Abstract
In hippocampal pyramidal neurons, voltage-gated Ca2+ channels open in response to action potentials. This results in elevations in the intracellular concentration of Ca2+ that are maximal in the proximal apical dendrites and decrease rapidly with distance from the soma. The control of these action potential-evoked Ca2+ elevations is critical for the regulation of hippocampal neuronal activity. As part of Ca2+ signaling microdomains, small-conductance Ca2+-activated K+ (SK) channels have been shown to modulate the amplitude and duration of intracellular Ca2+ signals by feedback regulation of synaptically activated Ca2+ sources in small distal dendrites and dendritic spines, thus affecting synaptic plasticity in the hippocampus. In this study, we investigated the effect of the activation of SK channels on Ca2+ transients specifically induced by action potentials in the proximal processes of hippocampal pyramidal neurons. Our results, obtained by using selective SK channel blockers and enhancers, show that SK channels act in a feedback loop, in which their activation by Ca2+ entering mainly through L-type voltage-gated Ca2+ channels leads to a reduction in the subsequent dendritic influx of Ca2+. This underscores a new role of SK channels in the proximal apical dendrite of hippocampal pyramidal neurons.
Keywords: SK channel, afterhyperpolarization, apamin, pyramidal neuron, calcium imaging
the afterhyperpolarizing current IAHP is mediated by apamin-sensitive, small-conductance Ca2+-activated K+ (SK) channels that are voltage independent and activated by increases in intracellular Ca2+, thereby linking intracellular Ca2+ elevations to changes in the membrane potential in a variety of neurons (reviewed by Adelman et al. 2012; Pedarzani and Stocker 2008). The constitutive binding of calmodulin to SK channels is responsible for their high sensitivity to Ca2+ (Xia et al. 1998). However, the sources of Ca2+ leading to the activation of SK channels vary in different types of neurons (reviewed by Pedarzani and Stocker 2008; Stocker 2004).
SK channels are part of Ca2+ microdomains (Fakler and Adelman 2008; Lujan et al. 2009; Marrion and Tavalin 1998; Oliver et al. 2000) created by the functional coupling of Ca2+-permeable channels and Ca2+-sensitive channels, and may serve diverse roles depending on their subcellular localization. In the soma of CA1 neurons Ca2+ channels and small-conductance Ca2+-activated K+ channels are found within 50–150 nm of each other (Marrion and Tavalin 1998).
Synaptic NMDA receptors and SK channels are functionally coupled in the dendritic spines of hippocampal, amygdala, and striatal neurons (Bloodgood and Sabatini 2007; Faber et al. 2005; Higley and Sabatini 2010; Lujan et al. 2009; Ngo-Anh et al. 2005). The activation of SK channels in dendritic spines limits the influx of Ca2+ through NMDA receptors and decreases glutamatergic excitatory postsynaptic potentials (Bloodgood and Sabatini 2007; Faber et al. 2005; Ngo-Anh et al. 2005). Moreover, in distal apical dendrites of hippocampal neurons SK channel activation controls the duration of glutamate-induced Ca2+ plateau potentials (Cai et al. 2004). Thus SK channels are part of a negative feedback loop that limits Ca2+ influx through those Ca2+ sources that initially activated them, shaping the amplitude and duration of synaptically evoked Ca2+ transients and modulating glutamatergic synaptic responses.
The regulation by SK channels of Ca2+ sources that are not dependent on synaptic activation, however, has not been explored so far. This may be of particular relevance in the proximal apical dendrite of hippocampal neurons, where SK channels have also been localized (Lin et al. 2008; Lujan et al. 2009; Sailer et al. 2002) and elevations of intracellular Ca2+ induced by the activation of voltage-gated Ca2+ channels by somatic action potentials (APs), which backpropagate to the dendrites, are largest (Callaway and Ross 1995; Christie et al. 1995; Jaffe et al. 1992; Regehr et al. 1989; Regehr and Tank 1994; Spruston et al. 1995). The proximal dendritic compartment is different from the distal compartment in CA1 neurons also because it receives more effective GABAergic innervation (Papp et al. 2001), while most glutamatergic excitatory inputs converge on the distal portion. Indeed, proximal and distal compartments of apical dendrites have different synaptic plasticity thresholds, which may also reflect a different contribution of voltage-gated Ca2+ channels to plasticity induction mechanisms (Parvez et al. 2010).
