Abstract
The SLC6 family of secondary active transporters are integral membrane solute carrier proteins characterized by the Na+-dependent translocation of small amino acid or amino acid-like substrates. SLC6 transporters, which include the serotonin, dopamine, norepinephrine, GABA, taurine, creatine, as well as amino acid transporters, are associated with a number of human diseases and disorders making this family a critical target for therapeutic development. In addition, several members of this family are directly involved in the action of drugs of abuse such as cocaine, amphetamines, and ecstasy. Recent advances providing structural insight into this family have vastly accelerated our ability to study these proteins and their involvement in complex biological processes.
Keywords: Neurotransmitters, Biogenic amines, Amino acids, Epigenetics, Osmolyte balance, Cocaine, SLC6
1. Introduction
The solute carrier 6 (SLC6) transport family are secondary active co-transporters that utilize a chemiosmotic Na+ gradient to couple ‘downhill’ transport of Na+ with ‘uphill’ transport of their substrates across a biomembrane. Some members of the SLC6 family co-transport Cl− and accordingly were originally named Na/Cl-dependent transporters. Based on the substrate they translocate, SLC6 transporters can be divided in 4 subgroups. The neurotransmitter transporters (NTT) which include 3 -aminobutyric acid (GABA) transporters (GAT), 2 glycine transporters (GLY) and the monoamine (dopamine (DAT), serotonin (SERT) and norepinephrine (NET)) transporters; the amino acid transporters which include proline (PROT, IMINO), cationic and neutral amino acid transporters (AA0, AA0,+); the osmolyte transporters which include the betaine (BGT1), taurine (TauT) transporters and creatine transporters (CT) and 1 orphan transporter. The functional divisions of the SLC6 family closely match the groupings observed in phylogenetic analysis (Fig 1). Currently, the SLC6 family is comprised of > 300 prokaryotic and eukaryotic proteins of which 21 have been identified in humans (Beuming et al., 2006). It is worth noting that within the “Transporter Classification System” developed by Milton Saier’s group (Saier et al., 2009), the SLC6 transporters are members of the Neurotransmitter:Sodium Symporter (NSS; 2.A.22) family within the Amino acid – Polyamine - Organocation (APC) superfamily. In eukaryotes, the SLC6 carriers that have been characterized to fulfill important, if not critical, roles in amino acid transport, (Bröer and Palacín, 2011) homeostasis of osmolytes levels, modulation of neurotransmitter signaling in the central and peripheral nervous system (Kristensen et al., 2011) and spermatogenesis (Chatterjee et al., 2011). Within the intestinal and kidney tubule epithelium these transporters are key in the absorption and reabsorption, respectively, of amino acids and osmolytes that are essential for several physiological processes. Within the nervous system SLC6 transporters are critical in the termination of synaptic transmission for several amino acid and amino acid–derived neurotransmitters in addition to their role in providing essential nutrients and osmolytes to neurons and glial cells (Fig. 2).
Fig. 1.
Phylogenetic Tree Diagram of Human SLC6 Members. Phylogenetic tree was generated in Geneious Pro Software (Biomatters Ltd) using Blosum 62 scoring matrix, open gap penalty of 12 and gap extension penalty of 3, the Jukes-Cantor genetic distance model, and Neighbor-Joining tree building method. The genes are given as A1, A2, etc along with the common name. The sub-groupings within the tree are designated by different colored ovals and named as indicated. SLC6A21P which is a pseudogene is not shown.
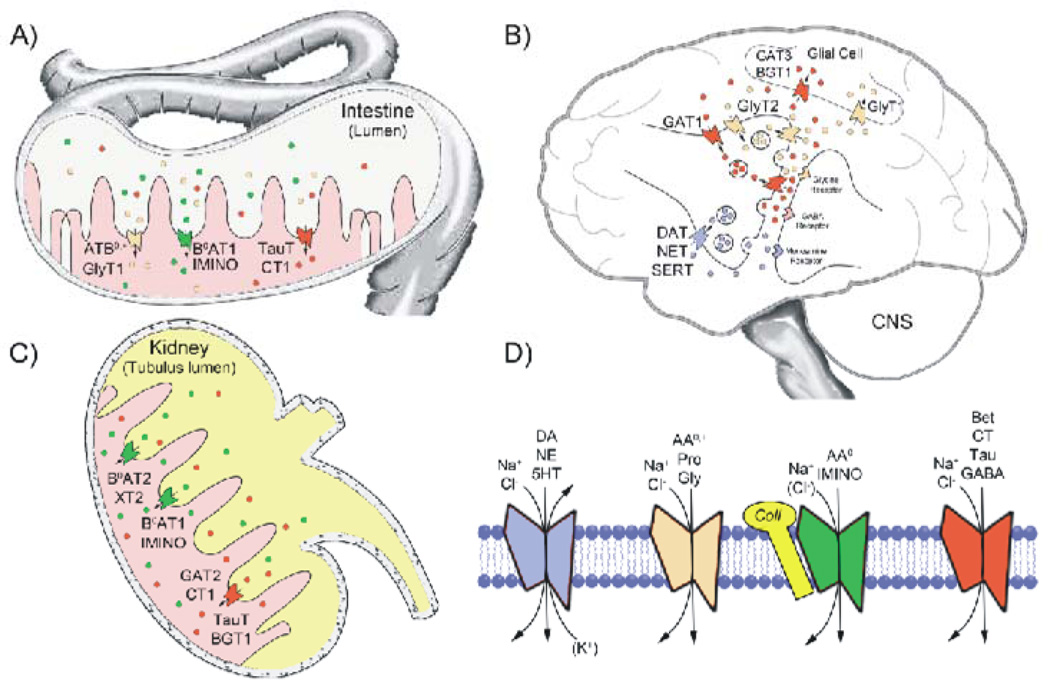
Fig. 2.
Tissue distribution, physiologic function and substrate specificity of several SLC6 family transporters. A schematic representation of the prevalent tissue distribution and physiologic function of several SLC6 transporters is shown in A–C. The prominent roles of these transporters in intestinal nutrient absorption (A), renal reabsorption (C) of several amino acid and osmolyte substrates, and the role of transporters in synaptic transmission in the central nervous system (B) are depicted. Substrate specificity and ion and binding partner dependence for the four subclasses of SLC6 transporters is shown in panel D. Substrate abbreviations are: dopamine (DA), norepinephrine (NE), serotonin (5-HT), neutral and cationic amino acids (AA0,+), proline (Pro), glycine (Gly), neutral amino acids (AA0), proline and hydroxyproline (IMINO), betaine (Bet), creatine (CT), taurine (Tau), and -aminobutyric acid (GABA). All SLC6 transporters are Na+-dependent and most are Cl−-dependent with the exception of B0AT2/SBAT1, NTT4/XT1 and B0AT1. Additionally, SERT utilizes the antiport of K+ in the translocation of 5-HT. Although SLC6 transporters have numerous binding partners that influence transport activity, B0AT1, B0AT3, and IMINO require expression of collectrin/TMEM27 or angiotensin-converting enzyme 2 (ACE2) for activity. For a detailed listing of transporter tissue distribution, substrate specificity and disease association, see Table 1.
In humans, the SLC6 family of transporters defines one of the most clinically relevant protein groups with links to orthostatic intolerance, attention deficit hyperactivity disorder (ADHD) (Mazei-Robison et al., 2008), addiction, osmotic imbalance, X-linked mental retardation (Martínez-Muñoz et al., 2008), Hartnup disorder, hyperekplexia, Tourette syndrome, schizophrenia, Parkinson disease (PD), autism (Hahn and Blakely, 2007) and mood disorders such as depression, anxiety, obsessive compulsive disorder (OCD), and post-traumatic stress disorder (PTSD) (Hahn and Blakely, 2007).
This review will focus on the structure-function aspects of the mammalian SLC6 transporters, their regulation by both classical as well as emerging epigenetic/transgenerational mechanisms and what impact these properties may have on disease and the use of biomarkers to detect these proteins in disease states. For a more comprehensive view of the SLC6 family of proteins see the recent review by (Kristensen et al., 2011).
2. Structure
The identification of the high-resolution structure of the SLC6 bacterial leucine transporter, LeuT (Yamashita et al., 2005), along with a wealth of supportive biochemical studies (Kristensen et al., 2011) has provided a framework for interpreting SLC6 structure-function relationships. In general, SLC6 proteins have 12 membrane spanning domains (TM) with intracellular N and C termini. In eukaryotic members, the N and C termini are significantly longer and have been shown to mediate complex regulatory processes such as protein trafficking, ion stoichiometry and function (Kristensen et al., 2011). By comparison, prokaryotic SLC6 proteins lack structural features present in eukaryotic members such as: (1) the extensive N and C termini, (2) an extended extracellular loop 2 domain (EL2) between TMs 3 and 4 containing a critical disulfide bond (3) consensus intracellular phosphorylation sites and (4) the post-translational modifications such as glycosylation and palmitoylation (Foster and Vaughan, 2011) (Fig. 3A and 4). Nevertheless, the NTTs and LeuT share a modest 20–25% overall sequence identity that increases to greater than 50% identity when focusing on the first shell of amino acids (within 7Å) of the bound substrate in the LeuT crystal (Yamashita et al., 2005). The LeuT crystal structure has reveal that SLC6 transporters are based on a 5 + 5 helical architecture where TMs 1–5 and TMs 6–10 form two pentahelical bundles aligned antiparallel to one another yielding a pseudo two-fold axis of symmetry (Fig. 3A). TMs 11 and 12 in LeuT reside on the periphery of the 5 + 5 core and may play a less critical role in transport but important functions in other aspects of structure or regulation a notion supported by the fact that 70% of the prokaryotic SLC6 members, including the functional Na+-dependent tyrosine transporter Tyt1 from Fusobacterium nucleatum, lack TM12 altogether (Quick et al., 2006).
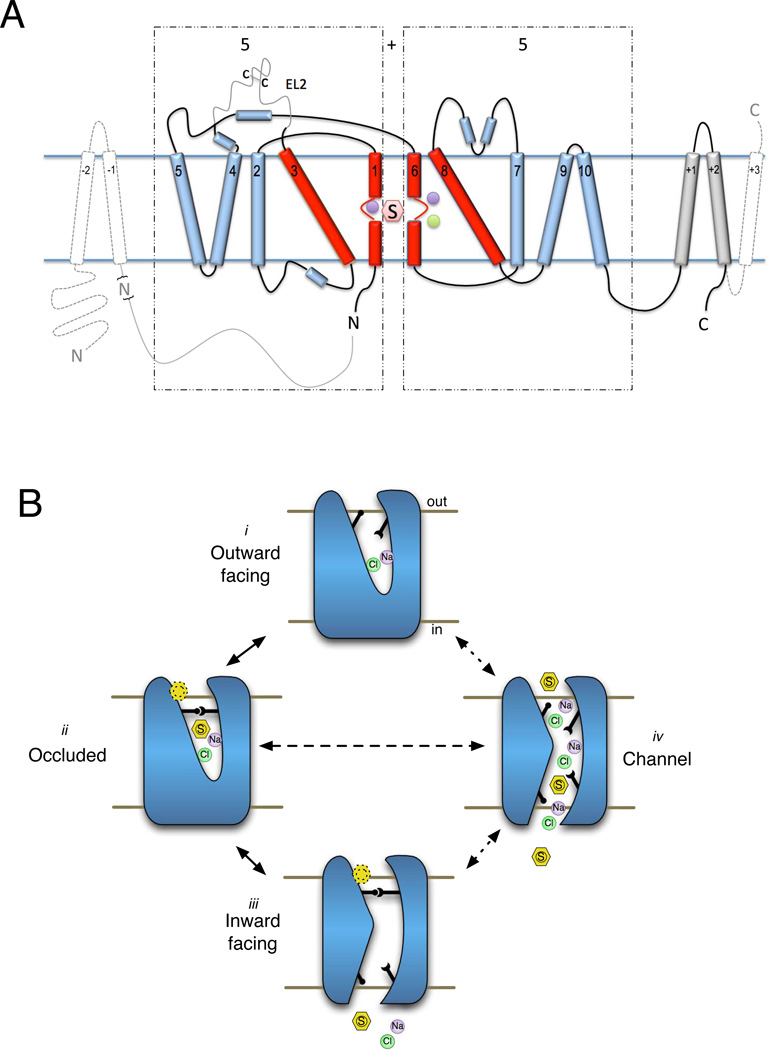
Fig. 3.
Structural aspects of the SLC6 family. (A) Helical architecture of the LeuT fold transporters. The 5+5 (LeuT fold) motif is represented as 2D where TMs 1 and 3 and 6 and 8 form the substrate binding helices (red cylinders) and combine with TMs 2, 4 and 5 and 7, 9 and 10 (blue), respectively, to form the antiparallel heptahelical domains (demarcated by the dashed boxes). TMs 11 and 12 (dark gray cylinders, labeled +1 and +2, respectively) are located peripheral to the 5+5 motif. The black lines represent densities resolved in the LeuT crystal structures. Gray solid lines represent additional regions found in eukaryotic SLC6 proteins. Other LeuT fold proteins, such as vSGLT, possess the 5+5 core but also have additional N and C terminal helices (dashed gray lines and light gray cylinders). Substrate (S), Na+ (purple spheres) and Cl− (green sphere) are also represented. (B) Illustration of alternating access and channel modes of transport. Substrate and ions access the binding site of the outward facing transporter (i) via the open outer gate (black sticks), outer gate closes yielding the occluded state (ii) which then transitions to the inward facing state (iii) releasing substrate and ions to cytosol (though controversial, this transition is proposed to be driven by a second substrate molecule (dashed line hexagon) binding to the S2 site). The transporter then rectifies to the outward facing structure (i). The transporter may undergo a lower probability transition (dashed lines) to a channel state (iv) where both gates are open simultaneously.
Fig. 4.
