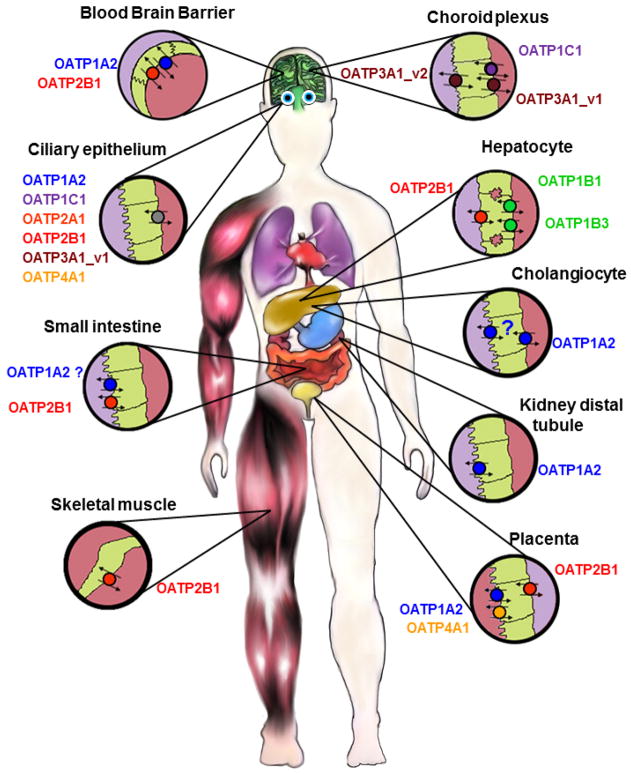
In humans, the OATP1 family has four members, OATP1A2, OATP1B1, OATP1B3 and OATP1C1 (Figure 1). Messenger RNA for SLCO1A2 encoding OATP1A2 has been detected in numerous tissues including brain, kidney, liver, lung, testes, placenta and prostate (Roth et al., 2012). At the protein level, OATP1A2 has been shown to be expressed in endothelial cells of the blood-brain barrier (Bronger et al., 2005; Gao et al., 2000; Lee et al., 2005), at the brush-border side in the distal nephron (Lee et al., 2005), in cholangiocytes (Lee et al., 2005), in the pars plana of the ciliary body epithelium (Gao et al., 2005), and in syncytiotrophoblasts (Loubiere et al., 2010) (Figure 2). As already mentioned above, OATP1B1 and OATP1B3 are considered to be liver specific transporters (Roth et al., 2012). Under normal conditions they are expressed at the sinusoidal membrane of human hepatocytes with OATP1B3 expression being stronger around the central vein than around the portal vein (Konig et al., 2000a). In a recent study protein expression of OATP1B1 and OATP1B3 was measured using a multiplex UPLC-MRM MS method and the results demonstrated that both proteins were found in equal amounts in membrane fractions isolated from human hepatocytes (Ji et al., 2012). SLCO1B3 mRNA and OATP1B3 protein expression was also documented in several cancers (reviewed in (Obaidat et al., 2012)) and the high level of mRNA could be due to an alternatively spliced variant (Nagai et al., 2012). The last member in the OATP1 family, OATP1C1, is expressed in glial cells throughout the hypothalamus (Alkemade et al., 2011), at the blood-brain barrier, in the choroid plexus (Roberts et al., 2008), in Leydig cells of testes (Pizzagalli et al., 2002), and in the pars plana of the ciliary epithelium (Gao et al., 2005) (Figure 2).
Figure 2.
Distribution of OATPs in selected human epithelial tissues. The OATPs within the same subfamily are labelled with the same color. For more details see text.
The prostaglandin transporter, OATP2A1, is one of the ubiquitously expressed OATPs. SLCO2A1 mRNA has been detected in almost every tissue tested (Roth et al., 2012). At the protein level, human OATP2A1 has been shown to be expressed in retinal epithelial cells and in epithelial and endothelial cell layers of different eye tissues including the ciliary body (Kraft et al., 2010) (Figure 2), in the endometrium (Kang et al., 2005), in neurons, astrocytes, and microglia (Choi et al., 2008), as well as in the parietal cells of the gastric corpus and the pyloric glands of the antrum (Mandery et al., 2010). The second transporter in the OATP2 family, OATP2B1 encoded by the SLCO2B1 gene, is also abundantly expressed in multiple organs at the mRNA level (Tamai et al., 2000). At the protein level OATP2B1 was detected at the sinusoidal membrane of hepatocytes (Kullak-Ublick et al., 2001), at the basolateral membrane of syncytiotrophoblasts (St Pierre et al., 2002), at the brush-border membrane in the small intestine (Kobayashi et al., 2003), in keratinocytes (Schiffer et al., 2003), in the mammary gland (Pizzagalli et al., 2003), at the luminal membrane of endothelial cells of the blood-brain barrier (Bronger et al., 2005), in the pars plicata and pars plana of the ciliary body (Gao et al., 2005; Kraft et al., 2010), in endothelial cells in the heart (Grube et al., 2006b), in human platelets (Niessen et al., 2009), and in the skeletal muscle (Knauer et al., 2010) (Figure 2).
SLCO3A1 mRNA levels were found in numerous tissues with highest levels in testes, brain, heart and lung (Huber et al., 2007). At the protein level, OATP3A1 is localized in the ciliary body epithelium (Gao et al., 2005), testes, in the choroid plexus, in neurons in the frontal cortex (Huber et al., 2007) and at the plasma membrane of epithelial cells of the lactiferous ducts in normal breast tissue (Kindla et al., 2011). In testes and in the brain two splice variants were shown to be expressed in a cell type-specific pattern (Huber et al., 2007). In testes, OATP3A1_v1 is expressed in germ cells while OATP3A1_v2 is expressed in Sertoli cells. In the choroid plexus variant 1 is expressed at the basolateral membrane while variant 2 is expressed at the apical and sub-apical membrane. In the frontal cortex, OATP3A1_v1 is localized in neuroglial cells of the grey matter and OATP3A1_v2 in cell bodies and axons of the neurons (Huber et al., 2007) (Figure 2).
SLCO4A1 mRNA was found in numerous tissues including the heart, placenta, lung, liver, skeletal muscle, kidney and pancreas (Fujiwara et al., 2001; Tamai et al., 2000). At the protein level OATP4A1 was detected in the ciliary body epithelium (Gao et al., 2005) and in syncytiotrophoblasts (Loubiere et al., 2010; Sato et al., 2003) (Figure 2). Based on northern blot analysis, OATP4C1 was predicted to be a kidney-specific transporter (Mikkaichi et al., 2004) but the human protein has not been localized yet. In addition, microarray studies suggest that mRNA from the SLCO4C1 gene is also detectable in the liver (Bleasby et al., 2006).
SLCO5A1 mRNA was reported in fetal brain, prostate, skeletal muscle and thymus (Bleasby et al., 2006). At the protein level, OATP5A1 was detected at the plasma membrane of epithelial cells of the lactiferous ducts in normal breast tissue (Kindla et al., 2011).
The expression of SLCO6A1 mRNA was detected in testes, spleen, brain and placenta (Lee et al., 2004; Suzuki et al., 2003). The expression of the different OATPs in various tumors has recently been summarized (Obaidat et al., 2012).
5. Transport Mechanisms of OATPs
The mechanism(s) by which OATPs transport is(are) not fully understood, but OATPs are believed to act as organic anion exchangers (Hagenbuch and Gui, 2008). In 1997 bicarbonate was identified as the first counterion in experiments with rat OATP1A1 expressed in HeLa cells (Satlin et al., 1997). Additional experiments demonstrated that reduced glutathione and glutathione conjugates could act as counter ions. Transport of taurocholate and leukotriene C4 mediated by rat OATP1A1 was trans-stimulated by glutathione (Li et al., 1998), while taurocholate transport by rat OATP1A4, but not by rat OATP1A1, was also trans-stimulated by the efflux of a conjugate of glutathione (Li et al., 2000), suggesting that no general transport mechanism exists for OATPs. OATP1B3 mediated cotransport of glutathione (Briz et al., 2006) could not be confirmed (Mahagita et al., 2007) and such a transport mechanism would be hard to reconcile for the uptake of organic anions into hepatocytes given the rather steep in-to-out glutathione gradient across the hepatocyte plasma membrane.