In view of the feedback regulation of synaptically evoked Ca2+ entry by SK channels shown in distal dendrites and spines, the present study addresses the question as to whether SK channels can modulate AP-induced Ca2+ transients in the proximal apical dendrites of hippocampal pyramidal neurons. We demonstrate that pharmacological modulation of SK channel activity regulates the amplitude and duration of AP-induced intracellular Ca2+ elevations mainly triggered by L-type voltage-gated Ca2+ channels in the proximal neurites of hippocampal neurons.
METHODS
Chemicals.
Tetrodotoxin (TTX) was obtained from Alomone Laboratories (Jerusalem, Israel); apamin from Laxotan (Rosans, France); dl-AP5, NBQX, picrotoxin, and 1-ethyl-2-benzimidazolinone (1-EBIO) from Tocris Cookson (Bristol, UK) or Ascent Scientific (Weston-super-Mare, UK); and tetraethylammonium (TEA), Na2-ATP, Na3-GTP, and 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate (CPT-cAMP) from Sigma-Aldrich (Poole, UK); all other salts and chemicals were obtained from Fluka (Sigma-Aldrich).
Cell culture.
Rats were handled in accordance with the UK Home Office Animal Procedures Act (1986), and protocols were reviewed and approved by the University College London Animal Ethical Committee. Primary hippocampal neurons were cultured from 0- to 1-day old rats (Goslin and Banker 1991) according to a modified protocol. Briefly, after dissection, the hippocampi were treated with 2.5% trypsin (Invitrogen) and mechanically dissociated with a flame-polished Pasteur pipette. Cells were plated onto poly-d-lysine-coated (0.1 mg/ml) glass or plastic (Nalgene) coverslips at a density of 35,000 cells/cm2 (for electrophysiology and imaging recordings) or 21,000 cells/cm2 (for immunofluorescence staining) in minimum essential medium (Invitrogen, Paisley, UK) supplemented with 10% horse serum, 1 mM pyruvic acid, and 0.59% glucose. After 4–14 h, the medium was substituted with Neurobasal medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), 0.59% glucose, and B27 supplement (Invitrogen). Neurons were kept at 5% CO2, 37°C, and 95% humidity for a variable number of days before the experiments.
Electrophysiology.
For recordings, coverslips were mounted in a custom-built recording chamber placed on the stage of a Nikon E600FN upright microscope. Cells were continuously superfused by a 2.5 ml/min flow of extracellular solution containing (in mM) 140 NaCl, 3.5 KCl, 10 HEPES, 20 glucose, 2.5 CaCl2, and 1.5 MgCl2 (pH 7.4, 300–305 mosmol/kgH2O) (20°C). CaCl2 was reduced to 1.5 mM for current-clamp recordings with trains of four APs. Borosilicate patch pipettes (5–6 MΩ; TW100F-4 glass, World Precision Instruments) were filled with a solution containing (in mM) 135 KMeSO4 (voltage-clamp and current-clamp experiments) or 135 K-gluconate (voltage-clamp experiments), 10 KCl, 10 HEPES, 1 MgCl2, 2 Na2-ATP, 0.4 Na3-GTP (pH 7.2–7.3, 280–290 mosmol/kgH2O). CPT-cAMP (50 μM) was included in the majority of the voltage-clamp experiments to inhibit the slow IAHP (sIAHP).