SLC6 transporter regulatory components. A representative topological diagram of SLC6 transporters is shown indicting major and common regulatory features and their location or site of action within the carrier protein. Identified Ser and Thr phosphorylation (S or T), ubiquitination (Ub), glycosylation (branched lines) and palmitoylation (Pal) sites as well as the location of the internalization motif (FREK) and selected known coding variants that alter transport function are shown. Common interacting proteins with their functional binding sites that are known to alter transport activity and trafficking are also indicted. The transmembrane domain of Syn 1A has been omitted for clarity.
2.1 The 5+5 architecture in Non-SLC6 Transporters
Following the crystallization of LeuT, several transporters from other SLC families were crystalized including amino acid, polyamine, and organocation transporter, ApcT; betaine transporter BetP; galactose transporter, vSGLT; arginine/agmatine antiporter, AdiC; amino acid, and sodium-benzylhydantoin transporter, Mhp1 and whereas these transporters share no sequence homology with LeuT, they possess the same 5 + 5 architecture suggesting a functional convergence to this structural configuration for many transport proteins (Boudker and Verdon, 2010). Interestingly, these transporters have been found to have up to 2 or 3 TMs preceding or following the 5 + 5 helix bundle (Fig. 3A). This observation is grounds for excitement in the field as we look forward with anticipation to understanding the contribution these helices have to the overall structure, function and regulation of these transporters.
2.2 Coupling chemiosmotic gradients to substrate uptake
In the “LeuT fold”, as it is being termed, the centrally located TMs 1 and 6 are unwound mid-way through the membrane and participate with TMs 3 and 8 in forming the substrate binding site (S1) (Yamashita et al., 2005). Two Na+ ions lie proximal to the substrate consistent with the coupling of the Na+ gradient with substrate translocation. Whereas, the LeuT structure did not provide any obvious insight into the location of the Cl− binding site for the NTT proteins, eloquent biochemical studies have revealed that Cl− binds proximal to the Na1 site in the NTT providing a negative charge that is functionally equivalent to E290 in LeuT which does not require Cl− (Forrest et al., 2007; Zomot et al., 2007). The range of ion stoichiometries and dependencies (Na+, Cl−, K+, H+) within the NTT proteins suggests variation in ion coupling mechanisms may allow for optimal binding, specificity and transport of the various substrates (Yu et al., 2010). In fact, single mutations have been shown to dramatically alter the ion/substrate coupling in these transporters converting Na+/Cl−-dependent transporters to be solely Na+-dependent (Henry et al., 2011; Tavoulari et al., 2011). Interestingly, of the two Na binding sites in LeuT, only the Na2 site is conserved among transporters that have the LeuT fold. With any luck, future studies will provide greater mechanistic insight as to how ion binding sites are utilized to drive substrate binding and uptake.
The crystal structures from the LeuT fold proteins revealed a gating mechanism involving two pairs of charged residues positioned above and below the S1 site that open and close allowing coordinated substrate and ion entry and exit from S1 (Krishnamurthy et al., 2009) supporting the alternating access model (Fig. 3B). Interestingly, molecular dynamic, biochemical and crystallographic analyses suggest that a second substrate molecule binds at a more cytoplasmic site (S2) in LeuT and mechanistically promotes substrate release from S1 (Reyes and Tavoulari, 2011). This claim is in contrast to findings of Piscitelli et al. (2010) which supports a single substrate site. In addition, in other solved LeuT fold structures an occupied S2 site has not been recovered (Krishnamurthy et al., 2009). The studies indicate the mechanisms of substrate binding and translocation may be more complex than anticipated warranting further data to determine the roles or existence of S1 and S2.
2.3 Inhibition of SLC6 transport at the molecular level
The continuing illumination of substrate translocation through these biological motors has also garnered more insight into how transport in NTTs can be disrupted by compounds such as antidepressants, cocaine and amphetamines. A co-crystal of LeuT with the competitive inhibitor tryptophan suggests that binding of Trp at the S1 site blocks the transition from an ‘open-to-out’ form to the ‘occluded’ (both gates closed) form preventing conformational changes that would allow the ligand to gain intracellular access (Singh et al., 2008) (Fig. 3B). However, alteration of a single residue in the S1 site of LeuT can convert Trp from an inhibitor to a substrate by altering the preferred binding pose of the Trp and allowing the appropriate conformational transitions for transport (Piscitelli and Gouaux, 2012). Non-competitive inhibition of substrate transport has also been observed with co-crystals of LeuT and several tricyclic antidepressants (TCA) and selective serotonin reuptake inhibitors (SSRI). Interestingly, the non-competitive blockers bind to a separate extracellular vestibular binding site which is essentially the S2 site on LeuT (Singh et al., 2007; Zhou et al., 2009). As with the S2 site and substrate binding, significant debate exists as to whether or not these co-crystal structures represent the mechanism of antidepressant binding in NTTs. The affinity for these inhibitors for LeuT is relatively low compared to the high affinities they possess for the NTTs (Singh et al., 2007). Based on studies with Trp inhibition of LeuT (Singh et al., 2008), one could speculate that in NTTs, the S2 binding site may be a low-affinity intermediate binding-site that becomes populated as the ligand transitions to the high-affinity S1 site. In addition, a large amount of biochemical, mutagenesis and pharmacological data show that mutations at S1 often lead to loss of high affinity binding for SSRIs and TCAs (Henry et al., 2006; Andersen et al., 2009; 2010). However, mutations at a few residues in the S2 site have shown dramatic impact on the potency of some inhibitors (Nyola et al., 2010) and a halogen binding pocket has been reported to lie between the S1 and S2 sites and play a role in determining inhibitor specificity (Zhou et al., 2009). These findings as well as data showing that S1 and S2 mutation exhibit differential impact on certain inhibitors (Henry et al., 2006; Andersen et al., 2010; Nyola et al., 2010).
2.4 Transporter oligomerization
Each NTT protomer is thought to be independent in regard to substrate translocation and thus far, the discussion has focused on the structure of the transporter as a monomer. However, there is increasing evidence that the oligomeric state of the transporters can impact proper trafficking to the plasma membrane (Sitte et al., 2004) and that crosstalk within each oligomer may modulate function, regulation (Gärtner et al., 2011) and efflux (reverse transport) in response to drugs like amphetamine. Given the apparent core structural similarities between SLC6 and other SLC families, mechanistic and structure-function insights will cross family boundaries suggesting that we pay close attention to emerging structural findings throughout the superfamily as this may lead to breakthroughs in our ability to intervene in the disease processes related to this family.
2.5 Structure: a pathway to transport mechanism
The emerging crystal structures of the LeuT fold family of proteins have provided snap shots of the transporter in the various stages of substrate translocation such as the outward-facing, occluded and inward-facing conformations. By assuming that the conformational changes necessary to move substrate will be conserved among the LeuT-like transporters, several groups have put the snap shots together to describe the alternating access process in these proteins. Two prevalent models are the “rocking bundle” (Forrest and Rudnick, 2009), where a bundle (TMs 1, 2, 6 and 7) and scaffold (TMs 3, 4, 5, 8, 9 and 10) rock in relation to one another resulting in transition from outward to inward facing, and the other involves a “hinge” model (Krishnamurthy et al., 2009) where flexing movements in TM1 and 6 followed by side chain rearrangement is sufficient to open and close the outer and inner gating densities allowing coordinated passage of substrate. A more complete set of crystal structures for Mhp1 representing the outward-facing, occluded and inward-facing conformations has suggested the transporter undergoes rigid body movements similar to those proposed by the rocking bundle (Shimamura et al., 2010). Recently, using antibody stabilization and mutagenesis primarily at the Na2 binding site, Krisnamurthy and Gouaux captured LeuT crystals representing the substrate-free outward facing and apoinward-facing conformations (Krishnamurthy and Gouaux, 2012). The authors propose these new structures reveal a hybrid mechanism of transport for LeuT that involves a combination of both hinge and rigid-body movements to permit substrate and ion passage across the membrane. Indeed, as more LeuT fold crystal structures develop so too will a clearer picture of the transport mechanism(s) used by this family. However, it should be noted that as we have observed how sequence dissimilarity can give rise to a strikingly similar 3-D fold (LeuT fold), co-existing mechanisms to achieve alternating access are not unreasonable.
3. Function
Consistent with their diverse physiological role, the SLC6 transporters are found in many tissues including nervous system, kidney, intestine, pancreas, adrenal gland and testis. However, because of their physiological relevance most functional studies have been performed on the NTT subgroup. In the central and peripheral nervous system, the NTTs can regulate signaling among neurons and are a site of action for various drugs.
3.1 Transporter Subgroups
3.1.1 GABA transporters
GAT1 (encoded by the SLC6A1) is the main GABA transporter in the brain and thus plays an important role in regulating GABAergic signaling (Madsen et al., 2010). It localizes within the GABAergic neurons in the neocortex, hippocampus, cerebellum, basal ganglia, brainstem, spinal cord, olfactory bulb and retina. GAT1 is strongly inhibited by anti-epileptic drugs, such as tiagabine and nipecotic acid that likely exert their effects by elevating extracellular level of GABA. GAT3 (SLC6A11) is predominantly expressed in glia cells, and its localization to GABAergic synapses suggests a role in regulating GABA signaling. GAT2 (SLC6A13), like GAT3, shares 52% homology with GAT1, however its expression does not overlap with markers of GABAergic signaling though it is found in the brain and retina as well as liver and kidney. Similar to GAT1, GAT3 is a target of anticonvulsant drugs that are nipecotic acid derivates.
3.1.2 Monoamine transporters
The NET (SLC6A2), DAT (SLC6A3) and SERT (SLC6A4) are mainly found in the brain. NET and SERT are also found in a subset of adrenal chromaffin cells, mast cells and blood platelets (Kristensen et al., 2011). They regulate many central and sympathetic nervous system functions including learning, mood, attention, movement, appetite, sleep and reward. Besides moving endogenous substrates, monoamine transporters can translocate amphetamines and neurotoxins including MDMA (ecstasy) and 1-methyl-4-phenylpyridinium (MPP+). Selective inhibitors of the monoamine transporters include cocaine analogs, mazindol, benztropine and antidepressants such as fluoxetine (Prozac). Thus, an understanding of the molecular mechanisms underlying transport by these proteins is of considerable interest.
3.1.3 Glycine transporter
The glycine transporter 2 (GlyT2) is highly expressed in neurons of the brainstem, cerebellum and spinal cord as well as in macrophages and mast cells in peripheral tissues; whereas GlyT1 is found only in glia cells (Supplisson and Roux, 2002). Two isoforms of GlyT2 exist. The GlyT2b does not exhibit functional glycine uptake and differs from GLYT2a by five amino acids in the N-terminal tail. Inactivation of GlyT2 in knockout mice is lethal during the second post-natal week as the absence of GlyT2 disrupts inhibitory transmission by reducing glycine release. In humans, GlyT2 is encoded by the SLC6A5 gene and mutations in this gene are responsible for a presynaptic form of hyperekplexia, a genetic disease causing increased startle reflex. GlyT1 (SLC6A9) has been associated with hypertension as well as elevation of extracellular synaptic glycine concentration by blockade of GlyT1 has been hypothesized to potentiate NMDA receptor function in vivo and to represent a rational approach for the treatment of schizophrenia and cognitive disorders.
3.1.4 L-Proline transporter
Brain-specific L-proline transporter, PROT (SLC6A7), is expressed by subpopulations of putative glutamatergic neurons which are particularly enriched with this protein in synaptosomal membrane fractions (Shafqat et al., 1995). These findings raise the possibility of a specialized role for PROT and its presumed natural substrate, L-proline, in the modulation of excitatory synaptic transmission in specific excitatory pathways within the CNS.
3.1.5 Neutral and cationic amino acid transporters
The SLC6 family includes a number of neutral and cationic amino acid transporters which are expressed in blastocysts, oocytes, intestine, kidney, pituitary gland, lung and brain. Members of the neutral amino acid transport subfamily (i.e. IMINO (SLC6A20), B0AT1 (SLC6A19), B0AT3 (SLC6A18), NTT4/XT1 (SLC6A20)) are found on the apical surface of epithelial cells (Fig. 2A and C) where they are important for the absorption of amino acids from the duodenum, jejunum and ileum and their reabsorption within the proximal tubule of the nephron, as well as in brain tissue (SBAT1 (SLC6A15), IMINO) (Bröer and Palacín, 2011). Remarkably, B0AT3, whose lack of function has been linked to imino- and hyperglycinuria, is the only member of the SLC6 family that requires co-expression of either collectrin or angiotensin-converting enzyme 2 (ACE2) to be active (Fig. 2D). However, although the mouse B0AT3 is active when expressed in vitro together with these ‘helper’ proteins, a functional human B0AT3 transporter has yet to be identified (Bröer and Palacín, 2011). Interestingly, several mutations have been reported to occur at a very high frequency in the B0AT3 gene such that a considerable proportion of the human population carries a non-functional version of the protein (see Table 1).
Table 1.