Uptake by various OATPs has been shown to be stimulated by a low extracellular pH, such as for example transport mediated by OATP2B1, which in expressed among other organs in the small intestine (Kobayashi et al., 2003). The influence of extracellular pH was tested with 13 different OATPs, out of which 12 were stimulated by a low extracellular pH. This stimulation was found to be due to a lowered Km value at pH 6.5 compared to pH 7.4 demonstrating increased affinity of the transport process at low pH. Mutagenesis of a highly conserved histidine in the third transmembrane domain to a glutamine abolished the pH dependency of rat OATP1A1. In contrast, replacing the glutamine found at this conserved position in the pH-insensitive OATB1C1 with a histidine rendered OATP1C1 pH sensitive (Leuthold et al., 2009). Hence, an acidic microclimate like in the intestine or lowering the pH in the microclimate adjacent to OATPs by an active Na+/H+-exchanger could stimulate their transport compared to uptake at neutral pH.
In addition to this modulation of transport at low pH, i.e. at higher proton concentrations, a similar modulation of OATP mediated substrate uptake by another substrate has also been observed. This was first reported for rat OATP1A4, where estradiol-17β-glucuronide stimulated transport of taurocholate but not of digoxin (Sugiyama et al., 2002). A similar stimulation has also been observed for OATP2B1, where prostaglandins A1 and A2 stimulated the transport of dehydroepiandrosteron sulfate (Pizzagalli et al., 2003). Interestingly, OATP2B1-mediated transport of dehydroepiandrosterone and estrone-3-sulfate was stimulated at low progesterone concentrations, while at higher concentrations of progesterone as modulator transport activity returned to control values (Grube et al., 2006a). In the same study, testosterone and mifepristone inhibited rather than stimulated transport activity of OATP2B1. Dietary and herbal components have also been documented to stimulate OATP-mediated transport, such as rutin, which stimulated OATP1B1-mediated dehydroepiandrosterone sulfate uptake (Wang et al., 2005) and green tea extracts or the green tea epigallocatechin gallate, which stimulated OATP1B3-mediated uptake of estrone-3-sulfate (Roth et al., 2011). Finally, OATP1B3, but not OATP1B1-mediated transport of estradiol-17β-glucuronide was stimulated by clotrimazole (Gui et al., 2008). However, such a stimulation was not observed for estrone-3-sulfate transport, which was not affected by clotimazole or for fluo-3 transport, which was inhibited. Several additional drugs tested in this study did not stimulate either OATP1B1 or OATP1B3. These results clearly show that OATPs have more than one substrate binding site, which may or may not interact with each other. This has been formally demonstrated with kinetic experiments that revealed a high and a low affinity component for estrone-3-sulfate uptake for OATP1B1 (Gui and Hagenbuch, 2009; Noe et al., 2007; Tamai et al., 2001). In summary, the transport mechanism of OATPs is complex, may vary for the same transporter for different substrates and clearly more work is needed to elucidate it in detail.
Understanding the exact transport mechanisms of OATPs is highly relevant as depending on the transport mechanism, OATPs may or may not be able to concentrate a substrate within cells over the respective extracellular concentration, like for example drugs in hepatocytes. For illustration, the concentration of the antidiabetic drug glibenclamide is 50 times higher in rat livers than in plasma (Kellner et al., 1969). While glibenclamide has been shown to be transported by OATP2B1 (Fuchikami et al., 2006), for OATP1B1 and OATP1B3 only inhibition by glibenclamide, but not direct uptake, has been demonstrated (Bednarczyk, 2010). We would like to emphasize that although such inhibition indicates that glibenclamide could be a substrate of OATP1B1 and/or OATT1B3 it does not necessarily imply that it is a substrate. This has recently been worked out for the liver function test marker indocyanine green, which is a potent inhibitor of all three hepatocellular OATPs and of the sodium taurocholate cotransporting polypeptide NTCP, but is only transported by NTCP and OATP1B3 (de Graaf et al., 2011).
6. Regulation of expression and modulation of function
Regulation of OATP expression has been documented at the transcriptional as well as at the post-translational level. Early studies demonstrated that expression of the liver specific OATP1B1 and OATP1B3 was controlled by the liver enriched hepatocyte nuclear factor 1α (HNF1α) (Jung et al., 2001). In hepatocellular carcinoma (HCC), expression of OATP1B3 was shown to be decreased while expression of OATP1B1 was normal at the mRNA level or slightly decreased at the protein level (Cui et al., 2003; Vavricka et al., 2004). It was also shown that HNF3β, which was overexpressed in HCC, could repress transcriptional expression of OATP1B3 but not of OATP1B1, potentially explaining the selective down-regulation of OATP1B3 in HCC (Vavricka et al., 2004). It is interesting to note that in ovarian cancer, but not in healthy ovarian tissue, expression of the liver specific OATP1B1 and OATP1B3 was reported (Svoboda et al., 2011). In addition several transcription factors in the family of the nuclear receptors have been shown to regulate OATP expression. Studies in breast carcinoma and breast cancer cell lines demonstrated a positive correlation between human PXR and OATP1A2 expression (Miki et al., 2006). This correlation was confirmed and a direct effect of PXR activity on OATP1A2 expression could be demonstrated (Meyer zu Schwabedissen et al., 2008). The same study also confirmed that the SLCO1A2 promoter could be activated by the constitutive androstane receptor (CAR) (Meyer zu Schwabedissen et al., 2008). Using isolated human hepatocytes it was shown that treatment with the prototypic activator of the aryl hydrocarbon receptor (AhR) 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and with the CAR activator phenobarbital resulted in a decreased expression of OATP1B3 and OATP2B1 (Jigorel et al., 2006). The promoter of human SLCO4C1 was also activated via AhR by 3-methylcholanthrene and by fluvastatin (Suzuki et al., 2011). Oltipraz, which activates NFE2-related factor 2 (Nrf2), also down-regulated OATP1B3, while the PXR agonist rifampicin led to an increase of OATP1B1 (Suzuki et al., 2011). Furthermore, OATP1B1 was shown to be regulated by the liver receptor α (LXRα) and by the farnesoid X receptor (FXR) (Meyer Zu Schwabedissen et al., 2010) and earlier it was also shown that OATP1B3 is under the control of FXR (Jung et al., 2002). In addition to these studies there are many other reports that show that OATP expression is influenced by different growth factors, cytokines and chemicals. Hepatocyte growth factor (HGF) down-regulated SLCO1B1 and SLCO2B1 mRNA and protein while mRNA for SLCO1B3 was not affected (Le Vee et al., 2009). Similarly, down-regulation of SLCO1B1, SLCO1B3 and SLCO2B1 mRNA as well as OATP1B1 at the protein level was shown by interleukin 1β (Le Vee et al., 2008).
Post-translational regulation was demonstrated for OATP2B1. Protein kinase C activation by a phorbol ester resulted in increased phosphorylation of OATP2B1 with a decrease in maximal transport rate suggesting a rapid internalization of the transporter (Kock et al., 2010).
All these regulations at the transcriptional and post-translational levels could lead to increased or decreased OATP-mediated uptake. However, as outlined above, modulation of OATP-mediated transport could also be due to direct effects of substances, e.g. by acting from the cis-side either as inhibitors or as stimulators of substrate transport. Furthermore, several of these modulators have been shown to act in a substrate-dependent way, making predictions of potential interactions more complicated. For example, rifampicin at 10 μM had no effect on OATP1B1-mediated uptake of bromosulfophthalein (BSP) (Vavricka et al., 2002) but inhibited estradiol-17β-glucuronide uptake by 90 % (Tirona et al., 2003). In another study, 100 μM gemfibrozil inhibited OATP1B1-mediated uptake of taurocholate, fluvastatin and simvastatin by about 60 % but had no effect on the uptake of estrone-3-sulfate or troglitazone-sulfate (Noe et al., 2007). Clotrimazole, epigallocatechin gallate, diclofenac and ibuprofen are among the chemicals that have been shown to have stimulatory, inhibitory or no effects on OATP-mediated transport in a substrate-dependent way (Gui et al., 2008; Kindla et al., 2011; Roth et al., 2011). Thus, because the molecular mechanisms of these modulations are not known yet it is crucial to test more than just a single OATP substrate when screening for potential interactions, e.g. to predict pharmacokinetic interactions during drug development.