Voltage-clamp experiments were performed on pyramidal cells at 10–18 days in vitro (DIV). Neurons were clamped at a membrane holding potential of −50 mV and repetitively depolarized to +30 mV for 100–200 ms at a frequency of 0.033 Hz to activate voltage-gated Ca2+ channels. After each depolarization the membrane potential was stepped back to −50 mV, where the apamin-sensitive IAHP was observed as an outward current. Voltage-clamp experiments were conducted in the presence of 0.5 μM TTX and 1 mM TEA to block voltage-gated Na+ channels and some voltage-gated K+ channels. Series resistance (range 15–25 MΩ) was monitored at regular intervals throughout the recording and presented minimal variations (≤15%) in the analyzed cells. Data are reported without corrections for liquid junction potentials.
Data were acquired with an Axopatch 1-D amplifier (Axon Instruments, Foster City, CA) controlled by the Strathclyde Electrophysiology Software WinWCP v.3.2.9 (John Dempster, University of Strathclyde, Glasgow, UK). Data were filtered at 1 kHz and sampled at 3 kHz with a Micro 1401 interface (Cambridge Electronic Design). Data were analyzed by pCLAMP9 Clampfit routine (Axon Instruments) and Origin 7.0 (MicroCal software).
APs were elicited in the presence of glutamate receptor blockers (dl-AP5 25 μM; NBQX 5 μM) in the whole cell current-clamp mode, and data were filtered at 10 kHz and digitized at 20 kHz. Somatic current injections of 10 ms, which evoked single APs, were delivered at a frequency of 20 Hz. Between stimulations the membrane potential of the cells was kept at −60 mV by DC current injection. The resting membrane potential was frequently checked, and only cells with a stable resting potential more hyperpolarized than −50 mV were included in the analysis.
Ca2+ transients were imaged with 20 μM fluo-4 (Molecular Probes, Eugene, OR) dissolved in the intracellular recording solution; 1 mM fluo-4 stock solution was prepared in purified H2O (Super Purity Reagent, Romil) and stored at −20°C (Yasuda et al. 2004). Neurons were filled with fluo-4 via the patch electrode for 10–15 min before imaging to allow dye equilibration in the proximal neurites (Helmchen et al. 1996; Maravall et al. 2000). Drugs were bath applied for 5–10 min. Because of the pharmacological accessibility of cultured neurons, drug effect was already maximal at 5 min.
Two-photon imaging.
Two-photon Ca2+ imaging was performed with a Bio-Rad multiphoton microscope based on a 1024 scan head mounted on a Nikon E600FN upright microscope equipped with a Nikon ×60 NA 1.0 water-immersion objective. A Millennia V pump laser coupled to a mode-locked Ti:sapphire infrared laser (Tsunami, Spectra Physics) was used for fluorescence excitation, tuned to 790 nm. For most experiments the laser power at the sample was 3 mW, but for those cells with a weak dye loading a higher power (up to 7 mW) was used to obtain a clear image. The fluorescence emission was collected with an external photomultiplier detector and was not descanned.
Since two-dimensional scans are too slow for accurate determination of the time course and amplitude of the calcium transients, line scans were used. These consisted of successive sweeps at 6-ms intervals across a single line in the field of view. Images were collected with Lasersharp software (Bio-Rad) and analyzed with ImageJ (National Institutes of Health) and Origin 7.0 (MicroCal software). For each recording, background fluorescence was determined from a cell-free area of size comparable to that of the line scan image. After the averaged background signal was subtracted, fluorescence values were taken 300 ms before the triggering of APs and averaged to measure the basal fluorescence (Fbasal). The amplitude of the fluorescence transients at the recording sites was expressed as the fractional change in basal fluorescence, (F − Fbasal)/Fbasal= (ΔF/F), which is approximately proportional to the changes in intracellular Ca2+ (Maravall et al. 2000). Fbasal did not change by more than two times the standard deviation of Fbasal measured under control conditions over the course of the experiment. For data analysis, transients were digitally filtered off-line (adjacent-averaging routine, smoothing factor n = 5; Origin 7). Peak fluorescence was calculated averaging data points 30–60 ms around the maximum. The decay time course of Ca2+ transients was fitted by a single-exponential function.