Association of SLC6 family members with human disease including genetic variation, biomarkers, epigenetic factors and tissue distribution
| Human Gene |
Substrate | Disease Association |
Aliases | Human Gene Locus |
SNP | Disease Association from SNOs |
Biomarker Imaging Technique |
Biomarker Disease Association |
Biomarker Utility |
Epigenetic Link | Tissue Distribution |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SLC6A1 | GABA | Epilepsy, Schizo- phrenia, Anxiety, ADHD |
GAT1 | 3p25-p24 | GABAergic neurons in Bra (Neocortex, hippocampus, cerebellum, basal ganglia |
||||||
| SLC6A2 | Norepinephrine | Depression, Orthostatic intolerance, Anorexia nervosa, Cardiovascular dis*., ADHD |
NAT1 NET1 NET |
16q12.2 | F528C1 A457P2 R121Q2 T283M3 V245I3 rs5569 (exon 9 G1287A)4 T182C, rs22424462 rs360175 T182C, rs22424466 rs7471077 T182C, rs22424467 rs28386840, −3081(T)8 T182C,rs22424468 |
Major depression1 Orthostatic tolerance2 Long QT syndrome2 ADHD3,4 Depression2 Amphetamine response5 Reward dependence6 Panic diso.7 Blood pressure8 |
PET | Alzheimer dis.35 Parkinson dis.36 |
Diagnosis, Drug Pharmaco- dynamics |
Hypertension and Panic diso.50 |
Brain (Non adrenergic neuronal somata, axons, dendrites) and Peripheral nervous system, Adrenal gland (Chromaffin cells), Placenta |
| SLC6A3 | Dopamine | Parkinson dis., Tourette syndrome, ADHD, addiction, Major affective diso**. |
DAT DAT1 |
5p15.3 | A559V2 rs40184, C/T9 A559V2 E602G2 intron 810 intron 82 rs6347, A1343G11 VNTR 92 rs40184, C/T12 rs40184, C/T13 intron 814 VNTR 102 VNTR 915 VNTR 112 VNTR 916 VNTR 92 |
Bipolar disorder2,9 ADHD2,10 Cocaine addiction2 Schizophrenia11 Smoking2 Depression12 Migraine13 OCD14 Alcoholism2 Epilepsy15 Parkinson dis.2 Tourette syndrome16 Methylphenidate response2 |
PET/ SPECT |
Parkinson dis.36 Dementia with lewy bodies37 ADHD38 Schizophrenia39 Sleep behaviour diso.40 Bipolar diso.41 Autism42 |
Diagnosis, Disease severity, Treatment Response |
Alcohol Dependence51 Anorexia nervosa and Bulimia nervosa52 Depression and anxiety53 Hyperactivity and impulsivity54 |
Brain (Dopaminergic neurons), Gut |
| SLC6A4 | Serotonin | Anxiety, Depression, Autism Gastrointestinal disorders, Premature ejaculation Obesity Schizophrenia OCD |
5-HTT SERT |
17q11.1-q12 | I425V2,18 F465L17 L550V17 I425V18 G56A2 I425L2 F465L2 L550V2 5HTTLPR s2,19,20,21,23 5HTTLPRl2,24,25 VNTR 922 VNTR 1026 |
OCD2,17,18 Anorexia nervosa18 Rigid compulsive L550V2 behavior2 Autism2 Autism2 Suicide behavior2 Antidepressant response2 Depression2 Alcoholism2 PTSD19 Drug abuse20 Smoking21 Antidepressant induced OCD2 Alcoholism2 Suicide behavior2 Antidepressant response2 Smoking22 24 25 Migraine26 |
PET/SPECT | Depression43 Neuroticism43 Mood diso.44 Bipolar diso.45 Impulsive aggression46 Depression with bipolar diso.47 Parkinson dis.48 Major depressive diso.49 |
Diagnosis | Depression55 Bipolar diso.56 Child abuse57 Unresolved loss or trauma58 Mood diso.59 Emotion regulation & Social cognition55 Anxiety and affective diso.55 Major depressive diso.60 Suicidal behavior55 Unipolar diso.55 Schizophrenia55 Response to cocaine61 Personality diso.62 PTSD63 Alcoholism64 Substance use65 Sleep disturbance65 Premature ejaculation67 Affective diso.21a Psychiatric diso.68 |
Brain (Extrasynaptic axona membranes, dendrites) and Peripheral nervous system Placenta, Epithelium Cells, Platelets |
| SLC6A5 | Glycine | Pain, Spasticity Hyperekplexia |
GlyT2 | 11p15.2-15.1 | rs89474727 L306V28 N509S28 S510R28 |
Schizophrenia27 Hyperekplexia28 |
Brain (Glycinergic neurons golgi cells, brain stem, cerebellum), Spinal cord |
||||
| SLC6A6 | Taurine | Taurine deficiency diseases, Retinal blindness, Abnormal renal development |
TauT | 3p25-p24 | Brain, Retina, Liver, Kidney Heart, Spleen, Pancreas, Placenta, Skeletal muscle, Lung |
||||||
| SLC6A7 | Proline | ND | PROT | 5q31–q32 | 11431T>C+12213C>T+129 27A>G+20113T>C29 |
Bronchodilator response in asthma29 |
Brain (Glutamatergic neuro hippocampus) | ||||
| SLC6A8 | Creatine | Creatine deficiency syndrome, Mental retardation, Musculoskeletal disorders |
CRTR, CT1 |
Xq28 | R514X2 | Mental retardation2 | Ubiquitous | ||||
| SLC6A9 | Glycine | Schizophrenia | GlyT1 | 1p33 | rs228624530 | Hypertension30 | Brain, Retina, Liver, Spleen Kidney, Pancreas, Uterus, Stomach, Lung, Placenta |
||||
| SLC6A10 | CT2 | 16p11.2 | Testis | ||||||||
| SLC6A11 | GABA | Epilepsy | GAT-B, GAT-3 |
3p25.3 | Brain (GABAergic neurons | ||||||
| SLC6A12 | Betaine, GABA | Epilepsy | BGT1 | 12p13 | rs49936831 rs55788131 |
Aspirin tolerant asthma31 rs21625032 Schizophrenia32 |
Brain, Kidney, Liver, Heart Skeletal muscle, Placenta |
||||
| SLC6A13 | GABA | Epilepsy | GAT2 | 12p13.3 | Brain (Meninges, Ependym Choroid plexus), Retina, Li Kidney, Lung |
||||||
| SLC6A14 | Neutral, cationic amino acids |
Obesity? | ATB0+ beta- alanine carrier |
Xq23–q24 | Obesity28 | Lung, Trachea, Salivary gla Mammary gland, Stomach Pituitary gland, Intestine, Uterus, Prostate, Testis |
|||||
| SLC6A15 | Large neutral amino acids |
ND | B0AT2, v7- 3, NTT7-3 |
12q21.3 | rs154584333 rs103168133 |
Major depression33 | Brain (Amygdala, Putamen Corpus callosum), Kidney |
||||
| SLC6A16 | Unknown | ND | NTT5 | 19q13.1 – q13.4 |
Testis, Pancreas, Prostate | ||||||
| SLC6A17 | Neutral amino acids |
ND | NTT4, XT1 |
1p13.3 | Brain, Heart, Skeletal musc | ||||||
| SLC6A18 | Neutral amino acids |
Hyperglycinuria? Hypertension? |
B0AT3, XT2 |
5p15.33 | rs744781534 | Myocardial infarction34 | Kidney (Proximal tubule) | ||||
| SLC6A19 | Neutral amino acids |
Hartnup disorder, hypertension?, Hyperglycinuria |
B0AT1, HND |
5p15.33 | R240X28 | Hartnup disorder28 | Intestine (Duodenum, Jejunum, Ileum), Stomach, Kidney, Liver, Prostate |
||||
| SLC6A20 | Proline, pipecolate, sarcosine |
Iminoglycinuria | system IMINO XT3, Xtrp3 |
3p21.3 | Brain, Kidney, Small Intest Thymus, Spleen, Ovary, Lu |
||||||
| SLC6A21 | NA | ND | psuedogene | 19q13.33 |
- disease,
- disorder
NA – Not Applicable, ND – No Association Determined
References for Biomarkers, Epigenetics and SNPs link to Disease:
1 – Haenisch et al., 2008, 2- Hahn and Blakely, 2007, 3 – Hahn et al., 2009, 4 - Song et al., 2011, 5 – Dlugos et al., 2007, 6 – Ham et al., 2005, 7 – Buttenschon et al., 2011, 8 – Kohli et al., 2011, 9 – Mick et al., 2008, 10 – Silva et al., 2009, 11 – Cordeiro et al., 2010, 12 – Pattarachotanant et al., 2010, 13 – Todt et al., 2009, 14 – Miguita et al., 2007, 15 - Sander et al., 2000, 16 – Tarnok et al., 2007, 17 - Prasad et al., 2009, 18 – Delorome et al., 2005, 19 - Wang et al., 2011, 20- Gerra et al., 2010, 21- Bloch et al., 2010, 22- Lima et al., 2011, 23 – Daray et al., 2010, 24 - Bosia et al., 2010, 25 – Retz et al., 2008, 26 – Schurks et al., 2010, 27 - Deng et al., 2008, 28 – Broer and Palacin, 2011, 29 – Kim et al., 2010, 30 - Ueno et al., 2009, 31 - Pasaje et al., 2010, 32 - Park et al., 2011, 33 - Kohli et al., 2011, 34 – Matsumoto et al., 2001, 35 – Gulyas et al., 2010, 36 - Remy et al., 2005, 37- McKeith et al., 2007, 38- Volkow et al., 2009, 39 – Mane et al., 2011, 40 - Iranzo et al., 2011, 41- Anand et al., 2011, 42- Nakamura et al., 2010, 43- Takano et al., 2007, 44 - Ichimiya et al., 2002, 45 - Cannon et al., 2006, 46 - Frankle et al., 2005, 47 - Oquendo et al., 2007, 48 – Guttman et al., 2007, 49 – Parsey et al., 2006, 50 - Esler et al., 2010, 51 – Hillemacher et al., 2009, 52 - Frieling et al., 2010, 53 – Haeffel et al., 2008, 54 - Becker et al., 2008, 55 – Lesch 2011, 56 – Chotai et al., 2003, 57 - Beach et al., 2009, 58 - vanIJzendoorn et al., 2010, 59 - Kinnally et al., 2010, 60 - Miller et al., 2009, 61 - Vasiliou et al., 2011, 62 - Jacob et al., 2010, 63 - Xie et al., 2009, 64 - Covault et al., 2007, 65 - Brody et al., 2009, 66 - Brummett et al., 2007, 67 - Safarinejad 2009, 68 - Maeney 2010
B0,+/ATB0+ (SLC6A14) is a unique amino acid transporter. It recognizes both neutral and cationic amino acids. Moreover, the ATB0+ can transport antiviral drugs such as acyclovir and ganciclovir when they are covalently coupled to the side chain of anionic amino acids. Thus it can function as a potential drug delivery system. Interestingly, it has been found that the ATB0+ expression is upregulated in cancer.
The NTT5 (SLC6A16) is an orphan transporter whose mRNA is highly expressed in peripheral tissues, particularly in testis, pancreas, and prostate. Transient transfection with epitope-tagged transporter constructs demonstrated NTT5 is predominantly intracellular, suggestive of a vesicular location. Although the substrates transported by this transporter remain unknown, its specific distribution suggests that it may mediate distinct and important functions.
3.1.6 Osmolyte transporters
The taurine and betaine transporters (TauT and BGT1, encoded by SLC6A6 and SLC6A12) are both found in brain, kidney and other non-neuronal tissues (Fig. 2A–C). Both transporters are thought to play a role in osmoregulation across basolateral membranes of epithelial cells and expression of BGT1 and TauT are regulated by changes in osmolarity (Kwon, 1996). By moving osmolytes such as taurine and betaine, TauT and BGT1 adjust the intracellular solute content to compensate for gain or loss of water. BGT1 is a betaine/GABA transporter that osmoregulates cells in the hypertonic inner medulla by mediating basolateral betaine accumulation. BGT1 also transports GABA and, in fact, has a higher affinity for GABA than betaine. Although there is no direct evidence for a role of TauT in pathological disorders, TauT may be associated with taurine deficiency diseases including abnormal renal development and retinal-related blindness, as well as regulation of bile-acid conjugation in hepatocytes (Sturman, 1990).
Creatine is important in maintaining ATP homeostasis thus the creatine transporter (CT1, SLC6A8), which is ubiquitously located in mammals, is thought to provide creatine in cells that cannot synthesize their own (Nash et al., 1994). An X chromosome-linked creatine deficiency syndrome has been identified in subjects carrying a mutation in the CT1 gene (Hahn and Blakely, 2007). This naturally occurring mutation leads to a non-functional CT1 that clinically manifests with moderate to severe intellectual disability, severe language problems, and increased rate of epilepsy. Unlike CT1, transcripts for the putative CT2 protein, which shares 97% homology with CT1 (Iyer et al., 1996), are present exclusively in the testis and brain, and therefore, it is speculated that CT2 provides creatine for its conversion to phosphocreatine and ultimately ATP for normal sperm mobility when sperm would lack an X chromosome after meiosis because the gene that encodes for CT2 resides in chromosome 16. However this SLC6A10P gene is considered to be a pseudogene by some groups and evidence for the CT2 protein has not yet been found.
3.2 Electrogenic transport
The central role of all SLC6 transporters is to mediate a rapid transport of substrate across cellular membranes against very large concentration gradients. Radiolabeled tracer flux studies have long been the standard technique employed to study transporter kinetics. These studies showed that the relationship between concentrations of the SLC6 substrate as well as Na+ and Cl−, and transport activity follows Michaelis-Menten kinetics with Km values in the lower micromolar range for the substrate and millimolar range for the two ions (Supplisson and Roux, 2002; Kristensen et al., 2011). To explain transport data radiolabeled tracer flux studies focused on the “alternating access” hypothesis, in which binding sites for ligands and ions alternately face extracellular or cytoplasmic compartments (Fig. 3B). Within this framework, coupling of substrates and ion movement results from conformational changes in the transporter induced by substrate and ion binding. Furthermore, flux experiments have suggested that substrate transport is electrogenic for some transporters (DAT, NET, GAT, GLYT) and electroneutral for others (SERT) (Amara and Sonders, 1998). Because the presence of transporters leads to additional currents, and because these currents depend on membrane potential, electrogenic transporters add conductance to the membrane. However, several studies have shown the conducting properties of transporters go well beyond the electrogenity predicted by the transport mechanism (Sonders and Amara, 1996). In addition to their ability to couple the uphill fluxes of their neurotransmitter substrate to the downhill movement of Na+ and other ions, NTT proteins also catalyze an uncoupled ion flux (Sonders and Amara, 1996). For example, 5-HT transport is electroneutral (exchange of internal K+ with Na+, Cl− and 5-HT+) (Amara and Sonders, 1998), yet it displays at least four distinct conducting states (Mager et al., 1994). Moreover, when measurements of macroscopic current during transport are integrated, the charge exceeds the current predicted from measurements of the expression level, turnover rate, and net charges moved per coupled transporter cycle according to putative alternating access schemes (Sonders et al., 1997; Carvelli et al., 2004). A second possible mechanism for coupled co-transport, also supported by tracer flux data as well as electrophysiological data, postulates that substrate and ions share a complex pore (DeFelice et al., 2001). In this scenario, coupling occurs as a result of interactions between substrate and ion in the pore lumen, referred to as flux coupling. A simplified example of coupling in a single-file, knock-through channel, was reported by Adams et al. (2002) in which Na+ ions move down their electrochemical gradient and push 5HT up its gradient.