7. Structure function relationship
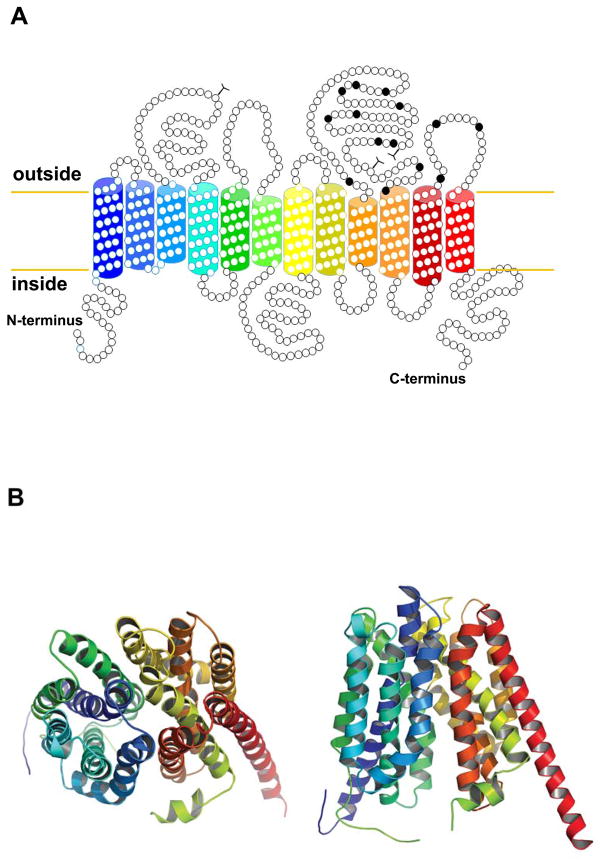
Based on hydrophobicity analyses, experimental data available from rat OATP1A1 (Jacquemin et al., 1994; Wang et al., 2008) and homology modeling, OATPs are 12 transmembrane domain proteins with the amino- and the C-terminal ends located at the cytoplasmic side of the membrane (Figure 3A). Because so far no crystal structure data are available, homology modeling was used to predict a putative three-dimensional model (Figure 3B). Furthermore, several groups have used chimeric approaches combined with site-directed mutagenesis and homology modeling to investigate what regions or what amino acids would be important for OATP function. Based on such studies it became clear that cysteine residues in the large extracellular loop 5 are involved in disulfide bonds and are required for proper surface expression of OATP2B1 (Hanggi et al., 2006) (Figure 3A). Transmembrane domains 1, 8, 9 and 10 as well as extracellular loop 6 have been shown to be important for OATP1B1 and OATP1B3 substrate transport (Degorter et al., 2012; Gui and Hagenbuch, 2008, 2009; Miyagawa et al., 2009). Furthermore, site-directed mutagenesis of conserved positively charged amino acids identified several residues in transmembrane domains 1, 7 and 10 that affect substrate transport in OATP1B1 and OATP1B3 (Glaeser et al., 2010; Mandery et al., 2011; Weaver and Hagenbuch, 2010). A recent study demonstrated that when the three amino acids A45, L545 and T615 in OATP1B1 were replaced to their corresponding residues in OATP1B3, OATP1B1 was able to transport the OATP1B3-selective substrate cholecystokinin-8 (CCK-8) (Degorter et al., 2012), suggesting that these amino acids are indeed involved in substrate recognition or transport by OATP1B3. These studies are important because they will help to eventually understand the molecular mechanisms of the broad substrate specificity of OATPs and potentially allow predicting and thus preventing drug-drug interactions at the OATP levels.
Figure 3.
Topology models for OATP1B1. A) The predicted secondary structure model for OATP1B1 is shown with 12 transmembrane domains. The extracellular conserved cysteine residues are labelled in black. B) Homology modelling was performed as described (Roth et al., 2012) based on the E. coli glycerol-3-phosphate transporter. OATP1B1 is shown from the extracellular side (left) and from within the lipid bilayer (right). Colors of the transmembrane domains match the colors used in the secondary structure model.
8. Mutations and Polymorphisms of OATPs
So far, few pathophysiologic conditions related to mutations in SLCO genes have been reported. Mesolemia-synosteses syndrome (OMIM600383) is a rare disease and includes mesomelic limb shortening and acral synosthoses (Isidor et al., 2009). This syndrome has been linked to a disturbance in sulfate metabolism and/or homoestasis (Dawson, 2011). Cytogenetic analysis of 5 patients from four families with this disease identified a submicroscopic microdeletion on chromosome 8q13 (Isidor et al., 2010). This deletion spans the two genes SULF1 (heparin sulphate 6-O-endosulfatase 1) and SLCO5A1 (OATP5A1). OATP5A1 is expressed in adult heart and in fetal brain and heart (Isidor et al., 2010), but its function has not been characterized so far. As in all patients deletions spanned both the SULF1 and the SLCO5A1 gene, the contribution of missing or malfunctioning OATP5A1 remains to be worked out, in particular as in a single healthy individual a partial deletion of SLCO5A1 was reported (de Smith et al., 2007). Rotor syndrome is a rare, benign syndrome presenting with conjugated and unconjugated hyperbilirubinemia in conjunction with coproporphyrinuria and a massively altered BSP clearance (Strassburg, 2010). Its inheritance is autosomal recessive. A recent investigation of individuals with Rotor syndrome from eight different families revealed mutations in the SLCO1B1 and SLCO1B3 genes, rendering the respective OATPs non-functional (van de Steeg et al., 2012). Mice with a disrupted Slco1a/1b locus also presented with unconjugated and conjugated hyperbilirubinemia. (van de Steeg et al., 2012). A study with healthy volunteers associated elevated conjugated and unconjugated bilirubin with the *15 allele of SLCO1B1, which is known to display reduced transport activity of the corresponding protein (Zhang et al., 2007). A study investigating the effect of the p.V174A variant of OATP1B1 on thyroid and estrogen metabolite levels in serum found total bilirubin, estrone-3-sulfate and thyroxine sulphate levels to be higher in individuals with the p.174A variant (van der Deure et al., 2008a). A genome-wide association study associated SLCO1B3 variants with elevated total and unconjugated serum bilirubin (Sanna et al., 2009), while a meta-analysis of three studies associated a SLCO1B1 variant with elevated serum bilirubin (Johnson et al., 2009). The finding of the latter study was recently confirmed in an independent genome-wide association study (Bielinski et al., 2011). Hence, there is now ample genetic evidence for a role of OATP1B1 and OATP1B3 in hepatocellular uptake of unconjugated and conjugated bilirubin. Finally, serum levels of reverse triiodothyronine have also been reported to be elevated in carriers of the OAPT1A2 p.172D variant (van der Deure et al., 2010).
A genome-wide association study in a cohort of individuals with progressive supranuclear palsy found a suggestive association with SLCO1A2 in addition to other loci (Hoglinger et al., 2011). Additionally, a genome-wide association study of Crohn’s diseases in an Ashekenazi Jewish population found a variant of the SLCO6A1 to be disease associated (Kenny et al., 2012).