Statistical analysis.
Data were analyzed with Prism (GraphPad Software, La Jolla, CA), Student's t-test, paired or unpaired as appropriate, or the nonparametric Mann-Whitney test was used for statistical comparisons between two groups (α = 0.05). For comparisons between more than two groups one-way ANOVA or one-way repeated-measures ANOVA followed by the Bonferroni post hoc test was used. All values are expressed as means ± SE.
Immunofluorescence.
Hippocampal neurons were fixed in phosphate saline buffer (PBS: 10 mM sodium phosphate, 130 mM NaCl, pH 7.2) containing 4% paraformaldehyde and 4% sucrose for 10 min at room temperature, rinsed twice in PBS, and permeabilized in 0.3% Triton X-100 for 15 min, followed by two more washes in PBS. After a 1-h incubation in 2% H2O2 to block the activity of the endogenous peroxidase, immunodetection was performed with the tyramide signal amplification method (Invitrogen). In short, the fixed, permeabilized, and peroxide-treated neurons were incubated overnight at 4°C with the affinity-purified anti-NSK2 antibody (0.25 μg/ml; see also Cingolani et al. 2002) diluted in blocking buffer. Controls were performed in parallel either by omitting the purified anti-NSK2 or by using the anti-NSK2 preadsorbed to a SK2 fusion protein (TRX-NSK2, 20 μg/ml). After repeated washes with PBS to remove the unbound primary antibodies, a 1-h incubation with the HRP-conjugated anti-rabbit secondary antibody (1:200) in blocking buffer was performed. After the cultures were washed with PBS, the tyramide-Alexa Fluor 488 reagent was added and incubated in the dark for 5 min. Slides were washed, mounted with ProLong Antifade (Invitrogen), and examined with a fluorescence microscope (Axiophot, Zeiss). Pictures were taken with a MicroPublisher camera (QImaging).
RESULTS
Apamin-sensitive IAHP in cultured hippocampal neurons.
Whole cell recordings were performed on morphologically identified hippocampal pyramidal neurons in primary culture. After 10–18 DIV, neurons showed a mean resting potential of −59 ± 1 mV (n = 38); 100- to 200-ms-long somatic depolarizations to +30 mV from a holding potential of −50 mV, delivered in the presence of 0.5 μM TTX and 1 mM TEA, activated voltage-gated Ca2+ currents, followed by an outward current. The observed currents decayed with either a time constant (τ) of 222 ± 17 ms when elicited by a 100-ms-long depolarization (n = 8), or a τ of 485 ± 46 ms for 200-ms-long depolarizations (n = 17). The mean amplitude of the outward current was 83 ± 20 pA (n = 8) in response to 100-ms-long depolarizing pulses and 140 ± 23 pA (n = 17) after 200-ms-long pulses.