3.3 Co-existence of transporter and channel mechanisms
Turnover rates for known NTTs are clustered in the range of 1 to 15 substrate molecules/sec. That is, a single transporter requires at least 60 ms to complete its cycle. Yet within a few ms after release at fast chemical synapses, the transmitter is cleared from the extra-cellular space (Thompson et al., 2011). As a result, receptors begin to deactivate and the synaptic event cannot spill over to neighboring synapses. How is this possible given the very modest turnover rate of neurotransmitter transporter? Some studies have suggested that efficient removal of the transmitter from the synapse arises from the fact that each transporter molecule may form a channel (Fig. 3B) (Carvelli et al., 2004). The formation of channels provides an alternative path for current flow and may explain why current is not always proportional to substrate flux. However, the low open probability of the transporter suggests that perhaps only a subpopulation of NTT exhibits a channel state when activated by the substrate, while most of the transporters behave classically (alternating access), and these states may interconvert. Several studies have shown that syntaxin 1A (Syn 1A), a protein involved in synaptic vesicle docking and in the regulation of several ion channels and transporters, binds and regulates the transport activity and conductance of SERT, DAT, NET and GAT-1 (Quick, 2003; Carvelli et al., 2008). These studies demonstrate that the interaction with Syn 1A converts the transporter from an electrogenic carrier with a voltage-dependent and variable stoichiometry to one with a fixed and tightly coupled stoichiometry suggesting that both cell excitability and psychostimulant-mediated effects are contingent to the state of association among the transporter and its interacting partners (Quick, 2003). Particularly, Carvelli et al. reported that interaction between Syn1A and DAT suppresses the channel state of the transporter, and the loss of interaction between the two proteins leads to enhanced synaptic DA levels (Carvelli et al., 2008). As electrogenic substrate transport and inward currents have also been described for other SLC6 members, which do not necessarily translocate neurotransmitters (amino acid, creatine and taurine transporters) it is conceivable that in addition to moving substrates across membranes this protein family also control ion concentrations within cellular microdomains.
The ability of a single molecular architecture to support both a transporter and a channel mechanism has been observed in Chloride channels where a single amino acid substitution converts the transporter to a channel (Accardi and Miller, 2004). In addition, the ability to form channels may allow transporters to produce large electrical signals and have a cellular function beyond the uptake and release of substrate. Indeed, data from native neurons suggest that DAT-mediated conductances generated by DA or amphetamine increase excitability of midbrain dopaminergic neurons (Ingram et al., 2002; Carvelli et al., 2004). These transport-associated currents provide useful tools for quantitative functional characterization, as well as for qualitative distinctions between substrates and blockers. For example, amphetamine-like dopamine-releasing drugs produce transport-associated currents at dopamine transporters, but cocaine-like drugs block such currents (Sonders and Amara, 1996).
4. Regulation
Transporter activity is dependent on the presence of the carrier at the cell surface and the transport capacity of each carrier molecule in this cell surface environment. Although transporter activity can be influenced greatly by changes in transcriptional activity, splice site usage, and mRNA translation and stability, these aspects of SLC6 transporter regulation will not be discussed here but briefly in a later section (see section 5.1 and (Hahn and Blakely, 2007). Additionally, cellular events that influence SLC6 NTT activity have been covered in detail in a recent comprehensive review (Kristensen et al., 2011) and we will limit our discussion here to transporter down-regulation which has been more thoroughly studied than up-regulation.
With the determination of the first high-resolution X-ray crystal structure of LeuT (Yamashita et al., 2005) came many advances and insight into the transport mechanism of this family of carriers. However, the highly conserved primary sequence between LeuT and the mammalian transporters is limited to TM domains and to a lesser extent the intracellular sequence joining these domains. LeuT has very limited N- and C-terminal domains while the mammalian transporters have extensive and diverse N- and C-terminal domains with differences in both amino acid sequence and length among the SLC6 family. With the exception of two highly conserved phosphorylation consensus sequences in the second and third intracellular loops, all of the identified phosphorylation, ubiquitination and known protein–protein interaction sites reside within the N- and C-terminal domains (Fig. 4). However, the differences in sequence and length of these domains among the SLC6 NTTs suggest the potential for a high degree of transporter-specific regulation.
4.1 PKC-dependent transporter down-regulation
Several studies have reported the involvement of several signaling pathways in the regulated trafficking of SLC6 transporters with some promoting trafficking toward the cell surface and others promoting internalization from the cell surface (see (Kristensen et al., 2011) for detailed information). It is clear from these studies that SLC6 NTTs traffic toward and away from the plasma membrane in constitutive and regulated manners. The most thoroughly studied kinase-dependent SLC6 trafficking mechanism is PKC-mediated transporter internalization (Fig. 5). It was originally thought that this internalization process would be analogous to GPCR internalization where a phosphorylation event is necessary to initiate internalization following agonist binding. However, this does not appear to be the case with the SLC6 NTT family because elimination of the major PKC phosphorylation sites in the first 21 or 22 residues of the DAT N-terminus abolished detectable phosphorylation without altering PKC-mediated internalization (Figs. 4 and 5). Although this result seemingly suggests that phosphorylation is not involved in DAT down-regulation, caution should be taken in this interpretation because it only indicates that phosphorylation is not necessary for transporter internalization but says little about the role of phosphorylation in modulating surface transporter activity because this mode of regulation may be masked by an independent internalization event and may vary significantly in different cellular environments. The majority of these studies have been conducted in heterologous non-neuronal cell systems that may differ in the basic components of transporter regulation and the time frame in which they occur. It is very plausible that an initial rapid trafficking-independent inactivation of transporters occurs at the plasma membrane prior to or in addition to internalization of the transporter as has been suggested for DAT (Mazei-Robison et al., 2008) and SERT (Jayanthi et al., 2005).
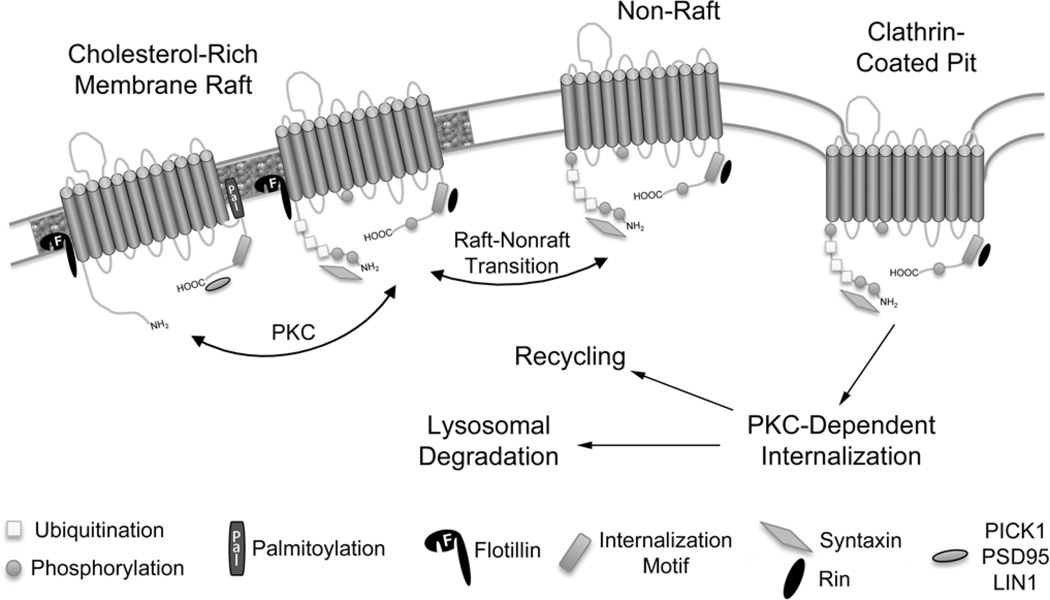
Fig. 5.
Modes of SLC6 transporter down-regulation. Plasma membrane residing SLC6 transporters are depicted with the many factors that influence their activity and internalization. Two modes of PKC-dependent transporter down-regulation that result in functional loss of substrate transport are depicted. This includes well-known clathrin/dynamin-dependent transporter endocytosis and the more recently uncovered endocytosis-independent mode that is dependent on membrane cholesterol. It is postulated that loss of substantial transport activity occurs prior to endocytosis, which removes transporters from the cell surface. Several post-translational modifications and binding partner interactions as well as cholesterol-rich membrane raft localization and PKC-dependent raft to non-raft transition are depicted with hypothetical roles for these participants and events in the down-regulation process. The transmembrane domain of Syn 1A and extended dimerization domain of Flot1 have been omitted for clarity.
4.2 Modes of transporter down-regulation
Along these same lines, an additional mode of transport down-regulation was proposed recently for DAT based on substantial retention of PKC-induced down-regulation following treatments that blocked the clathrin/dynamin-dependent endocytotic mode of down-regulation (Foster et al., 2008). Both modes of regulation were PKC-dependent but the time frame in which these events occurred was not explored. It is unclear if this recently uncovered endocytosis–independent mode of regulation involves transporter phosphorylation, localization to membrane raft domains, interactions with binding partners or combinations of these mechanisms including interaction between N- and C-terminal domains as has been demonstrated for the BetP transporter (Ressl et al., 2009) (Fig. 5). Elevated levels of PMA-stimulated phosphorylation were noted for DATs localized to cholesterol-rich membrane raft domains suggesting that rafts may be the location of PKC-dependent DAT phosphorylation (Foster et al., 2008). Additionally, the localization of DAT to membrane rafts is reported to be dependent on the raft protein Flotillin-1/Reggie-1 (Flot-1) and PKC–triggered DAT endocytosis as well as AMPH-induce DA efflux in neurons were both dependent on the presence of this raft-associated protein (Cremona et al., 2011). The specific mechanism in which Flot-1 interacts with DAT thereby stabilizing its raft localization and concurrently participates in internalization of the transporter is not known but is suggested to involve PKC-mediated phosphorylation of Flot-1 at Ser315 (Fig. 5) (Cremona et al., 2011). Interestingly, SERT and NET, which also have been found in membrane rafts (Jayanthi et al., 2004; Magnani et al., 2004), display raft to nonraft redistribution following PMA treatment (Samuvel et al., 2005; Jayanthi et al., 2006) but this redistribution was not observed with DAT (Foster et al., 2008) (Fig. 5). However, a clear dependence of transporter activity on membrane cholesterol levels has been established for DAT (Adkins et al., 2007; Foster et al., 2008; Hong and Amara, 2010) and SERT (Magnani et al., 2004) suggesting that the lipid membrane environment may be important for transporter conformation and activity. Acute cholesterol depletion results in decreased transporter activity that is not the result of altered surface expression or ion gradients required to drive substrate translocation (Foster et al., 2008). This phenomenon was also observed in Erlich Lettré tumor cells that displayed decreased taurine uptake after cholesterol depletion (Villumsen et al., 2010) suggesting that TauT and possibly the entire SLC6 family may require cholesterol for optimal transport activity. Other than transition into and out of membrane rafts it is not currently known how cholesterol/transporter interactions may be modulated resulting in altered transport activity.
4.3 Transporter palmitoylation
Another lipid-mediated form of regulation, S-palmitoylation, has been reported recently for DAT (Foster and Vaughan, 2011). Palmitoylation is reversible and dynamic, with addition and removal of palmitic acid via a thioester linkage to cysteine that is rapidly catalyzed by palmitoyl acyltransferases (PATs) and protein palmitoyl thioesterases (PPTs), respectively. These modifications confer the ability of target proteins to respond to regulatory signals in a manner analogous to phosphorylation/dephosphorylation and strongly influence DAT transport kinetics, degradation, and PKC-mediated down-regulation. Site-directed mutagenesis indicates that palmitoylation of rat DAT occurs at Cys580 at the intracellular end of TM12 (Figs. 4 and 5) and at one or more additional unidentified site (s) (Foster and Vaughan, 2011). This C-terminal site is conserved in human DAT (hDAT) as well as TauT and GlyT1 and a nearby TM12 cysteine residue in GAT3, but is not conserved in closely related SERT and NET suggesting that palmitoylation and any resulting regulatory events may occur in several but not all SLC6 transporters. However, identification of the remaining palmitoylation site (s) in DAT may reveal conserved sites and other SLC6 family members may have unique sites for this modification. Although the mechanism in which palmitoylation influences DAT activity, structural conformation and trafficking is unclear, the proximity to TM12 (Figs. 4 and 5) may impact DAT oligomerization, as TM12 of LeuT forms a dimer interface and palmitoylation may stabilize protein dimeric interactions (see Kristensen et al. 2011) in addition to possibly targeting DAT to membrane raft domains (Foster and Vaughan, 2011).
4.4 Regulatory components found in intracellular loops
Two relatively well-conserved intracellular loops (IL) in SLC6 transporters have been implicated in the PKC-dependent mechanism for transporter internalization. IL2 contains a highly conserved pair of S/T residues and Ser259 within IL 2 in NET (Fig. 4) appears to be a site of PKC action, but mutation of both Thr258 and Ser259 was required to abolish PKC-mediated down-regulation of NE transport (Jayanthi et al., 2006). In Gly 2, mutation of Ser 420 or an adjacent Lys residue in IL2 to the phosphomimetic residue Glu abolished PMA-induced down-regulation including plasma membrane GlyT 2 internalization while mutation of Ser420 to non-phosphorylatable Ala had little affect (Fornes et al., 2004). These two studies suggest that this region may be important in PKC-mediated SLC6 transporter internalization but the role of phosphorylation in this process remains unclear. IL3 contains well-conserved Tyr and Ser residues that have also been associated with PKC-dependent transporter regulation (Fig. 4). GAT1 is phosphorylated on unidentified serine residues in response to PKC activation but only if Tyr phosphorylation on residues 107 and 317 is eliminated or greatly reduced. Tyr317 is highly conserved within IL3 in the SLC6 family including TauT where an adjacent Ser residue (322) was identified as a PKC-dependent phosphorylation site (Han et al., 1999). Mutation of this site to Ala resulted in loss of PKC-mediated transporter down-regulation but was accompanied by a 2-3-fold increase in basal taurine transport activity. It is interesting to note that GAT1 also has a potential PKC phosphorylation site at Ser320 just C-terminal to Tyr317. These studies suggest that IL3 may also be important in SLC6 transporter down-regulation, but again, the role of phosphorylation in this process remains unclear.