While evidence for the involvement of SLCO genes in the pathogenesis of diseases is starting to emerge, a multitude of studies have investigated the role of SLCO variants on drug disposition with a particular focus on pharmacokinetics of drugs. Many reviews have covered the role of genetic SLCO variants on pharmacokinetics of drugs (Fahrmayr et al., 2010; Franke et al., 2010; Kerb, 2006; Konig, 2011; Niemi et al., 2011; Sissung et al., 2010; Stieger and Meier, 2011; Zair et al., 2008). Understanding the impact of SLCO pharmacogenomics is not only relevant for understanding alterations in pharmacokinetics of drugs in patients with different SLCO genotypes (Kalliokoski and Niemi, 2009), but also contributes to an understanding of adverse drug reactions. This is exemplified in the case of SLCO1B1, which in an elegant genome-wide association study was associated with simvastatin-induced myotoxicity (Link et al., 2008). This and other studies have been the basis to suggest a dosing regimen for statins, which takes the SLCO1B1 genotype into account (Niemi, 2010).
9. Conclusion and Outlook
Since the cloning of the first OATP, the SLCO family members have made it to center stage in drug development (Giacomini et al., 2010) and in the understanding of drug disposition (Fenner et al., 2012). While the progress in developing tools for understanding the role of OATPs in handling of endo- and xenobiotics has been enormous, knowledge on their molecular transport mechanisms and on their structure is clearly lagging behind. Both areas are however highly relevant for developing better models to predict their impact in physiology and pathophysiology as well as in drug disposition. In addition, OATPs are increasingly recognized as important transporters in cancer therapy (Obaidat et al., 2012) and in understanding clearance tests like e.g. liver function tests (Stieger et al., 2012).
Acknowledgments
The authors would like to acknowledge the National Institutes of Health grants RR021940 and GM077336, the Swiss National Science Foundation Grant # 31003A_124652 for their support, and thank Melanie Hagenbuch for her help with the artwork
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, Matsuno S, Yawo H. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem. 1999;274:17159–17163. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, Nunoki K, Sato E, Kakyo M, Nishio T, Sugita J, Asano N, Tanemoto M, Seki M, Date F, Ono K, Kondo Y, Shiiba K, Suzuki M, Ohtani H, Shimosegawa T, Iinuma K, Nagura H, Ito S, Matsuno S. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120 (7):1689–1699. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- Adachi H, Suzuki T, Abe M, Asano N, Mizutamari H, Tanemoto M, Nishio T, Onogawa T, Toyohara T, Kasai S, Satoh F, Suzuki M, Tokui T, Unno M, Shimosegawa T, Matsuno S, Ito S, Abe T. Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol. 2003;285 (6):F1188–1197. doi: 10.1152/ajprenal.00402.2002. [DOI] [PubMed] [Google Scholar]
- Alkemade A, Friesema EC, Kalsbeek A, Swaab DF, Visser TJ, Fliers E. Expression of thyroid hormone transporters in the human hypothalamus. J Clin Endocrinol Metab. 2011;96 (6):E967–971. doi: 10.1210/jc.2010-2750. [DOI] [PubMed] [Google Scholar]
- Bednarczyk D. Fluorescence-based assays for the assessment of drug interaction with the human transporters OATP1B1 and OATP1B3. Anal Biochem. 2010;405 (1):50–58. doi: 10.1016/j.ab.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinski SJ, Chai HS, Pathak J, Talwalkar JA, Limburg PJ, Gullerud RE, Sicotte H, Klee EW, Ross JL, Kocher JP, Kullo IJ, Heit JA, Petersen GM, de Andrade M, Chute CG. Mayo Genome Consortia: a genotype-phenotype resource for genome-wide association studies with an application to the analysis of circulating bilirubin levels. Mayo Clin Proc. 2011;86 (7):606–614. doi: 10.4065/mcp.2011.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasby K, Castle JC, Roberts CJ, Cheng C, Bailey WJ, Sina JF, Kulkarni AV, Hafey MJ, Evers R, Johnson JM, Ulrich RG, Slatter JG. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36 (10–11):963–988. doi: 10.1080/00498250600861751. [DOI] [PubMed] [Google Scholar]
- Bossuyt X, Muller M, Hagenbuch B, Meier PJ. Polyspecific drug and steroid clearance by an organic anion transporter of mammalian liver. J Pharmacol Exp Ther. 1996a;276 (3):891–896. [PubMed] [Google Scholar]
- Bossuyt X, Muller M, Meier PJ. Multispecific amphipathic substrate transport by an organic anion transporter of human liver. J Hepatol. 1996b;25 (5):733–738. doi: 10.1016/s0168-8278(96)80246-7. [DOI] [PubMed] [Google Scholar]
- Briz O, Romero MR, Martinez-Becerra P, Macias RI, Perez MJ, Jimenez F, San Martin FG, Marin JJ. OATP8/1B3-mediated cotransport of bile acids and glutathione: an export pathway for organic anions from hepatocytes? J Biol Chem. 2006;281 (41):30326–30335. doi: 10.1074/jbc.M602048200. [DOI] [PubMed] [Google Scholar]
- Briz O, Serrano MA, MacIas RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003;371 (Pt 3):897–905. doi: 10.1042/BJ20030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, Keppler D, Nies AT. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65 (24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- Cattori V, Hagenbuch B, Hagenbuch N, Stieger B, Ha R, Winterhalter KE, Meier PJ. Identification of organic anion transporting polypeptide 4 (Oatp4) as a major full-length isoform of the liver-specific transporter-1 (rlst-1) in rat liver. FEBS Lett. 2000;474 (2–3):242–245. doi: 10.1016/s0014-5793(00)01596-9. [DOI] [PubMed] [Google Scholar]
- Cattori V, van Montfoort JE, Stieger B, Landmann L, Meijer DK, Winterhalter KH, Meier PJ, Hagenbuch B. Localization of organic anion transporting polypeptide 4 (Oatp4) in rat liver and comparison of its substrate specificity with Oatp1, Oatp2 and Oatp3. Pflugers Arch. 2001;443 (2):188–195. doi: 10.1007/s004240100697. [DOI] [PubMed] [Google Scholar]
- Choi K, Zhuang H, Crain B, Dore S. Expression and localization of prostaglandin transporter in Alzheimer disease brains and age-matched controls. J Neuroimmunol. 2008;195 (1–2):81–87. doi: 10.1016/j.jneuroim.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology. 2011;53 (1):272–281. doi: 10.1002/hep.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Konig J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276 (13):9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- Cui Y, Konig J, Nies AT, Pfannschmidt M, Hergt M, Franke WW, Alt W, Moll R, Keppler D. Detection of the human organic anion transporters SLC21A6 (OATP2) and SLC21A8 (OATP8) in liver and hepatocellular carcinoma. Lab Invest. 2003;83 (4):527–538. doi: 10.1097/01.lab.0000065015.02412.48. [DOI] [PubMed] [Google Scholar]
- Dawson PA. Sulfate in fetal development. Semin Cell Dev Biol. 2011;22 (6):653–659. doi: 10.1016/j.semcdb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- de Graaf W, Hausler S, Heger M, van Ginhoven TM, van Cappellen G, Bennink RJ, Kullak-Ublick GA, Hesselmann R, van Gulik TM, Stieger B. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol. 2011;54 (4):738–745. doi: 10.1016/j.jhep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- de Smith AJ, Tsalenko A, Sampas N, Scheffer A, Yamada NA, Tsang P, Ben-Dor A, Yakhini Z, Ellis RJ, Bruhn L, Laderman S, Froguel P, Blakemore AI. Array CGH analysis of copy number variation identifies 1284 new genes variant in healthy white males: implications for association studies of complex diseases. Hum Mol Genet. 2007;16 (23):2783–2794. doi: 10.1093/hmg/ddm208. [DOI] [PubMed] [Google Scholar]