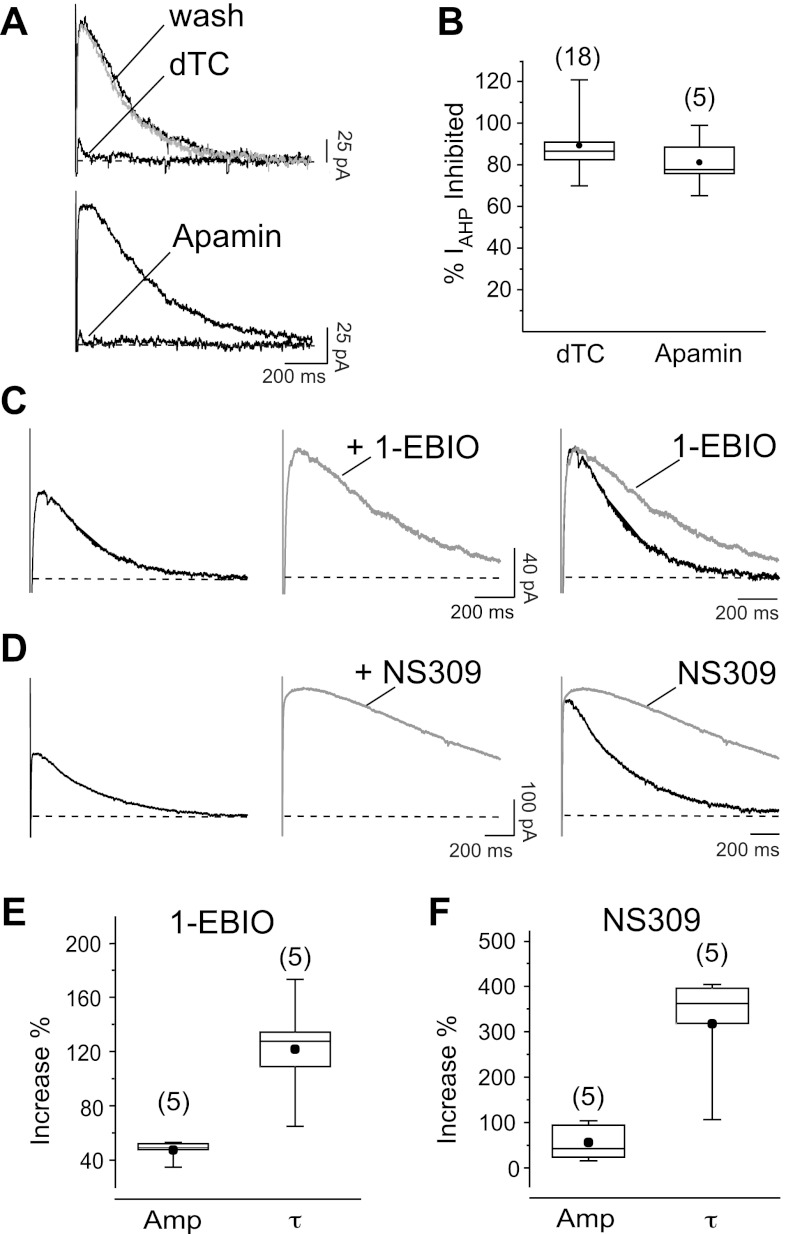
d-Tubocurarine (dTC, 100 μM), which blocks SK channels in a reversible manner, inhibited the outward current (Fig. 1, A and B). Similarly, the specific SK channel blocker apamin (5 nM) produced a strong suppression of the outward current (Fig. 1, A and B), demonstrating that it is mediated by SK channels.
Fig. 1.
Electrophysiological characterization of afterhyperpolarizing current (IAHP) in cultured hippocampal neurons. A: inhibition of the IAHP, seen as an outward tail current, by d-tubocurarine (dTC, 100 μM; top) and the specific small-conductance Ca2+-activated K+ (SK) channel blocker apamin (5 nM; bottom). B: box and whisker diagrams summarizing effects of the 2 blockers on the IAHP. ●, Mean values of current inhibited by apamin (81 ± 6%; n = 5) and dTC (89 ± 3%; n = 18). C and D: effect of 250 μM 1-ethyl-2-benzimidazolinone (1-EBIO; C) and 5 μM 6,7-dichloro-1H-indole-2,3-dione 3-oxime (NS309; D) on the amplitude and time course of decay of the IAHP. The increase in current amplitude in the presence of the drug is clearly visible (center). In the superimposition (right) IAHP traces have been normalized to peak to show the prolongation of the time course. E and F: box and whiskers diagrams summarizing the effects of 1-EBIO (E) and NS309 (F) on the peak amplitude (1-EBIO: 47 ± 3% increase, P < 0.001; NS309: 56 ± 18% increase, P = 0.01) and time constant of decay (τ; 1-EBIO: 122 ± 18% increase, P = 0.03; NS309: 317 ± 55% increase, P = 0.01) of IAHP measured from 5 cells.