4.5 C-Terminal regulatory components
Several C-terminal motifs of DAT have been linked to endocytotic trafficking and/or degradation. These include a binding site for the scaffolding protein Hic5 at hDAT residues 571–580 adjacent to the potential hDAT palmitoylation site (Carneiro et al., 2002), neighboring residues 587–596 that have been identified as a nonconventional internalization motif (FREKLAYAIA) that binds the plasma membrane–associated GTPase Rin and impacts constitutive and PKC-dependent trafficking (Boudanova et al., 2008), and a distal C-terminal binding site for the PDZ protein PICK1 that impacts transport surface targeting (Torres et al., 2001; Bjerggaard et al., 2004) (Figs. 4 and 5). The distal C-termini of NET and SERT were also identified as PICK1 binding sites with NET displaying strong and SERT week interaction with PICK1 (Torres et al., 2001).
In terms of phosphorylation, the only C-terminal phosphorylation site that has been identified is found in BGT1 (Massari et al., 2005). Both serine and threonine phosphorylation are stimulated in BGT1 in response to phorbol ester treatment and Thr612 was identified as the major phosphorylation site after this treatment (Fig. 4). Mutation of this residue resulted in impaired interaction with the PDZ protein LIN7 at the distal C-terminus of BGT1 analogous to PICK1 interactions with NET and DAT (Torres et al., 2001) and PDZ protein PSD-95 interaction with GLYT1 (Cubelos et al., 2005). No phosphorylatable residues are found in the PICK1 binding sites on DAT and NET but a PKC consensus sequence is found just upstream from this site in DAT suggesting that phosphorylation at this residue could potentially modulate PICK 1 binding (Fig. 4).
The C-terminal internalization motif (Figs. 4 and 5) is relatively well conserved among the SLC6 NTT family and mutation of this motif in NET also results in impaired internalization. This internalization domain is reported to be necessary for binding of Rin to DAT in a PKC-dependent manner (Navaroli et al., 2011). Mutation of residues 587–589 (REK) in the internalization motif resulted in loss of the PMA-induced increase in DAT-Rin interaction, however, the basal DAT-Rin interaction was increased by 300% in the mutant suggesting that these residues modulate DAT-Rin interaction in a PKC-dependent manner. It remains to be determined the exact role of all these regulatory features in SLC6 down-regulation but it appears that PICK1, Hic5, and LIN1 binding as well as the internalization motif and Rin interaction are likely involved in regulated trafficking/internalization with posttranslational modifications such as phosphorylation and palmitoylation potentially impacting these mechanism.
4.6 N-Terminal regulatory components
A broad body of evidence suggests that cellular kinases and phosphatases regulate SLC6 transporter expression, activity, trafficking and degradation. Although several of these transporters are known phosphoproteins a number of the specific phosphorylation sites within these proteins have not be identified. The N- and C-terminal tails have received considerable interest because of the presence of a number of kinase consensus sequences as potential phosphorylation sites in these domains. DAT has been well characterized to undergo phosphorylation by PKC within a serine cluster at the distal end of the N-terminus and proline-directed phosphorylation at the membrane proximal residue Thr53 also within this N-terminal domain (Foster et al., 2002; Gorentla et al., 2009) (Figs. 4 and 5). Serine cluster phosphorylation in not required for DAT clathrin/dynamin-dependent endocytosis but may play a role in the cholesterol associated non-endocytic mode of DAT down-regulation recently uncovered (Foster et al., 2008) in addition to modulating AMPH-stimulated DA efflux (Khoshbouei et al., 2004; Fog et al., 2006). It is not known if phosphorylation at Thr53 is involved PKC-mediated DAT down-regulation or DA efflux but several recent studies have indicated that DAT and SERT N-termini are flexible and that altered conformations induced by mutations at Thr62 near Thr53 or membrane tethering of the N-terminus strongly impact uptake, binding, and efflux functions (Guptaroy et al., 2009; Sucic et al., 2010; Guptaroy et al., 2011), supporting the idea that N-terminal phosphorylation may be altering DAT and SERT conformation.
The N-terminal domain of DAT, SERT, NET and GAT1 is also the binding site of the SNARE protein Syn 1A (reviewed in Kristensen et al. (2011)) which influences the activity and cell surface availability of these transporters. Evidence suggests that binding may be mediated through the SNARE H3 motif and closely-spaced negatively charged N-terminal Asp residues in GAT1 and SERT. For DAT, the interaction site is contained within the first 33 N-terminal residues (Binda et al., 2008) that contains few negatively charged amino acids but does include a closely-spaced PKC-mediated phosphorylation serine cluster (Ser2, 4, 7, 12 and 13) (Foster et al., 2002) (Fig. 4). Additionally, cleavage of Syn 1A with botulinum toxin C1 in striatal slices results in elevated transport Vmax and reduced DAT phosphorylation (Cervinski et al., 2010) suggesting that Syn 1A-DAT interaction suppresses DA uptake and may be dependent on DAT N-terminal phosphorylation which would provide the negative charge necessary for interaction as seen with SERT and GAT1. Furthermore, DAT phosphorylation within the N-terminal Ser cluster and Syn 1A interaction with the distal N-terminus both promote AMPH-stimulated DA efflux (Khoshbouei et al., 2004; Cervinski et al., 2005; Fog et al., 2006; Binda et al., 2008) suggesting the potential for phosphorylation-dependent DAT-Syn1A interaction as the mechanism responsible for this efflux event. Although distal phosphorylation of the DAT N-terminus is not required for transporter internalization, another N-terminal post-translational modification is required for receptor endocytosis. DAT is constitutively ubiquitinated and PMA treatment increases this modification (Figs. 4 and 5), which is dependent on the presence of three N-terminal lysines (Lys19, Lys27, and Lys35) (Miranda and Sorkin, 2007). Mutation of these residues to Arg essentially abolishes both ubiquitination and PKC-dependent DAT internalization suggesting that this modification is an essential component of DAT endocytosis and the endocytotic mode of transporter down-regulation (Miranda and Sorkin, 2007)
4.7 Disruption of transporter regulation
Although great progress has been made in determining the ways in which SLC6 transporters can be regulated it is clear that the mechanisms involved are complex and multifaceted and may differ slightly between members of this family. Much more analysis is necessary to elucidate the complex manner in which all of these regulatory elements interact resulting in altered transporter function and trafficking. In addition, multiple SLC6 transporter coding variants have been identified but functional analysis of these variants is limited to a select few including those found in SERT and DAT. Polymorphisms in SERT and DAT have been associated with psychiatric disorders, such as autism, bipolar and attention deficit/hyperactivity disorder (Hahn and Blakely, 2007), and many of the identified mutations occur in the N- and C-terminal regions in close proximity to many regulatory components in these transporters (Fig. 4). This suggests that these mutations may alter the normal regulatory mechanisms in these proteins and thereby compromise synaptic signaling related to these transporters leading to psychiatric disturbances.
5. Therapeutic Development
In this section, we provide information on studies related to epigenetics, mouse models, biomarkers, single nucleotide polymorphisms (SNPs) and small molecule development for their utilization in discovery of novel therapeutics for neurological disorders and drug addiction therapy.
5.1 Epigenetics
Epigenetics, a mechanism of rapid environmental-initiated adaptation through changes in gene expression patterns that do not require changes in DNA sequence, has come to the forefront as a possible way to understand diseases that do not have a clear genetic etiology. Epigenetic control of gene expression occurs through DNA methylation, histone modification and RNA silencing, and this programming can exhibit transgenerational inheritance (Franklin et al., 2011) whereby expression modulated traits acquired in germ line genes can be transmitted to subsequent generations. Given the relationship of SLC6 transporters to mood disorders such as depression, addiction, aggression, PTSD, anxiety, OCD and disorders like ADHD, autism, the possibility that these illnesses may be beyond simple genetic control where environmental imprinting on gene expression could modulate the severity or propensity for developing these conditions suggests epigenetics could provide an exciting new path for therapeutic intervention. Here, we present information related to epigenetic studies of SLC6 transporters. The majority of epigenetic studies have been conducted on the NTTs, particularly on SERT, DAT and NET (see Table 1).
In humans and primates, evidence for environmental factor induced neurological diseases have been obtained by measuring the CpG methylation of the promoter region of candidate genes and correlating results with the disrupted mRNA expression levels in specific pathologies (Kinnally et al., 2010; Sugawara et al., 2011). Gene and environment (GXE) interactions such as those between the SERT polymorphisms and environmental adversities (i.e. early life stress, war repatriation, maternal separation, psychosocial adversity) have been associated with depression, anxiety, aggressive behavior, suicide and other mental disorders (Meaney, 2010; Artero et al., 2011; Lesch, 2011). A classic example of such a GXE relationship involves the short and long alleles of a SERT promoter polymorphism (5-HT transporter-linked promoter region or 5HTTLPR) where the short form combined with life stress was associated with increased depression and suicidal behavior (Caspi et al., 2003). Similarly, the DAT gene is also sensitive to epigenetic regulation and DNA methylation of the DAT promoter regions affects behavior in eating disorders and alcohol dependence and craving (Hillemacher et al., 2009). While there is very limited information of GXE interaction at NET, methylation of SLC6A2 has been implicated in essential hypertension and panic disorder (Esler et al., 2008).
Comprehensive evaluation of epigenetic influences on SERT from different animal paradigms support the idea of environmental impact on SERT expression patterns and its contribution to complex diseases and traits (Caspi et al., 2010). However, the relationship between the NTTs and epigenetics is not definitive as a study by Park et al. (2011a) failed to find a significant difference when comparing methylation of SERT promoters in alcoholic versus control patients. The discrepancy between positive and negative association for GXE to SERT expression have been linked to experimental variables such as sample age, allelic variation, heterozygocity, environmental paradigm and trait assessment (Uher and McGuffin, 2008) suggesting that addressing these experimental issues may unmask the impact epigenetics has on NTT function. Epigenetic research provides compelling evidence from cohort based studies to explain the epigenetic regulation of gene expression for better understanding of the disease etiology. However, due to brain tissue accessibility issues with the participants, exploration of more easily attainable peripheral tissue biomarkers becomes a future strategy for such epigenetic studies. Attempts are underway to develop epigenetic biomarkers associated with the SERT gene that could be associated with neuropathological conditions like depression (Olsson et al., 2010) and thus help in early diagnosis and disease treatment. Such a strategy may also enable GXE effects to be examined for other SLC6 members. Importantly, testing the known epigenetic regulation of disease states in animal models and identifying the cellular components that control chromatin and histone modifications could lead to discovery of drug treatments that target gene modifying proteins involved in disease processes.
5.2 Role of murine models in the development of psychiatric therapeutics
The complex and overlapping roles of monoamine systems in a number of mood and behavioral disorders has made deciphering their individual contributions problematic. Transgenic NTT mouse models have provided key insights in differentiating the roles among the aminergic systems. Early experiments involving transgenic null mice where one of the NTT genes had been eliminated were enlightening because they sometimes yielded unexpected results. For instance, in the DAT (Slc6a3) knockout mice, self-administration of cocaine remains (Rocha et al., 1998) indicating involvement of an intricate compensatory mechanism regulated by other transporters (SERT and NET). However, complete knockout of the DAT gene may cause developmental and adaptive changes that disturb dopamine homeostasis. SERT (Slc6a4) knockout models for autism revealed that 5-HT levels in this animal model were contrary to those actually observed in autism (Veenstra-VanderWeele et al., 2009) suggesting that SERT is not the only target regulating 5-HT levels in autism. Similar limitations for data interpretation were evident for null, over-expressing or polymorphic variant mouse models upon examination of their effects on SERT protein expression, function and sensitivity to selective serotonin receptor inhibitors (SSRI) (Homberg, 2011).
An alternative approach has been implemented to generate mutant NTT knock-in mouse models that do not alter the target protein expression levels but exhibit increased substrate transport or unaltered substrate transport with changes in antagonist binding. A DAT mouse model was generated by introducing three mutations into DAT that maintains DA transport activity but loses cocaine-mediated transport inhibition (Chen et al., 2006). This cocaine-insensitive DAT mouse model has been utilized to weigh and discriminate DAT involvement from other neurotransporters (SERT and NET) in the neurochemical and behavioral profile of molecules like cocaine and methylphenidate (Tilley and Gu, 2008; Tilley et al., 2009) in a system that should better mimic the wild type mouse.
Likewise, identification of a single residue mutation in human SERT (I172M) that dramatically alters the potency of many antidepressants and cocaine (Henry et al., 2006) led to the development of an antidepressant-insensitive mouse (Thompson et al., 2011). The I172M mice displayed unaltered SERT protein levels and function while exhibiting decreased sensitivity for SSRIs and recently have been employed in identification of another target (i.e. sigma receptors) for SSRIs (Ye and Blakely, 2011). Finally, a transgenic mouse carrying a G56A mutation in SERT which corresponds to a non-synonymous single nucleotide polymorphism (NS-SNP) linked with autism was generated. While not yet characterized, it may provide clues suggesting how this N-terminal mutation in SERT can contribute to autism.