- Degorter MK, Ho RH, Leake BF, Tirona RG, Kim RB. Interaction of Three Regiospecific Amino Acid Residues Is Required for OATP1B1 Gain of OATP1B3 Substrate Specificity. Mol Pharm. 2012;9 (4):986–995. doi: 10.1021/mp200629s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson PD, Lanthaler K, Oliver SG, Kell DB. Implications of the dominant role of transporters in drug uptake by cells. Curr Top Med Chem. 2009;9 (2):163–181. doi: 10.2174/156802609787521616. [DOI] [PubMed] [Google Scholar]
- Eckhardt U, Schroeder A, Stieger B, Hochli M, Landmann L, Tynes R, Meier PJ, Hagenbuch B. Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am J Physiol. 1999;276 (4 Pt 1):G1037–1042. doi: 10.1152/ajpgi.1999.276.4.G1037. [DOI] [PubMed] [Google Scholar]
- Fahrmayr C, Fromm MF, Konig J. Hepatic OATP and OCT uptake transporters: their role for drug-drug interactions and pharmacogenetic aspects. Drug Metab Rev. 2010;42 (3):380–401. doi: 10.3109/03602530903491683. [DOI] [PubMed] [Google Scholar]
- Fenner KS, Jones HM, Ullah M, Kempshall S, Dickins M, Lai Y, Morgan P, Barton HA. The evolution of the OATP hepatic uptake transport protein family in DMPK sciences: from obscure liver transporters to key determinants of hepatobiliary clearance. Xenobiotica. 2012;42 (1):28–45. doi: 10.3109/00498254.2011.626464. [DOI] [PubMed] [Google Scholar]
- Franke RM, Gardner ER, Sparreboom A. Pharmacogenetics of drug transporters. Curr Pharm Des. 2010;16 (2):220–230. doi: 10.2174/138161210790112683. [DOI] [PubMed] [Google Scholar]
- Friesema EC, Docter R, Moerings EP, Stieger B, Hagenbuch B, Meier PJ, Krenning EP, Hennemann G, Visser TJ. Identification of thyroid hormone transporters. Biochem Biophys Res Commun. 1999;254 (2):497–501. doi: 10.1006/bbrc.1998.9974. [DOI] [PubMed] [Google Scholar]
- Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y. Effects of herbal extracts on the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2006;34 (4):577–582. doi: 10.1124/dmd.105.007872. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, Onogawa T, Suzuki T, Asano N, Tanemoto M, Seki M, Shiiba K, Suzuki M, Kondo Y, Nunoki K, Shimosegawa T, Iinuma K, Ito S, Matsuno S, Abe T. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology. 2001;142:2005–2012. doi: 10.1210/endo.142.5.8115. [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- Gao B, Huber RD, Wenzel A, Vavricka SR, Ismair MG, Reme C, Meier PJ. Localization of organic anion transporting polypeptides in the rat and human ciliary body epithelium. Exp Eye Res. 2005;80 (1):61–72. doi: 10.1016/j.exer.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9 (3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser H, Mandery K, Sticht H, Fromm MF, Konig J. Relevance of conserved lysine and arginine residues in transmembrane helices for the transport activity of organic anion transporting polypeptide 1B3. Br J Pharmacol. 2010;159 (3):698–708. doi: 10.1111/j.1476-5381.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Kock K, Karner S, Reuther S, Ritter CA, Jedlitschky G, Kroemer HK. Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol. 2006a;70 (5):1735–1741. doi: 10.1124/mol.106.026450. [DOI] [PubMed] [Google Scholar]
- Grube M, Kock K, Oswald S, Draber K, Meissner K, Eckel L, Bohm M, Felix SB, Vogelgesang S, Jedlitschky G, Siegmund W, Warzok R, Kroemer HK. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006b;80 (6):607–620. doi: 10.1016/j.clpt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Gui C, Hagenbuch B. Amino acid residues in transmembrane domain 10 of organic anion transporting polypeptide 1B3 are critical for cholecystokinin octapeptide transport. Biochemistry. 2008;47 (35):9090–9097. doi: 10.1021/bi8008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Hagenbuch B. Role of transmembrane domain 10 for the function of organic anion transporting polypeptide 1B1. Protein Sci. 2009;18 (11):2298–2306. doi: 10.1002/pro.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, Hagenbuch B. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584 (1):57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica. 2008;38 (7–8):778–801. doi: 10.1080/00498250801986951. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: Phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Hanggi E, Grundschober AF, Leuthold S, Meier PJ, St-Pierre MV. Functional analysis of the extracellular cysteine residues in the human organic anion transporting polypeptide, OATP2B1. Mol Pharmacol. 2006;70 (3):806–817. doi: 10.1124/mol.105.019547. [DOI] [PubMed] [Google Scholar]
- Hazard SE, Patel SB. Sterolins ABCG5 and ABCG8: regulators of whole body dietary sterols. Pflugers Arch. 2007;453 (5):745–752. doi: 10.1007/s00424-005-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447 (5):465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311 (1):139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab Dispos. 2006;34 (7):1229–1236. doi: 10.1124/dmd.106.009290. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L, Rademakers R, de Silva R, Litvan I, Riley DE, van Swieten JC, Heutink P, Wszolek ZK, Uitti RJ, Vandrovcova J, Hurtig HI, Gross RG, Maetzler W, Goldwurm S, Tolosa E, Borroni B, Pastor P, Cantwell LB, Han MR, Dillman A, van der Brug MP, Gibbs JR, Cookson MR, Hernandez DG, Singleton AB, Farrer MJ, Yu CE, Golbe LI, Revesz T, Hardy J, Lees AJ, Devlin B, Hakonarson H, Muller U, Schellenberg GD. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43 (7):699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- Huber RD, Gao B, Sidler Pfandler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, Folkers G, Meier PJ, Stieger B. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol. 2007;292 (2):C795–806. doi: 10.1152/ajpcell.00597.2005. [DOI] [PubMed] [Google Scholar]
- Ishizuka H, Konno K, Naganuma H, Nishimura K, Kouzuki H, Suzuki H, Stieger B, Meier PJ, Sugiyama Y. Transport of temocaprilat into rat hepatocytes: role of organic anion transporting polypeptide. J Pharmacol Exp Ther. 1998;287 (1):37–42. [PubMed] [Google Scholar]
- Isidor B, Hamel A, Plasschaert F, Claus L, Mercier JM, Mortier GR, Leroy JG, Verloes A, David A. Mesomelic dysplasia with acral synostoses Verloes-David-Pfeiffer type: follow-up study documents progressive clinical course. Am J Med Genet A. 2009;149A (10):2220–2225. doi: 10.1002/ajmg.a.32926. [DOI] [PubMed] [Google Scholar]
- Isidor B, Pichon O, Redon R, Day-Salvatore D, Hamel A, Siwicka KA, Bitner-Glindzicz M, Heymann D, Kjellen L, Kraus C, Leroy JG, Mortier GR, Rauch A, Verloes A, David A, Le Caignec C. Mesomelia-synostoses syndrome results from deletion of SULF1 and SLCO5A1 genes at 8q13. Am J Hum Genet. 2010;87 (1):95–100. doi: 10.1016/j.ajhg.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismair MG, Stieger B, Cattori V, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. Hepatic uptake of cholecystokinin octapeptide by organic anion-transporting polypeptides OATP4 and OATP8 of rat and human liver. Gastroenterology. 2001;121 (5):1185–1190. doi: 10.1053/gast.2001.28704. [DOI] [PubMed] [Google Scholar]
- Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression cloning of a rat liver Na(+)-independent organic anion transporter. Proc Natl Acad Sci U S A. 1994;91 (1):133–137. doi: 10.1073/pnas.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J, Friesema EC, Milici C, Visser TJ. Thyroid hormone transporters in health and disease. Thyroid. 2005;15 (8):757–768. doi: 10.1089/thy.2005.15.757. [DOI] [PubMed] [Google Scholar]