To further validate the molecular identity of the outward current and complete its pharmacological characterization, we tested the SK channel enhancer 1-EBIO (250 μM) (Pedarzani et al. 2001), which increased the IAHP peak amplitude (Fig. 1, C and E). A similar increase in IAHP amplitude was observed with the more specific and potent SK channel enhancer 6,7-dichloro-1H-indole-2,3-dione 3-oxime (NS309) at 5 μM (Fig. 1, D and F) (Pedarzani et al. 2005). In addition, both 1-EBIO (Fig. 1, C and E) and NS309 (Fig. 1, D and F) prolonged the decay τ of the IAHP.
Taken together, these results demonstrate that the IAHP is expressed in cultured primary hippocampal neurons and has properties similar to the SK-mediated IAHP recorded in pyramidal neurons from acute hippocampal slices (Sailer et al. 2002; Stocker et al. 1999).
SK2 channel expression in hippocampal neurons.
Evidence obtained in pharmacological and biochemical studies, and work performed on genetically modified animals, point to the SK2 (KCa2.2) subunit as a main contributor to the formation of SK channels mediating the IAHP in hippocampal neurons (Bond et al. 2004; Sailer et al. 2002; Stocker et al. 1999).
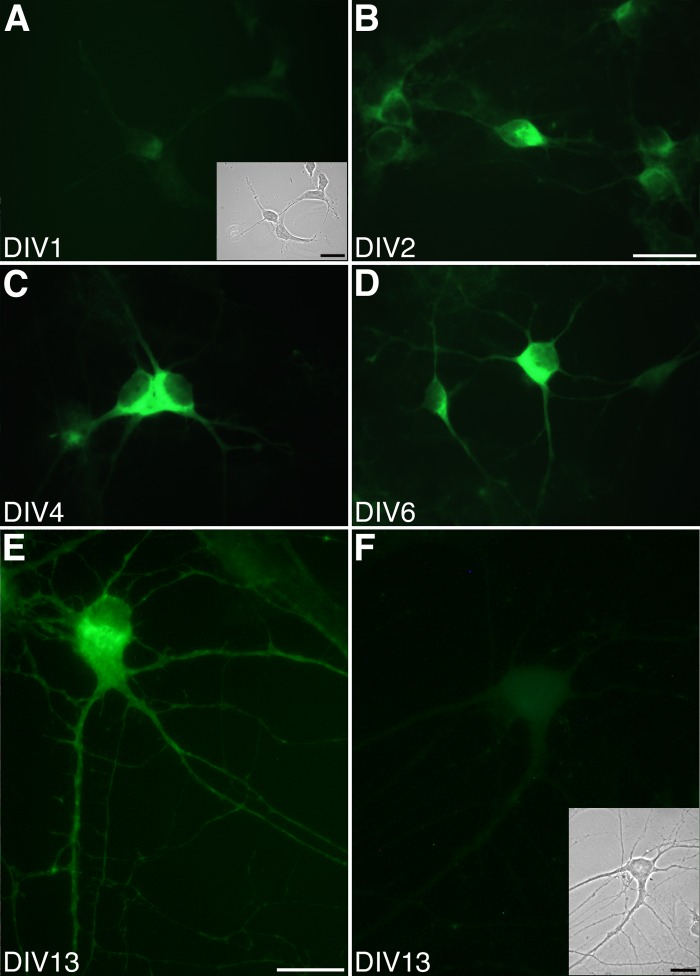
Therefore, the distribution of SK2 channel subunits in postnatal hippocampal neurons was investigated with a specific antibody (anti-NSK2) raised against the NH2-terminal region of the SK2 protein (Cingolani et al. 2002). Clear SK2 immunostaining was observed in the soma of the neurons from DIV 2 to DIV 13 (Fig. 2, B–E). The staining increased progressively from DIV 2. Maximal expression was seen at DIV 10–13 (Fig. 2E). For this reason, neurons between DIV 10 and DIV 18 were used for electrophysiological and imaging experiments. SK2 immunoreactivity was visible in the soma of the hippocampal pyramidal neurons and in the proximal and distal portions of neurites (Fig. 2E). Preadsorption of the anti-NSK2 antibodies (Fig. 2F at DIV 13) and omission of the primary antibody (data not shown) resulted in the lack of fluorescent staining at all developmental stages, confirming the specificity of the detected signal.