Use of transgenic animals that exhibit altered drug response while maintaining the natural function of the transporter offers a valuable experimental paradigm to help dissect complex and multifaceted disease systems that could be dramatically altered through compensatory mechanisms observed in knockout models. Pharmacologically ‘silencing’ these primary drug targets allows for the identification of contributions of secondary targets to the model and possible detection of novel therapeutic targets.
5.3 Biomarkers
A neuroimaging biomarker by definition aids in early and differential diagnosis of disease, evaluation of disease severity/progression and/or assessing treatment outcome. Use of genetic or epigenetic disease associations as biomarkers is problematic due to the lack of reproducibility and complex inter-individual genetic and epigenetic variation.
In PD, autism, ADHD and Alzheimer disease (AD) substantial neuronal loss occurs in the substantia nigra, anterior and posterior cingulate cortices, locus coeruleus and thalamus leading to altered dopaminergic, serotoninergic and noradrenergic transmission mediated by the NTTs (Zarow et al., 2003; Spencer et al., 2007; Nakamura et al., 2010). As such, the SLC6 family of proteins is well suited as neuroimaging markers for diagnosing these disorders as they estimate the integrity of these transporters.
Among the SLC6 transporters, DAT and SERT are well characterized for their potential application in the area of neuroimaging biomarker research (Tan et al., 2011) (Table 1). Many radiotracer ligands for NTTs are synthesized and utilized for positron emission tomography (PET) and single photon emission computed tomography (SPECT) brain imaging (Table 1). Numerous DAT and SERT neuroimaging radioligand markers currently available for PET and SPECT imaging exhibit varying properties including high-affinity and selectivity, increased target to background ratio, long half-lives, rapid kinetics, favorable metabolism and augmented imaging quality (Varrone and Halldin, 2010; Tan et al., 2011). NET radiotracer ligands used in PET imaging are still under development but a recent report defines the usefulness of the NET radioligand (S,S) - [18F]FMeNER-D2, the only acceptable and proven PET ligand for NET, in the early diagnosis of AD in humans (Gulyás et al., 2010).
Biomarkers can play an important role in early diagnosis and effective therapeutic intervention; however, comprehending complex neuropathologies is problematic. Due to the presence of large inter-individual variation contributed by numerous factors like genetic variability, environment, GXE interaction, etc, experiments dealing with interdisciplinary approaches, such as combining genetics and neuroimaging could benefit the development of more effective therapeutics.
5.4 Pharmacogenomics
Association of SNPs (non synonymous, synonymous, non-coding) and variable number of tandem repeats (VNTR) with disease states serves as a useful mechanism for identification of proteins and understanding how alteration of the protein’s structure, function and expression can be involved in a disease pathway. Defining the genetic link to disease is becoming increasingly important as health care transitions to more personalized medicine. Here, we will focus on the major genetic-disease associations (GDA) identified for SLC6 transporters but an expanded view of SLC6 SNPs and VNTRs can be found in Table 1 and recent reviews (Hahn and Blakely, 2007; Bröer and Palacín, 2011; Murphy and Moya, 2011).
5.4.1 SERT polymorphisms
Studies of genetic variation in the SERT gene (SLC6A4) have identified several polymorphisms, both coding and noncoding, associated with disease. One of the noncoding VNTR polymorphisms, 5HTTLPR, located in the SLC6A4 promoter gives rise to two alleles termed short (s) and long (l) forms with the s form being associated in some studies with increased occurrence of suicide-with violence, anxiety, depression, positive responsiveness to antidepressant treatment (Hahn and Blakely, 2007), PTSD (Wang et al., 2011), smoking (Bloch et al., 2010) and substance abuse (Gerra et al., 2010) and the l form associated with OCD, alcoholism, schizophrenia, suicidal behavior and ADHD (Hahn and Blakely, 2007; Retz et al., 2008; Bosia et al., 2010; Tartter and Ray, 2011). The impact of the 5HTTLPR polymorphism may be more pronounced on individuals having stressful life events and the homozygous ss genotype (Hahn and Blakely, 2007). A second VNTR in intron 2 has been associated with affective disorders and appears to modulate protein expression in a primary sequence dependent manner (Lovejoy et al., 2003). Numerous NS-SNPs have been identified in SERT; many of which impact transporter function. Variants G56A, F465L, L550V and I425L have been identified in affected individuals in a study of 341 autistic families and an I425V allele has been linked to OCD and Asperger syndrome (Hahn and Blakely, 2007; Voyiaziakis et al., 2011) and the I425L and L550V variants are associated with autism/OCD (Prasad et al., 2009).
5.4.2 NET polymorphisms
NET polymorphisms can also be found in coding, noncoding and promoter regions of the SLC6A2 gene (Table 1). Polymorphisms in SLC6A2 have been associated with anorexia nervosa, panic disorder, ADHD, depression, and chronicity of depression (Hahn and Blakely, 2007) and blood pressure response to exercise (Kohli et al., 2011b). NS-SNPs number around 20 and include A457P, F528C, R121Q, T283M and V245I which have been associated with occurrence of orthostatic intolerance, long QT syndrome, blood-pressure homeostasis, major depression and ADHD (Hahn and Blakely, 2007; Haenisch et al., 2009; Hahn et al., 2009)
5.4.3 DAT polymorphisms
Like SERT and NET, DAT polymorphisms occur in the coding, noncoding and promoter regions of the SLC6A3 gene (Table 1). The 3’ untranslated region of SLC6A3 contains a common VNTR (frequency of 0.70) and has been associated with substance dependence, paranoia in cocaine addicts, response to amphetamines, ADHD, PD and bipolar disorder, schizophrenia (Cordeiro et al., 2010) and major depression (Pattarachotanant et al., 2010). Coding variants in DAT include V55A, R237Q, L368Q, V382A, A559V, P395L and E602G. Of these, A559V and E602G have been associated with bipolar disorder with A559V also being linked to ADHD, and L368Q and P395L tagged with infantile parkinsonism-dystonia (IPD) syndrome (Kurian et al., 2009). As mentioned above, amphetamines promote reverse transport through DAT and are commonly used for treatment of ADHD. Interestingly, the A559V DAT mutation results in a transporter that exhibits a high level of reverse transport even in the absence of amphetamine (Mazei-Robison et al., 2008).
5.4.4 Non-NTT SLC6 polymorphisms
The link between disease and SLC6 transporters is not limited to the neurotransmitter transporters. In fact, other members of the SLC6 family have solid associations with disease. The creatine transporter (CT1, SLC6A8) represents one of the strongest correlations between SNPs and disease among SLC6 transporters where numerous SNPs in coding and non-coding regions of the CT1 (SLC6A8) gene have been connected to X-linked mental retardation which is thought to result from reduced or absent creatine transport in the brain (Hahn and Blakely, 2007). Similarly, variations in the neutral amino acid transporter (B0AT1, SLC6A19) are associated with Hartnup disorder, a metabolic disorder resulting from dysfunctional amino acid transport (Kleta et al., 2004). Glycine transporter (GlyT2, SLC6A5) SNPs have been associated with hyperekplexia (Rees et al., 2006) and schizophrenia (Deng et al., 2008). Schizophrenia has also been correlated with the betaine-GABA transporter (BGT1, SLC6A12) (Park et al., 2011b). Interestingly, SNPs for both BGT and the proline transporter (PROT, SLC6A7) are correlated with asthma (Kim et al., 2010; Pasaje et al., 2010). The amino acid transporter (B0AT2, SLC6A15), like the several NTT members, has been linked to major depression (Kohli et al., 2011a). Additionally, myocardial infarction, hypertension and obesity have been linked to SLC6A18 (XT2) (Matsumoto et al., 2011), SLC6A9 (GLYT1) (Ueno et al., 2009) and SLC6A14 (ATB0+) (Suviolahti et al., 2003) variants, respectively.
Although there are a large number of pathologies and drug treatments associated with SLC6A transporters, the identification of genetic markers and development of effective biomarkers for these conditions is a difficult task due to the enormous amount of genetic variation and inconsistency in experimental findings. In fact, the reliability of the associations drawn in these studies has been questioned in meta-analyses studies (Serretti et al., 2008; Uher and McGuffin, 2008). In spite of such drawbacks, there is an increasing interest in pharmacogenetics where the association of SNPs to disease states and therapeutic treatments contribute in understanding genetic variation within a specific population and designing effective individualized diagnostic techniques and pharmacotherapies.
5.5 Cocaine Pharmacotherapeutics - Behavioral Profile
DAT, NET and SERT are primary targets of the psychostimulant cocaine and the socio-economic impact of its abuse has been well documented (Volkow and Li, 2005). To date, substitution pharmacotherapy for cocaine has had little success (Grabowski et al., 2004). The reinforcing and behavioral properties such as self administration and locomotor activity, responsible for the abuse liability of cocaine and cocaine-like compounds and other DAT inhibitors are related to the binding affinity, selectivity and occupancy of DAT (Heikkila et al., 1979; Kuhar et al., 1991; Newman and Kulkarni, 2002; Lindsey et al., 2004; Kimmel et al., 2008; Newman and Katz, 2009).
Recently there has been excitement about the therapeutic potential of a class of compounds called Benztropines (3 alpha-diphenylmethoxytropanes; BZT) as they act on DAT, but yield a behavioral profile distinctive from cocaine (Newman and Kulkarni, 2002; Newman and Katz, 2009) as demonstrated by less abuse liability, altered self-administration and varied place conditioning. While the cause of the altered behavioral outcomes with BZT remains unclear, it has been proposed that the slower rate of onset of action, affinity to muscarinic M1 and histaminic H1 receptors, differential binding to DAT unique from cocaine (Reith et al., 2001; Chen and Zhen, 2004; Loland et al., 2008) or interaction with other receptors like 5HT1, 5HT2 and sigma receptors are plausible explanations for the characteristic behavioral (non-cocaine-like) effects (Agoston et al., 1997).
Another class of compounds, sigma receptor ligands (eg. BMY-14802, NPC 16377, rimcazole, SR 31742A), like BZT, also result in modified behavioral patterns by altering the locomotor stimulant effects of cocaine (Matsumoto et al., 2001; Katz et al., 2003). Initially, it was thought that binding of rimcazole analogs to sigma receptor, but not to the dopamine receptor, (Matsumoto et al., 2001) weakened cocaine-induced convulsive effects. However, the latest findings suggest a dual occupancy mode of action for rimcazole on DAT and sigma receptors for lowered cocaine self-administration (Hiranita et al., 2011). The potential of sigma receptor ligands as cocaine abuse therapeutics has been reviewed previously (Matsumoto, 2009). These findings from BZT and rimcazole compounds offer hope that a stimulant-replacement therapy can be achieved (Henry and Blakely, 2008).
6. Conclusion and future perspectives
Indeed, the emergence of crystal structures revealing the architecture of a prokaryotic SLC6 transporter complexed with substrates and inhibitors has resulted in an enormous leap forward in our understanding as well as provided a canvas on which to paint our biochemical and genetic studies. Incredibly, we found that this canvas is more universal than first thought and provides insights into unrelated transporters that possess the same LeuT fold core structural design. Nevertheless, the current crystal structures leave many unanswered questions as the prokaryotic structures lack important regulatory and functional domains found in the eukaryotic SLC6 proteins of which greater understanding into their roles will surely help unravel their connection to dysregulation/dysfunction and disease. Besides needing a fundamental understanding of the coupling of substrate and ion symport, the electrogenic nature of these transporters and the ability of some to transition to a channel mode suggests a possible role in functional regulation by modulation of the membrane potential. Further study is needed to determine if such a function could have pathological consequences. So while we wait for a eukaryotic SLC6 structure to emerge, there is still much to accomplish in the study of this group of proteins and their critical associations to human health and homeostasis.