- Ji C, Tschantz WR, Pfeifer ND, Ullah M, Sadagopan N. Development of a multiplex UPLC-MRM MS method for quantification of human membrane transport proteins OATP1B1, OATP1B3 and OATP2B1 in in vitro systems and tissues. Anal Chim Acta. 2012;717:67–76. doi: 10.1016/j.aca.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34 (10):1756–1763. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Kavousi M, Smith AV, Chen MH, Dehghan A, Aspelund T, Lin JP, van Duijn CM, Harris TB, Cupples LA, Uitterlinden AG, Launer L, Hofman A, Rivadeneira F, Stricker B, Yang Q, O’Donnell CJ, Gudnason V, Witteman JC. Genome-wide association meta-analysis for total serum bilirubin levels. Hum Mol Genet. 2009;18 (14):2700–2710. doi: 10.1093/hmg/ddp202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Hagenbuch B, Gresh L, Pontoglio M, Meier PJ, Kullak Ublick GA. Characterization of the human OATP-C (SLC21A6) gene promoter and regulation of liver-specific OATP genes by hepatocyte nuclear factor 1 alpha. J Biol Chem. 2001;276 (40):37206–37214. doi: 10.1074/jbc.M103988200. [DOI] [PubMed] [Google Scholar]
- Jung D, Podvinec M, Meyer UA, Mangelsdorf DJ, Fried M, Meier PJ, Kullak-Ublick GA. Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology. 2002;122 (7):1954–1966. doi: 10.1053/gast.2002.33583. [DOI] [PubMed] [Google Scholar]
- Kakyo M, Unno M, Tokui T, Nakagomi R, Nishio T, Iwasashi H, Nakai D, Seki M, Suzuki M, Naitoh T, Matsuno S, Yawo H, Abe T. Molecular characterization and functional regulation of a novel rat liver-specific organic anion transporter rlst-1. Gastroenterology. 1999;117 (4):770–775. doi: 10.1016/s0016-5085(99)70333-1. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158 (3):693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science. 1995;268 (5212):866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- Kang J, Chapdelaine P, Parent J, Madore E, Laberge PY, Fortier MA. Expression of human prostaglandin transporter in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2005;90 (4):2308–2313. doi: 10.1210/jc.2004-1482. [DOI] [PubMed] [Google Scholar]
- Kellner HM, Christ O, Rupp W, Heptner W. Resorption, distribution and excretion after administration of 14C-labelled HB 419 in rabbits, rats and dogs. Arzneimittelforschung. 1969;19(8 Suppl):1388–1400. [PubMed] [Google Scholar]
- Kenny EE, Pe’er I, Karban A, Ozelius L, Mitchell AA, Ng SM, Erazo M, Ostrer H, Abraham C, Abreu MT, Atzmon G, Barzilai N, Brant SR, Bressman S, Burns ER, Chowers Y, Clark LN, Darvasi A, Doheny D, Duerr RH, Eliakim R, Giladi N, Gregersen PK, Hakonarson H, Jones MR, Marder K, McGovern DP, Mulle J, Orr-Urtreger A, Proctor DD, Pulver A, Rotter JI, Silverberg MS, Ullman T, Warren ST, Waterman M, Zhang W, Bergman A, Mayer L, Katz S, Desnick RJ, Cho JH, Peter I. A genome-wide scan of ashkenazi jewish Crohn’s disease suggests novel susceptibility Loci. PLoS Genet. 2012;8 (3):e1002559. doi: 10.1371/journal.pgen.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerb R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006;234 (1):4–33. doi: 10.1016/j.canlet.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Kindla J, Rau TT, Jung R, Fasching PA, Strick R, Stoehr R, Hartmann A, Fromm MF, Konig J. Expression and localization of the uptake transporters OATP2B1, OATP3A1 and OATP5A1 in non-malignant and malignant breast tissue. Cancer Biol Ther. 2011;11 (6):584–591. doi: 10.4161/cbt.11.6.14533. [DOI] [PubMed] [Google Scholar]
- Knauer MJ, Urquhart BL, Meyer zu Schwabedissen HE, Schwarz UI, Lemke CJ, Leake BF, Kim RB, Tirona RG. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106 (2):297–306. doi: 10.1161/CIRCRESAHA.109.203596. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–708. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- Kock K, Koenen A, Giese B, Fraunholz M, May K, Siegmund W, Hammer E, Volker U, Jedlitschky G, Kroemer HK, Grube M. Rapid modulation of the organic anion transporting polypeptide 2B1 (OATP2B1, SLCO2B1) function by protein kinase C-mediated internalization. J Biol Chem. 2010;285 (15):11336–11347. doi: 10.1074/jbc.M109.056457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig J. Uptake transporters of the human OATP family. Molecular characteristics, substrates, their role in drug-drug interactions, and functional consequences of polymorphisms. Handb Exp Pharmacol. 2011;201:1–28. doi: 10.1007/978-3-642-14541-4_1. [DOI] [PubMed] [Google Scholar]
- Konig J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000a;275:23161–23168. doi: 10.1074/jbc.M001448200. [DOI] [PubMed] [Google Scholar]
- Konig J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000b;278:G156–164. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- Kouzuki H, Suzuki H, Ito K, Ohashi R, Sugiyama Y. Contribution of organic anion transporting polypeptide to uptake of its possible substrates into rat hepatocytes. J Pharmacol Exp Ther. 1999;288 (2):627–634. [PubMed] [Google Scholar]
- Kraft ME, Glaeser H, Mandery K, Konig J, Auge D, Fromm MF, Schlotzer-Schrehardt U, Welge-Lussen U, Kruse FE, Zolk O. The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Invest Ophthalmol Vis Sci. 2010;51 (5):2504–2511. doi: 10.1167/iovs.09-4290. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Fisch T, Oswald M, Hagenbuch B, Meier PJ, Beuers U, Paumgartner G. Dehydroepiandrosterone sulfate (DHEAS): identification of a carrier protein in human liver and brain. FEBS Lett. 1998;424 (3):173–176. doi: 10.1016/s0014-5793(98)00168-9. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109:1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Functional characterization of the basolateral rat liver organic anion transporting polypeptide. Hepatology. 1994;20 (2):411–416. [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120 (2):525–533. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. In vitro-in vivo extrapolation of transporter-mediated clearance in the liver and kidney. Drug Metab Pharmacokinet. 2009;24 (1):37–52. doi: 10.2133/dmpk.24.37. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Gripon P, Stieger B, Fardel O. Down-regulation of organic anion transporter expression in human hepatocytes exposed to the proinflammatory cytokine interleukin 1beta. Drug Metab Dispos. 2008;36 (2):217–222. doi: 10.1124/dmd.107.016907. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Lecureur V, Moreau A, Stieger B, Fardel O. Differential regulation of drug transporter expression by hepatocyte growth factor in primary human hepatocytes. Drug Metab Dispos. 2009;37 (11):2228–2235. doi: 10.1124/dmd.109.028035. [DOI] [PubMed] [Google Scholar]
- Lecerf JM, de Lorgeril M. Dietary cholesterol: from physiology to cardiovascular risk. Br J Nutr. 2011;106 (1):6–14. doi: 10.1017/S0007114511000237. [DOI] [PubMed] [Google Scholar]
- Lee SY, Williamson B, Caballero OL, Chen YT, Scanlan MJ, Ritter G, Jongeneel CV, Simpson AJ, Old LJ. Identification of the gonad-specific anion transporter SLCO6A1 as a cancer/testis (CT) antigen expressed in human lung cancer. Cancer Immun. 2004;4:13. [PubMed] [Google Scholar]
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, Leake BF, Kim RB. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280 (10):9610–9617. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am J Physiol Cell Physiol. 2009;296 (3):C570–582. doi: 10.1152/ajpcell.00436.2008. [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Meier PJ, Ballatori N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem. 1998;273 (26):16184–16191. doi: 10.1074/jbc.273.26.16184. [DOI] [PubMed] [Google Scholar]
- Li L, Meier PJ, Ballatori N. Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol. 2000;58 (2):335–340. doi: 10.1124/mol.58.2.335. [DOI] [PubMed] [Google Scholar]
- Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359 (8):789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- Loubiere LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta. 2010;31 (4):295–304. doi: 10.1016/j.placenta.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA(hPGT) J Clin Invest. 1996;98 (5):1142–1149. doi: 10.1172/JCI118897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Kambara M, Tian Y, Hofmann AF, Sugiyama Y. Uptake of ursodeoxycholate and its conjugates by human hepatocytes: role of Na(+)-taurocholate cotransporting polypeptide (NTCP), organic anion transporting polypeptide (OATP) 1B1 (OATP-C), and oatp1B3 (OATP8) Mol Pharm. 2006;3 (1):70–77. doi: 10.1021/mp050063u. [DOI] [PubMed] [Google Scholar]
- Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. Human organic anion transporter 1B1 and 1B3 function as bidirectional carriers and do not mediate GSH-bile acid cotransport. Am J Physiol Gastrointest Liver Physiol. 2007;293 (1):G271–278. doi: 10.1152/ajpgi.00075.2007. [DOI] [PubMed] [Google Scholar]
- Mandery K, Bujok K, Schmidt I, Wex T, Treiber G, Malfertheiner P, Rau TT, Amann KU, Brune K, Fromm MF, Glaeser H. Influence of Cyclooxygenase Inhibitors on the Function of the Prostaglandin Transporter Organic Anion-Transporting Polypeptide 2A1 Expressed in Human Gastroduodenal Mucosa. Journal of Pharmacology and Experimental Therapeutics. 2010;332 (2):345–351. doi: 10.1124/jpet.109.154518. [DOI] [PubMed] [Google Scholar]
- Mandery K, Sticht H, Bujok K, Schmidt I, Fahrmayr C, Balk B, Fromm MF, Glaeser H. Functional and structural relevance of conserved positively charged lysine residues in organic anion transporting polypeptide 1B3. Mol Pharmacol. 2011;80 (3):400–406. doi: 10.1124/mol.111.071282. [DOI] [PubMed] [Google Scholar]
- Masuda S, Ibaramoto K, Takeuchi A, Saito H, Hashimoto Y, Inui KI. Cloning and functional characterization of a new multispecific organic anion transporter, OAT-K2, in rat kidney. Mol Pharmacol. 1999;55 (4):743–752. [PubMed] [Google Scholar]
- Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. Impact of oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 2012;153 (3):1528–1537. doi: 10.1210/en.2011-1633. [DOI] [PubMed] [Google Scholar]
- Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997;26 (6):1667–1677. doi: 10.1002/hep.510260641. [DOI] [PubMed] [Google Scholar]
- Meyer Zu Schwabedissen HE, Bottcher K, Chaudhry A, Kroemer HK, Schuetz EG, Kim RB. Liver X receptor alpha and farnesoid X receptor are major transcriptional regulators of OATP1B1. Hepatology. 2010;52 (5):1797–1807. doi: 10.1002/hep.23876. [DOI] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res. 2008;68 (22):9338–9347. doi: 10.1158/0008-5472.CAN-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res. 2006;66 (1):535–542. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, Abe M, Satoh F, Unno M, Hishinuma T, Inui K, Ito S, Goto J, Abe T. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci U S A. 2004;101:3569–3574. doi: 10.1073/pnas.0304987101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa M, Maeda K, Aoyama A, Sugiyama Y. The eighth and ninth transmembrane domains in organic anion transporting polypeptide 1B1 affect the transport kinetics of estrone-3-sulfate and estradiol-17beta-D-glucuronide. J Pharmacol Exp Ther. 2009;329 (2):551–557. doi: 10.1124/jpet.108.148411. [DOI] [PubMed] [Google Scholar]
- Nagai M, Furihata T, Matsumoto S, Ishii S, Motohashi S, Yoshino I, Ugajin M, Miyajima A, Chiba K. Identification of a new organic anion transporting polypeptide 1B3 mRNA isoform primarily expressed in human cancerous tissues and cells. Biochem Biophys Res Commun. 2012;418 (4):818–823. doi: 10.1016/j.bbrc.2012.01.115. [DOI] [PubMed] [Google Scholar]
- Nakai D, Nakagomi R, Furuta Y, Tokui T, Abe T, Ikeda T, Nishimura K. Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J Pharmacol Exp Ther. 2001;297 (3):861–867. [PubMed] [Google Scholar]
- Niemi M. Transporter pharmacogenetics and statin toxicity. Clin Pharmacol Ther. 2010;87 (1):130–133. doi: 10.1038/clpt.2009.197. [DOI] [PubMed] [Google Scholar]
- Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63 (1):157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- Niessen J, Jedlitschky G, Grube M, Bien S, Schwertz H, Ohtsuki S, Kawakami H, Kamiie J, Oswald S, Starke K, Strobel U, Siegmund W, Rosskopf D, Greinacher A, Terasaki T, Kroemer HK. Human platelets express organic anion-transporting peptide 2B1, an uptake transporter for atorvastatin. Drug Metab Dispos. 2009;37 (5):1129–1137. doi: 10.1124/dmd.108.024570. [DOI] [PubMed] [Google Scholar]
- Nishio T, Adachi H, Nakagomi R, Tokui T, Sato E, Tanemoto M, Fujiwara K, Okabe M, Onogawa T, Suzuki T, Nakai D, Shiiba K, Suzuki M, Ohtani H, Kondo Y, Unno M, Ito S, Iinuma K, Nunoki K, Matsuno S, Abe T. Molecular identification of a rat novel organic anion transporter moat1, which transports prostaglandin D(2), leukotriene C(4), and taurocholate. Biochem Biophys Res Commun. 2000;275 (3):831–838. doi: 10.1006/bbrc.2000.3377. [DOI] [PubMed] [Google Scholar]
- Noe B, Hagenbuch B, Stieger B, Meier PJ. Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. Proc Natl Acad Sci U S A. 1997;94 (19):10346–10350. doi: 10.1073/pnas.94.19.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe J, Portmann R, Brun ME, Funk C. Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos. 2007;35 (8):1308–1314. doi: 10.1124/dmd.106.012930. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004;308:438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- Obaidat A, Roth M, Hagenbuch B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol. 2012;52:135–151. doi: 10.1146/annurev-pharmtox-010510-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow JD, Mukerjee P, Tiribelli C. Structure and binding of unconjugated bilirubin: relevance for physiological and pathophysiological function. J Lipid Res. 1994;35 (10):1715–1737. [PubMed] [Google Scholar]
- Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol. 2002;16 (10):2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV. Identification of steroid sulfate transport processes in the human mammary gland. J Clin Endocrinol Metab. 2003;88 (8):3902–3912. doi: 10.1210/jc.2003-030174. [DOI] [PubMed] [Google Scholar]
- Reichel C, Gao B, Van Montfoort J, Cattori V, Rahner C, Hagenbuch B, Stieger B, Kamisako T, Meier PJ. Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology. 1999;117 (3):688–695. doi: 10.1016/s0016-5085(99)70463-4. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149 (12):6251–6261. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Timmermann BN, Hagenbuch B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos. 2011;39 (5):920–926. doi: 10.1124/dmd.110.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Beatty JT, Goffeau A, Harley KT, Heijne WH, Huang SC, Jack DL, Jahn PS, Lew K, Liu J, Pao SS, Paulsen IT, Tseng TT, Virk PS. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1 (2):257–279. [PubMed] [Google Scholar]
- Sanna S, Busonero F, Maschio A, McArdle PF, Usala G, Dei M, Lai S, Mulas A, Piras MG, Perseu L, Masala M, Marongiu M, Crisponi L, Naitza S, Galanello R, Abecasis GR, Shuldiner AR, Schlessinger D, Cao A, Uda M. Common variants in the SLCO1B3 locus are associated with bilirubin levels and unconjugated hyperbilirubinemia. Hum Mol Genet. 2009;18 (14):2711–2718. doi: 10.1093/hmg/ddp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satlin LM, Amin V, Wolkoff AW. Organic anion transporting polypeptide mediates organic anion/HCO3- exchange. J Biol Chem. 1997;272 (42):26340–26345. doi: 10.1074/jbc.272.42.26340. [DOI] [PubMed] [Google Scholar]