Fig. 2.
Expression of SK2 in cultured postnatal hippocampal neurons at different stages of in vitro development. A: immunohistochemistry performed with a specific anti-NSK2 antibody at 1 day in culture (DIV 1) shows a fluorescence signal indistinguishable from the background signal. Inset: bright field picture of the stained neurons. B–D: by DIV 2 SK2 can be clearly detected (B), and its expression increases at DIV 4 (C) and DIV 6 (D). E: distribution of the SK2 subunit at DIV 13, showing a clear somato-dendritic localization of the channel. F: nonspecific staining with preadsorbed antibodies (+Trx-NSK2) shows a very weak signal in soma but not in neurites. Inset: bright field picture of the neurons. Scale bars: A–D, 20 μm (shown in B); E and F, 10 μm (shown in E); insets, 20 μm in A and 10 μm in F.
Imaging and modulation of Ca2+ transients elicited by action potentials backpropagating to proximal dendrite of hippocampal neurons.
In hippocampal pyramidal neurons there is plentiful evidence that SK channels can be activated by Ca2+ entering through voltage-gated Ca2+ channels opening during trains of APs (Cai et al. 2007; Empson and Jefferys 2001; Fernandez de Sevilla et al. 2006; Kaczorowski et al. 2007; Kramar et al. 2004; Oh et al. 2000; Shah et al. 2006; Stocker et al. 1999; but see Gu et al. 2005). This opens the possibility that the activation of SK channels regulates local, voltage-gated Ca2+ channel-mediated calcium signals.
Simultaneous two-photon Ca2+ imaging and whole cell current-clamp recordings allow the testing of this hypothesis. Recent measurements of Ca2+ transients evoked by single APs in small secondary to quaternary dendritic branches of CA1 pyramidal neurons demonstrated that the calcium-sensitive fluorophore fluo-4, with a Kd ranging from 340 nM (34°C) to 800 nM (24°C) (Yasuda et al. 2004) and its large dynamic range, is the dye of choice when used at low concentrations (Sabatini et al. 2002). A general concern with Ca2+-sensitive processes is the potential interference exerted by Ca2+-sensitive dyes because of their concentration and binding properties. In the case of SK channels activated by Ca2+ entering the cell via voltage-gated Ca2+ channels, the dye might act as an exogenous Ca2+ buffer that captures the incoming Ca2+, thereby reducing the activation of the SK channels.
The amplitude and the time course of decay of the IAHP in the absence and presence of fluo-4 were first tested in a subset of voltage-clamp experiments. Fluo-4 at 20 μM did not significantly alter the amplitude (control: 140 ± 23 pA, n = 17; fluo-4: 98 ± 13 pA, n = 14) and the time constant of decay (control: 458 ± 46 ms, n = 17; fluo-4: 574 ± 50 ms, n = 14) of the IAHP elicited by a 200-ms-long pulse (P = 0.1).