Acknowledgments
The authors are grateful for support from the National Institutes of Health to LKH (DA024797), LC (DA027845) and JDF (DA 031991) and the University North Dakota seed grant program to JDF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl-channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- Adams SV, DeFelice LJ. Flux coupling in the human serotonin transporter. Biophys J. 2002;83:3268–3282. doi: 10.1016/S0006-3495(02)75328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–10497. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- Agoston GE, Vaughan R, Lever JR, Izenwasser S, Terry PD, Newman AH. A novel photoaffinity label for the dopamine transporter based on N-substituted 3 -[bis(4'-fluorophenyl)methoxy]tropane. Bioorg Med Chem Lett. 1997;7:3027–3032. [Google Scholar]
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- Andersen J, Olsen L, Hansen KB, Taboureau O, Jørgensen FS, Jørgensen AM, Bang-Andersen B, Egebjerg J, Stromgaard K, Kristensen AS. Mutational mapping and modeling of the binding site for (S)-citalopram in the human serotonin transporter. J Biol Chem. 2010;285:2051–2063. doi: 10.1074/jbc.M109.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Taboureau O, Hansen KB, Olsen L, Egebjerg J, Stromgaard K, Kristensen AS. Location of the antidepressant binding site in the serotonin transporter: importance of Ser-438 in recognition of citalopram and tricyclic antidepressants. J Biol Chem. 2009;284:10276–10284. doi: 10.1074/jbc.M806907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero S, Touchon J, Dupuy A-M, Malafosse A, Ritchie K. War exposure, 5-HTTLPR genotype and lifetime risk of depression. Br J Psychiatry. 2011;199:43–48. doi: 10.1192/bjp.bp.110.087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol. 2008;74:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerggaard C, Fog JU, Hastrup H, Madsen K, Loland CJ, Javitch JA, Gether U. Surface targeting of the dopamine transporter involves discrete epitopes in the distal C terminus but does not require canonical PDZ domain interactions. J Neurosci. 2004;24:7024–7036. doi: 10.1523/JNEUROSCI.1863-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch B, Reshef A, Cohen T, Tafla A, Gathas S, Israel S, Gritsenko I, Kremer I, Ebstein RP. Preliminary effects of bupropion and the promoter region (HTTLPR) serotonin transporter (SLC6A4) polymorphism on smoking behavior in schizophrenia. Psychiatry Res. 2010;175:38–42. doi: 10.1016/j.psychres.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Bosia M, Anselmetti S, Pirovano A, Ermoli E, Marino E, Bramanti P, Smeraldi E, Cavallaro R. HTTLPR functional polymorphism in schizophrenia: executive functions vs. sustained attention dissociation. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:81–85. doi: 10.1016/j.pnpbp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, Stevens Z, Melikian HE. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Mol Cell Neurosci. 2008;39:211–217. doi: 10.1016/j.mcn.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudker O, Verdon G. Structural perspectives on secondary active transporters. Trends Pharmacol Sci. 2010;31:418–426. doi: 10.1016/j.tips.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S, Palacín M. The role of amino acid transporters in inherited and acquired diseases. Biochem J. 2011;436:193–211. doi: 10.1042/BJ20101912. [DOI] [PubMed] [Google Scholar]
- Carneiro AM, Ingram SL, Beaulieu JM, Sweeney A, Amara SG, Thomas SM, Caron MG, Torres GE. The multiple LIM domain-containing adaptor protein Hic-5 synaptically colocalizes and interacts with the dopamine transporter. J Neurosci. 2002;22:7045–7054. doi: 10.1523/JNEUROSCI.22-16-07045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Blakely RD, DeFelice LJ. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci. 2008;105:14192–14197. doi: 10.1073/pnas.0802214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, McDonald PW, Blakely RD, DeFelice LJ. Dopamine transporters depolarize neurons by a channel mechanism. Proc Natl Acad Sci. 2004;101:16046–16051. doi: 10.1073/pnas.0403299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Syntaxin 1A regulates dopamine transporter activity, phosphorylation and surface expression. Neuroscience. 2010;170:408–416. doi: 10.1016/j.neuroscience.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Rollins J, Mahowald AP, Bazinet C. Neurotransmitter Transporter-Like: a male germline-specific SLC6 transporter required for Drosophila spermiogenesis. PLoS ONE. 2011;6:e16275. doi: 10.1371/journal.pone.0016275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Zhen J. Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding interaction for GBR12909 and benztropine as opposed to cocaine. J Neurochem. 2004;89:853–864. doi: 10.1111/j.1471-4159.2004.02386.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou F-M, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro Q, Siqueira-Roberto J, Vallada H. Association between the SLC6A3 A1343G polymorphism and schizophrenia. Arq Neuropsiquiatr. 2010;68:716–719. doi: 10.1590/s0004-282x2010000500008. [DOI] [PubMed] [Google Scholar]
- Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubelos B, Gonzalez-Gonzalez IM, Gimenez C, Zafra F. The scaffolding protein PSD-95 interacts with the glycine transporter GLYT1 and impairs its internalization. J Neurochem. 2005;95:1047–1058. doi: 10.1111/j.1471-4159.2005.03438.x. [DOI] [PubMed] [Google Scholar]
- DeFelice LJ, Adams SV, Ypey DL. Single-file diffusion and neurotransmitter transporters: Hodgkin and Keynes model revisited. BioSystems. 2001;62:57–66. doi: 10.1016/s0303-2647(01)00137-x. [DOI] [PubMed] [Google Scholar]
- Deng X, Sagata N, Takeuchi N, Tanaka M, Ninomiya H, Iwata N, Ozaki N, Shibata H, Fukumaki Y. Association study of polymorphisms in the neutral amino acid transporter genes SLC1A4, SLC1A5 and the glycine transporter genes SLC6A5, SLC6A9 with schizophrenia. BMC Psychiatry. 2008;8:58. doi: 10.1186/1471-244X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Kaye D, El-Osta A, Guo L, Barton D, Pier C, Brenchley C, Dawood T, Jennings G, Lambert E. Human sympathetic nerve biology: parallel influences of stress and epigenetics in essential hypertension and panic disorder. Ann N Y Acad Sci. 2008;1148:338–348. doi: 10.1196/annals.1410.064. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Fornes A, Nunez E, Aragon C, Lopez-Corcuera B. The second intracellular loop of the glycine transporter 2 contains crucial residues for glycine transport and phorbol ester-induced regulation. J Biol Chem. 2004;279:22934–22943. doi: 10.1074/jbc.M401337200. [DOI] [PubMed] [Google Scholar]
- Forrest LR, Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 2009;24:377–386. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LR, Tavoulari S, Zhang Y-W, Rudnick G, Honig B. Identification of a chloride ion binding site in Na+/Cl -dependent transporters. Proc Natl Acad Sci. 2007;104:12761–12766. doi: 10.1073/pnas.0705600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem. 2002;277:25178–86. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Foster JD, Vaughan RA. Palmitoylation controls dopamine transporter kinetics, degradation and protein kinase C-dependent regulation. J Biol Chem. 2011;286:5175–5186. doi: 10.1074/jbc.M110.187872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Linder N, Russig H, Thöny B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS ONE. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner RM, Perez C, Koshy C, Ziegler C. Role of Bundle Helices in a Regulatory Crosstalk in the Trimeric Betaine Transporter BetP. J Mol Biol. 2011;414:327–336. doi: 10.1016/j.jmb.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Castaldini L, Garofano L, Manfredini M, Somaini L, Leonardi C, Gerra ML, Donnini C. Relevance of perceived childhood neglect, 5-HTT gene variants and hypothalamuspituitary-adrenal axis dysregulation to substance abuse susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:715–722. doi: 10.1002/ajmg.b.31038. [DOI] [PubMed] [Google Scholar]
- Gorentla BK, Moritz AE, Foster JD, Vaughan RA. Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry. 2009;48:1067–1076. doi: 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gulyás B, Brockschnieder D, Nag S, Pavlova E, Kása P, Beliczai Z, Légrádi Á, Gulya K, Thiele A, Dyrks T, Halldin C. The norepinephrine transporter (NET) radioligand (S,S)-[18F]FMeNER-D2 shows significant decreases in NET density in the human brain in Alzheimer's disease: A post-mortem autoradiographic study. Neurochem Int. 2010;56:789–798. doi: 10.1016/j.neuint.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Guptaroy B, Fraser R, Desai A, Zhang M, Gnegy ME. Site-Directed Mutations near Transmembrane Domain 1 Alter Conformation and Function of Norepinephrine and Dopamine Transporters. Mol Pharmacol. 2011;79:520–532. doi: 10.1124/mol.110.069039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptaroy B, Zhang M, Bowton E, Binda F, Shi L, Weinstein H, Galli A, Javitch JA, Neubig RR, Gnegy ME. A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Mol Pharmacol. 2009;75:514–524. doi: 10.1124/mol.108.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Linsel K, Brüss M, Gilsbach R, Propping P, Nöthen MM, Rietschel M, Fimmers R, Maier W, Zobel A, Höfels S, Guttenthaler V, Göthert M, Bönisch H. Association of major depression with rare functional variants in norepinephrine transporter and serotonin1A receptor genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1013–1016. doi: 10.1002/ajmg.b.30912. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annu. Rev. Pharmacol. Toxicol. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Steele A, Couch RS, Stein MA, Krueger JJ. Novel and functional norepinephrine transporter protein variants identified in attention-deficit hyperactivity disorder. Neuropharmacology. 2009;57:694–701. doi: 10.1016/j.neuropharm.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Budreau AM, Chesney RW. Ser-322 is a critical site for PKC regulation of the MDCK cell taurine transporter (pNCT) J Am Soc Nephrol. 1999;10:1874–1879. doi: 10.1681/ASN.V1091874. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Cabbat FS, Duvoisin RC. Motor activity and rotational behavior after analogs of cocaine: correlation with dopamine uptake blockade. Commun Psychopharmacol. 1979;3:285–290. [PubMed] [Google Scholar]
- Henry LK, Blakely RD. Distinctions between dopamine transporter antagonists could be just around the bend. Mol Pharmacol. 2008;73:616–618. doi: 10.1124/mol.107.044586. [DOI] [PubMed] [Google Scholar]
- Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou M-F, Newman AH, Blakely RD. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem. 2006;281:2012–2023. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- Henry LK, Iwamoto H, Field JR, Kaufmann K, Dawson ES, Jacobs MT, Adams C, Felts B, Zdravkovic I, Armstrong V, Combs S, Solis E, Rudnick G, Noskov SY, DeFelice LJ, Meiler J, Blakely RD. A conserved asparagine residue in transmembrane segment 1 (TM1) of serotonin transporter dictates chloride-coupled neurotransmitter transport. J Biol Chem. 2011;286:30823–30836. doi: 10.1074/jbc.M111.250308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillemacher T, Frieling H, Hartl T, Wilhelm J, Kornhuber J, Bleich S. Promoter specific methylation of the dopamine transporter gene is altered in alcohol dependence and associated with craving. J Psychiatr Res. 2009;43:388–392. doi: 10.1016/j.jpsychires.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH, Tanda G, Katz JL. Decreases in cocaine self-administration with dual inhibition of the dopamine transporter and receptors. J Pharmacol Exp Ther. 2011;339:662–677. doi: 10.1124/jpet.111.185025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR. A mouse model to address unresolved antidepressant issues. Proc Natl Acad Sci. 2011;108:3463–3464. doi: 10.1073/pnas.1100757108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong WC, Amara SG. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J Biol Chem. 2010;285:32616–32626. doi: 10.1074/jbc.M110.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- Iyer GS, Krahe R, Goodwin LA, Doggett NA, Siciliano MJ, Funanage VL, Proujansky R. Identification of a testis-expressed creatine transporter gene at 16p11.2 and confirmation of the X-linked locus to Xq28. Genomics. 1996;34:143–146. doi: 10.1006/geno.1996.0254. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Annamalai B, Samuvel DJ, Gether U, Ramamoorthy S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis and phosphorylation. Mol Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem. 2004;279:19315–19326. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- Katz JL, Libby TA, Kopajtic T, Husbands SM, Newman AH. Behavioral effects of rimcazole analogues alone and in combination with cocaine. Eur J Pharmacol. 2003;468:109–119. doi: 10.1016/s0014-2999(03)01638-8. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-H, Cheong HS, Park BL, Bae JS, Jung S, Yoon S-H, Park JS, Jang AS, Park SW, Uh S-T, Kim Y-H, Hwang H-K, Park C-S, Shin HD. A New Association Between Polymorphisms of the Slc6a7 Gene in the Chromosome 5q31–32 Region and Asthma. J Hum Genet. 2010;55:358–365. doi: 10.1038/jhg.2010.34. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol. Biochem. Behav. 2008;90:453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, Mann JJ. Epigenetic Regulation of Serotonin Transporter Expression and Behavior in Infant Rhesus Macaques. Genes Brain Behav. 2010;9:575. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleta R, Romeo E, Ristic Z, Ohura T, Stuart C, Arcos-Burgos M, Dave MH, Wagner CA, Camargo SRM, Inoue S, Matsuura N, Helip-Wooley A, Bockenhauer D, Warth R, Bernardini I, Visser G, Eggermann T, Lee P, Chairoungdua A, Jutabha P, Babu E, Nilwarangkoon S, Anzai N, Kanai Y, Verrey F, Gahl WA, Koizumi A. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat Genet. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- Kohli MA, Lucae S, Saemann PG, Schmidt MV, Demirkan A, Hek K, Czamara D, Alexander M, Salyakina D, Ripke S, Hoehn D, Specht M, Menke A, Hennings J, Heck A, Wolf C, Ising M, Schreiber S, Czisch M, Müller MB, Uhr M, Bettecken T, Becker A, Schramm J, Rietschel M, Maier W, Bradley B, Ressler KJ, Nöthen MM, Cichon S, Craig IW, Breen G, Lewis CM, Hofman A, Tiemeier H, van Duijn CM, Holsboer F, Müller-Myhsok B, Binder EB. The neuronal transporter gene SLC6A15 confers risk to major depression. Neuron. 2011a;70:252–265. doi: 10.1016/j.neuron.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli U, Hahn MK, English BA, Sofowora GG, Muszkat M, Li C, Blakely RD, Stein CM, Kurnik D. Genetic variation in the presynaptic norepinephrine transporter is associated with blood pressure responses to exercise in healthy humans. Pharmacogenet. Genomics. 2011b;21:171–178. doi: 10.1097/FPC.0b013e328344f63e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Piscitelli CL, Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature. 2009;459:347–355. doi: 10.1038/nature08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Kurian MA, Zhen J, Cheng S-Y, Li Y, Mordekar SR, Jardine P, Morgan NV, Meyer E, Tee L, Pasha S, Wassmer E, Heales SJR, Gissen P, Reith MEA, Maher ER. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J Clin Invest. 2009;119:1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HM. Transcriptional regulation of the betaine/gamma-aminobutyric acid transporter by hypertonicity. Biochem Soc Trans. 1996;24:853–856. doi: 10.1042/bst0240853. [DOI] [PubMed] [Google Scholar]