- Sato K, Sugawara J, Sato T, Mizutamari H, Suzuki T, Ito A, Mikkaichi T, Onogawa T, Tanemoto M, Unno M, Abe T, Okamura K. Expression of organic anion transporting polypeptide E (OATP-E) in human placenta. Placenta. 2003;24 (2–3):144–148. doi: 10.1053/plac.2002.0907. [DOI] [PubMed] [Google Scholar]
- Schiffer R, Neis M, Holler D, Rodriguez F, Geier A, Gartung C, Lammert F, Dreuw A, Zwadlo-Klarwasser G, Merk H, Jugert F, Baron JM. Active influx transport is mediated by members of the organic anion transporting polypeptide family in human epidermal keratinocytes. J Invest Dermatol. 2003;120 (2):285–291. doi: 10.1046/j.1523-1747.2003.12031.x. [DOI] [PubMed] [Google Scholar]
- Sissung TM, Baum CE, Kirkland CT, Gao R, Gardner ER, Figg WD. Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010;44 (2):152–167. doi: 10.1007/s12033-009-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab. 2002;87 (4):1856–1863. doi: 10.1210/jcem.87.4.8431. [DOI] [PubMed] [Google Scholar]
- Stieger B, Heger M, de Graaf W, Paumgartner G, van Gulik T. The emerging role of transport systems in liver function tests. Eur J Pharmacol. 2012;675 (1–3):1–5. doi: 10.1016/j.ejphar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Stieger B, Meier PJ. Pharmacogenetics of drug transporters in the enterohepatic circulation. Pharmacogenomics. 2011;12 (5):611–631. doi: 10.2217/pgs.11.53. [DOI] [PubMed] [Google Scholar]
- Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome) Best Pract Res Clin Gastroenterol. 2010;24 (5):555–571. doi: 10.1016/j.bpg.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Shitara Y, Abe T, Sugiyama Y. Effect of 17 beta-estradiol-D-17 beta-glucuronide on the rat organic anion transporting polypeptide 2-mediated transport differs depending on substrates. Drug Metab Dispos. 2002;30 (2):220–223. doi: 10.1124/dmd.30.2.220. [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem. 2003;278 (44):43489–43495. doi: 10.1074/jbc.M306933200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Onogawa T, Asano N, Mizutamari H, Mikkaichi T, Tanemoto M, Abe M, Satoh F, Unno M, Nunoki K, Suzuki M, Hishinuma T, Goto J, Shimosegawa T, Matsuno S, Ito S, Abe T. Identification and characterization of novel rat and human gonad-specific organic anion transporters. Mol Endocrinol. 2003;17 (7):1203–1215. doi: 10.1210/me.2002-0304. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Toyohara T, Akiyama Y, Takeuchi Y, Mishima E, Suzuki C, Ito S, Soga T, Abe T. Transcriptional regulation of organic anion transporting polypeptide SLCO4C1 as a new therapeutic modality to prevent chronic kidney disease. J Pharm Sci. 2011;100 (9):3696–3707. doi: 10.1002/jps.22641. [DOI] [PubMed] [Google Scholar]
- Svoboda M, Wlcek K, Taferner B, Hering S, Stieger B, Tong D, Zeillinger R, Thalhammer T, Jager W. Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport. Biomed Pharmacother. 2011;65 (6):417–426. doi: 10.1016/j.biopha.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Masuda S, Saito H, Abe T, Inui K. Multispecific substrate recognition of kidney-specific organic anion transporters OAT-K1 and OAT-K2. J Pharmacol Exp Ther. 2001;299 (1):261–267. [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nozawa T, Koshida M, Nezu J, Sai Y, Tsuji A. Functional characterization of human organic anion transporting polypeptide B (OATP-B) in comparison with liver-specific OATP-C. Pharm Res. 2001;18:1262–1269. doi: 10.1023/a:1013077609227. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther. 2003;304 (1):223–228. doi: 10.1124/jpet.102.043026. [DOI] [PubMed] [Google Scholar]
- van de Steeg E, Stranecky V, Hartmannova H, Noskova L, Hrebicek M, Wagenaar E, van Esch A, de Waart DR, Oude Elferink RP, Kenworthy KE, Sticova E, al-Edreesi M, Knisely AS, Kmoch S, Jirsa M, Schinkel AH. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest. 2012;122 (2):519–528. doi: 10.1172/JCI59526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE, Schinkel AH. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest. 2010;120 (8):2942–2952. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Deure WM, Friesema EC, de Jong FJ, de Rijke YB, de Jong FH, Uitterlinden AG, Breteler MM, Peeters RP, Visser TJ. Organic anion transporter 1B1: an important factor in hepatic thyroid hormone and estrogen transport and metabolism. Endocrinology. 2008a;149 (9):4695–4701. doi: 10.1210/en.2008-0169. [DOI] [PubMed] [Google Scholar]
- van der Deure WM, Hansen PS, Peeters RP, Kyvik KO, Friesema EC, Hegedus L, Visser TJ. Thyroid hormone transport and metabolism by organic anion transporter 1C1 and consequences of genetic variation. Endocrinology. 2008b;149 (10):5307–5314. doi: 10.1210/en.2008-0430. [DOI] [PubMed] [Google Scholar]
- van der Deure WM, Peeters RP, Visser TJ. Molecular aspects of thyroid hormone transporters, including MCT8, MCT10, and OATPs, and the effects of genetic variation in these transporters. J Mol Endocrinol. 2010;44 (1):1–11. doi: 10.1677/JME-09-0042. [DOI] [PubMed] [Google Scholar]
- Vavricka SR, Jung D, Fried M, Grutzner U, Meier PJ, Kullak-Ublick GA. The human organic anion transporting polypeptide 8 (SLCO1B3) gene is transcriptionally repressed by hepatocyte nuclear factor 3beta in hepatocellular carcinoma. J Hepatol. 2004;40 (2):212–218. doi: 10.1016/j.jhep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology. 2002;36 (1):164–172. doi: 10.1053/jhep.2002.34133. [DOI] [PubMed] [Google Scholar]
- Walters HC, Craddock AL, Fusegawa H, Willingham MC, Dawson PA. Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. Am J Physiol Gastrointest Liver Physiol. 2000;279 (6):G1188–1200. doi: 10.1152/ajpgi.2000.279.6.G1188. [DOI] [PubMed] [Google Scholar]
- Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. Am J Physiol Gastrointest Liver Physiol. 2008;294 (4):G1052–1059. doi: 10.1152/ajpgi.00584.2007. [DOI] [PubMed] [Google Scholar]
- Wang X, Wolkoff AW, Morris ME. Flavonoids as a novel class of human organic anion-transporting polypeptide OATP1B1 (OATP-C) modulators. Drug Metab Dispos. 2005;33 (11):1666–1672. doi: 10.1124/dmd.105.005926. [DOI] [PubMed] [Google Scholar]
- Weaver YM, Hagenbuch B. Several conserved positively charged amino acids in OATP1B1 are involved in binding or translocation of different substrates. J Membr Biol. 2010;236 (3):279–290. doi: 10.1007/s00232-010-9300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Sugie M, Okada M, Mikkaichi T, Toyohara T, Abe T, Goto J, Hishinuma T, Shimada M, Mano N. Transport of estrone 3-sulfate mediated by organic anion transporter OATP4C1: estrone 3-sulfate binds to the different recognition site for digoxin in OATP4C1. Drug Metab Pharmacokinet. 2010;25 (3):314–317. doi: 10.2133/dmpk.25.314. [DOI] [PubMed] [Google Scholar]
- Zaher H, Meyer zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, Palandra J, Stock JL, Kim RB, Ware JA. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol. 2008;74 (2):320–329. doi: 10.1124/mol.108.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zair ZM, Eloranta JJ, Stieger B, Kullak-Ublick GA. Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics. 2008;9 (5):597–624. doi: 10.2217/14622416.9.5.597. [DOI] [PubMed] [Google Scholar]
- Zhang W, He YJ, Gan Z, Fan L, Li Q, Wang A, Liu ZQ, Deng S, Huang YF, Xu LY, Zhou HH. OATP1B1 polymorphism is a major determinant of serum bilirubin level but not associated with rifampicin-mediated bilirubin elevation. Clin Exp Pharmacol Physiol. 2007;34 (12):1240–1244. doi: 10.1111/j.1440-1681.2007.04798.x. [DOI] [PubMed] [Google Scholar]