- Lesch K-P. When the serotonin transporter gene meets adversity: the contribution of animal models to understanding epigenetic mechanisms in affective disorders and resilience. Curr Top Behav Neurosci. 2011;7:251–280. doi: 10.1007/7854_2010_109. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou M-F, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- Lovejoy EA, Scott AC, Fiskerstrand CE, Bubb VJ, Quinn JP. The serotonin transporter intronic VNTR enhancer correlated with a predisposition to affective disorders has distinct regulatory elements within the domain based on the primary DNA sequence of the repeat unit. Eur J Neurosci. 2003;17:417–420. doi: 10.1046/j.1460-9568.2003.02446.x. [DOI] [PubMed] [Google Scholar]
- Madsen KK, White HS, Schousboe A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol Ther. 2010;125:394–401. doi: 10.1016/j.pharmthera.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Mager S, Min C, Henry DJ, Chavkin C, Hoffman BJ, Davidson N, Lester HA. Conducting states of a mammalian serotonin transporter. Neuron. 1994;12:845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- Martínez-Muñoz C, Rosenberg EH, Jakobs C, Salomons GS. Identification, characterization and cloning of SLC6A8C, a novel splice variant of the creatine transporter gene. Gene. 2008;418:53–59. doi: 10.1016/j.gene.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Massari S, Vanoni C, Longhi R, Rosa P, Pietrini G. Protein kinase C-mediated phosphorylation of the BGT1 epithelial gamma-aminobutyric acid transporter regulates its association with LIN7 PDZ proteins: a post-translational mechanism regulating transporter surface density. J Biol Chem. 2005;280:7388–7397. doi: 10.1074/jbc.M412668200. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Shimodaira M, Nakagawa T, Nakayama T, Nakazato T, Izumi Y, Soma M, Matsumoto K, Sato N, Aoi N. Association study: SLC6A18 gene and myocardial infarction. Clin. Biochem. 2011;44:789–794. doi: 10.1016/j.clinbiochem.2011.03.031. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol. 2009;2:351–358. doi: 10.1586/ecp.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Hewett KL, Pouw B, Bowen WD, Husbands SM, Cao JJ, Hauck-Newman A. Rimcazole analogs attenuate the convulsive effects of cocaine: correlation with binding to sigma receptors rather than dopamine transporters. Neuropharmacology. 2001;41:878–886. doi: 10.1016/s0028-3908(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the Biological Definition of Gene × Environment Interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Miranda M, Sorkin A. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol. Interv. 2007;7:157–167. doi: 10.1124/mi.7.3.7. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Moya PR. Human serotonin transporter gene (SLC6A4) variants: their contributions to understanding pharmacogenomic and other functional G×G and G×E differences in health and disease. Curr Opin Pharmacol. 2011;11:3–10. doi: 10.1016/j.coph.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, Matsuzaki H, Suda S, Sugiyama T, Takei N, Mori N. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch. Gen. Psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- Nash SR, Giros B, Kingsmore SF, Rochelle JM, Suter ST, Gregor P, Seldin MF, Caron MG. Cloning, pharmacological characterization and genomic localization of the human creatine transporter. Recept. Channels. 1994;2:165–174. [PubMed] [Google Scholar]
- Navaroli DM, Stevens ZH, Uzelac Z, Gabriel L, King MJ, Lifshitz LM, Sitte HH, Melikian HE. The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking. J Neurosci. 2011;31:13758–13770. doi: 10.1523/JNEUROSCI.2649-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Katz JL. Atypical dopamine uptake inhibitors that provide clues about cocaine's mechanism at the dopamine transporter. Transporters as Targets for Drugs. 2009;4:95–129. [Google Scholar]
- Newman AH, Kulkarni S. Probes for the dopamine transporter: New leads toward a cocaine-abuse therapeutic--A focus on analogues of benztropine and rimcazole. Med. Res. Rev. 2002;22:429–464. doi: 10.1002/med.10014. [DOI] [PubMed] [Google Scholar]
- Nyola A, Karpowich NK, Zhen J, Marden J, Reith ME, Wang D-N. Substrate and drug binding sites in LeuT. Curr Opin Struct Biol. 2010;20:415–422. doi: 10.1016/j.sbi.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson CA, Foley DL, Parkinson-Bates M, Byrnes G, McKenzie M, Patton GC, Morley R, Anney RJL, Craig JM, Saffery R. Prospects for epigenetic research within cohort studies of psychological disorder: a pilot investigation of a peripheral cell marker of epigenetic risk for depression. Biol Psychol. 2010;83:159–165. doi: 10.1016/j.biopsycho.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Park B-Y, Lee B-C, Jung KH, Jung MH, Park BL, Chai YG, Choi I-G. Epigenetic changes of serotonin transporter in the patients with alcohol dependence: methylation of an serotonin transporter promoter CpG island. Psychiatry Investig. 2011a;8:130–133. doi: 10.4306/pi.2011.8.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kim JW, Lee SK, Kim SK, Park JK, Cho AR, Chung J-H, Song JY. Association between the SLC6A12 gene and negative symptoms of schizophrenia in a Korean population. Psychiatry Res. 2011b;189:478–479. doi: 10.1016/j.psychres.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Pasaje CFA, Kim J-H, Park BL, Cheong HS, Chun J-Y, Park T-J, Lee J-S, Kim Y, Bae JS, Park JS, Yoon S-H, Uh S-T, Choi J-S, Kim Y-H, Kim M-K, Choi IS, Cho SH, Choi BW, Park C-S, Shin HD. Association of SLC6A12 variants with aspirin-intolerant asthma in a Korean population. Ann Hum Genet. 2010;74:326–334. doi: 10.1111/j.1469-1809.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- Pattarachotanant N, Sritharathikhun T, Suttirat S, Tencomnao T. Association of C/T polymorphism in intron 14 of the dopamine transporter gene (rs40184) with major depression in a northeastern Thai population. Genet. Mol. Res. 2010;9:565–572. doi: 10.4238/vol9-1gmr757. [DOI] [PubMed] [Google Scholar]
- Piscitelli CL, Gouaux E. Insights into transport mechanism from LeuT engineered to transport tryptophan. EMBO J. 2012;31:228–235. doi: 10.1038/emboj.2011.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli CL, Krishnamurthy H, Gouaux E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature. 2010;468:1129–1132. doi: 10.1038/nature09581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M, Yano H, Goldberg NR, Duan L, Beuming T, Shi L, Weinstein H, Javitch JA. State-dependent conformations of the translocation pathway in the tyrosine transporter Tyt1, a novel neurotransmitter:sodium symporter from Fusobacterium nucleatum. J Biol Chem. 2006;281:26444–26454. doi: 10.1074/jbc.M602438200. [DOI] [PubMed] [Google Scholar]
- Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40:537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Rees MI, Harvey K, Pearce BR, Chung S-K, Duguid IC, Thomas P, Beatty S, Graham GE, Armstrong L, Shiang R, Abbott KJ, Zuberi SM, Stephenson JBP, Owen MJ, Tijssen MAJ, van den Maagdenberg AMJM, Smart TG, Supplisson S, Harvey RJ. Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat Genet. 2006;38:801–806. doi: 10.1038/ng1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Berfield JL, Wang LC, Ferrer JV, Javitch JA. The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem. 2001;276:29012–29018. doi: 10.1074/jbc.M011785200. [DOI] [PubMed] [Google Scholar]
- Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- Retz W, Freitag CM, Retz-Junginger P, Wenzler D, Schneider M, Kissling C, Thome J, Rösler M. A functional serotonin transporter promoter gene polymorphism increases ADHD symptoms in delinquents: interaction with adverse childhood environment. Psychiatry Res. 2008;158:123–131. doi: 10.1016/j.psychres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Reyes N, Tavoulari S. To be or not to be two sites: that is the question about LeuT substrate binding. J Gen Physiol. 2011;138:467–471. doi: 10.1085/jgp.201110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nature Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Saier MH, Yen MR, Noto K, Tamang DG, Elkan C. The Transporter Classification Database: recent advances. Nucleic Acids Res. 2009;37:D274–D278. doi: 10.1093/nar/gkn862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Kato M, Kennedy JL. Pharmacogenetic studies in depression: a proposal for methodologic guidelines. Pharmacogenomics J. 2008;8:90–100. doi: 10.1038/sj.tpj.6500477. [DOI] [PubMed] [Google Scholar]
- Shafqat S, Velaz-Faircloth M, Henzi VA, Whitney KD, Yang-Feng TL, Seldin MF, Fremeau RT. Human brain-specific L-proline transporter: molecular cloning, functional expression and chromosomal localization of the gene in human and mouse genomes. Mol Pharmacol. 1995;48:219–229. [PubMed] [Google Scholar]
- Shimamura T, Weyand S, Beckstein O, Rutherford N, Hadden J, Sharples D, Sansom M, Iwata S, Henderson P, Cameron A. Molecular Basis of Alternating Access Membrane Transport by the Sodium-Hydantoin Transporter Mhp1. Science. 2010;328:470–473. doi: 10.1126/science.1186303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Farhan H, Javitch JA. Sodium-dependent neurotransmitter transporters: oligomerization as a determinant of transporter function and trafficking. Mol. Interv. 2004;4:38–47. doi: 10.1124/mi.4.1.38. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Amara SG. Channels in transporters. Curr Opin Neurobiol. 1996;6:294–302. doi: 10.1016/s0959-4388(96)80111-5. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, Meltzer PC, Martin J, Rauch S, Fischman AJ. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62:1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman JA. Taurine deficiency. Prog. Clin. Biol. Res. 1990;351:385–395. [PubMed] [Google Scholar]
- Sucic S, Dallinger S, Zdrazil B, Weissensteiner R, Jorgensen TN, Holy M, Kudlacek O, Seidel S, Cha JH, Gether U, Newman AH, Ecker GF, Freissmuth M, Sitte HH. The N terminus of monoamine transporters is a lever required for the action of amphetamines. J Biol Chem. 2010;285:10924–10938. doi: 10.1074/jbc.M109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H, Iwamoto K, Bundo M, Ueda J, Miyauchi T, Komori A, Kazuno A, Adati N, Kusumi I, Okazaki Y, Ishigooka J, Kojima T, Kato T. Hypermethylation of serotonin transporter gene in bipolar disorder detected by epigenome analysis of discordant monozygotic twins. Transl Psychiatry. 2011;1:e24. doi: 10.1038/tp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supplisson S, Roux MJ. Why glycine transporters have different stoichiometries. FEBS Lett. 2002;529:93–101. doi: 10.1016/s0014-5793(02)03251-9. [DOI] [PubMed] [Google Scholar]
- Suviolahti E, Oksanen LJ, Ohman M, Cantor RM, Ridderstrale M, Tuomi T, Kaprio J, Rissanen A, Mustajoki P, Jousilahti P, Vartiainen E, Silander K, Kilpikari R, Salomaa V, Groop L, Kontula K, Peltonen L, Pajukanta P. The SLC6A14 gene shows evidence of association with obesity. J Clin Invest. 2003;112:1762–1772. doi: 10.1172/JCI17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SKH, Hartung H, Sharp T, Temel Y. Serotonin-dependent depression in Parkinson's disease: a role for the subthalamic nucleus? Neuropharmacology. 2011;61:387–399. doi: 10.1016/j.neuropharm.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Tartter MA, Ray LA. The Serotonin Transporter Polymorphism (5-HTTLPR) and Alcohol Problems in Heavy Drinkers: Moderation by Depressive Symptoms. Front Psychiatry. 2011;2:49. doi: 10.3389/fpsyt.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavoulari S, Rizwan AN, Forrest LR, Rudnick G. Reconstructing a Chloride-binding Site in a Bacterial Neurotransmitter Transporter Homologue. J Biol Chem. 2011;286:2834–2842. doi: 10.1074/jbc.M110.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Jessen T, Henry LK, Field JR, Gamble KL, Gresch PJ, Carneiro AM, Horton RE, Chisnell PJ, Belova Y, McMahon DG, Daws LC, Blakely RD. Transgenic elimination of high-affinity antidepressant and cocaine sensitivity in the presynaptic serotonin transporter. Proc Natl Acad Sci. 2011;108:3785–3790. doi: 10.1073/pnas.1011920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Gu HH. The effects of methylphenidate on knockin mice with a methylphenidate-resistant dopamine transporter. J Pharmacol Exp Ther. 2008;327:554–560. doi: 10.1124/jpet.108.141713. [DOI] [PubMed] [Google Scholar]
- Tilley MR, O'Neill B, Han DD, Gu HH. Cocaine does not produce reward in absence of dopamine transporter inhibition. Neuroreport. 2009;20:9–12. doi: 10.1097/WNR.0b013e32831b9ce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, Staudinger J, Caron MG. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron. 2001;30:121–134. doi: 10.1016/s0896-6273(01)00267-7. [DOI] [PubMed] [Google Scholar]
- Ueno T, Tabara Y, Fukuda N, Tahira K, Matsumoto T, Kosuge K, Haketa A, Matsumoto K, Sato Y, Nakayama T, Katsuya T, Ogihara T, Makita Y, Hata A, Yamada M, Takahashi N, Hirawa N, Umemura S, Miki T, Soma M. Association of SLC6A9 gene variants with human essential hypertension. J. Atheroscler. Thromb. 2009;16:201–206. doi: 10.5551/jat.e125. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Varrone A, Halldin C. Molecular imaging of the dopamine transporter. J. Nucl. Med. 2010;51:1331–1334. doi: 10.2967/jnumed.109.065656. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Jessen TN, Thompson BJ, Carter M, Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Modeling rare gene variation to gain insight into the oldest biomarker in autism: construction of the serotonin transporter Gly56Ala knock-in mouse. J Neurodev Disord. 2009;1:158–171. doi: 10.1007/s11689-009-9020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villumsen KR, Duelund L, Lambert IH. Acute cholesterol depletion leads to net loss of the organic osmolyte taurine in Ehrlich Lettre tumor cells. Amino Acids. 2010;39:1521–1536. doi: 10.1007/s00726-010-0621-4. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li T-K. The neuroscience of addiction. Nat Neurosci. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Voyiaziakis E, Evgrafov O, Li D, Yoon H-J, Tabares P, Samuels J, Wang Y, Riddle MA, Grados MA, Bienvenu OJ, Shugart YY, Liang KY, Greenberg BD, Rasmussen SA, Murphy DL, Wendland JR, McCracken JT, Piacentini J, Rauch SL, Pauls DL, Nestadt G, Fyer AJ, Knowles JA. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol Psychiatry. 2011;16:108–120. doi: 10.1038/mp.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Baker DG, Harrer J, Hamner M, Price M, Amstadter A. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depress Anxiety. 2011;28:1067–1073. doi: 10.1002/da.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Ye R, Blakely RD. Natural and engineered coding variation in antidepressant-sensitive serotonin transporters. Neuroscience. 2011;197:28–36. doi: 10.1016/j.neuroscience.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Noskov SY, Roux B. Two mechanisms of ion selectivity in protein binding sites. Proc Natl Acad Sci. 2010;107:20329–20334. doi: 10.1073/pnas.1007150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith MEA, Wang D-N. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomot E, Bendahan A, Quick M, Zhao Y, Javitch JA, Kanner BI. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature. 2007;449:726–730. doi: 10.1038/nature06133. [DOI] [PubMed] [Google Scholar]