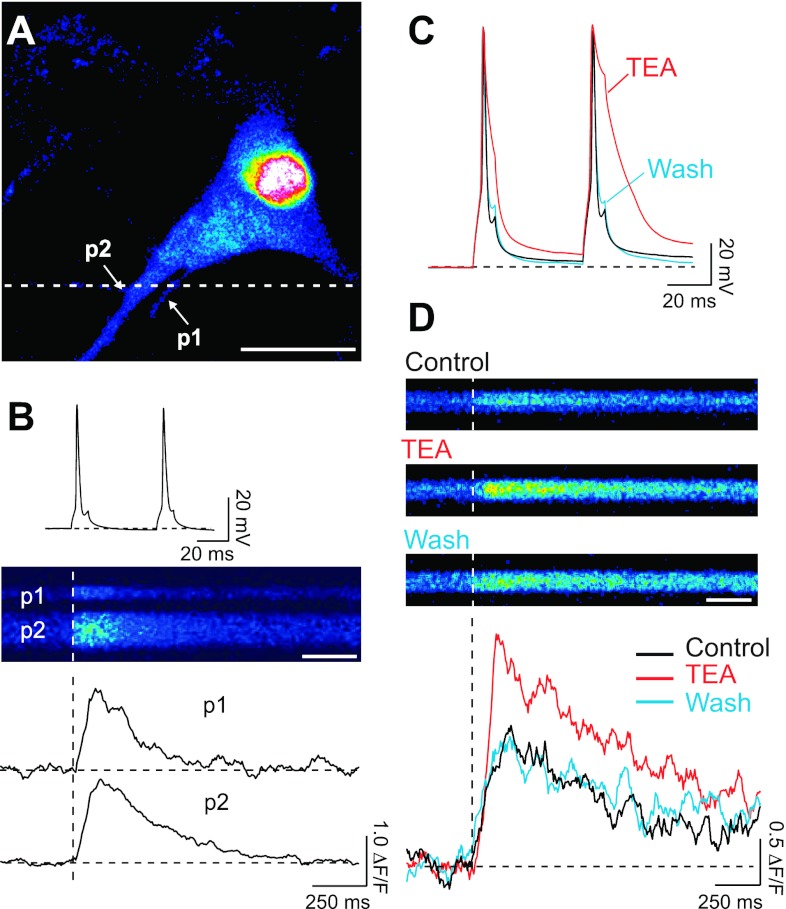
Consequently, this concentration of fluo-4 was used to study the effect of SK channels on the dynamics of intracellular free Ca2+ concentration in response to APs in the proximal dendrite. A typical filled pyramidal cell with two neuronal processes (p1, p2) in the field of vision is shown in Fig. 3A. For visualization, cells were subjected to a prolonged depolarization at the end of the experiment to increase the intracellular Ca2+ concentration. This was necessary because under resting conditions the low level of fluo-4 was too dim to reliably detect neuronal processes. APs were elicited by somatic current injections of 10-ms-long depolarizing pulses at 20 Hz (Fig. 3B, top), a firing frequency in the range observed in CA1 place cells when the animals are in proximity of their place field center (Dragoi and Buzsaki 2006; O'Keefe and Dostrovsky 1971). The two APs induced an increase in fluorescence, indicative of Ca2+ entry, in the proximal neurites of the cell (<50 μm from soma; Fig. 3B). The fluorescence signal was collected by line scans along the dashed line in Fig. 3A with a two-photon imaging system with a photomultiplier as a detector to measure the dynamics of intracellular free Ca2+ concentration (Fig. 3B, middle). The time course of decay of the evoked fluorescence transients (Fig. 3B, bottom) showed cell-to-cell variability with an average τ of 443 ± 34 ms (range: 221–987 ms in 28 processes of 25 cells). To test whether increasing the AP duration (Fig. 3C) saturates the fluo-4 fluorescence signal elicited by two APs at 20 Hz, the K+ channel blocker TEA was applied by bath perfusion. TEA at 10 mM reversibly prolonged the duration of the APs (Fig. 3C) and doubled the amplitude of the fluorescence transients (198 ± 46%, n = 4, Mann-Whitney test, P < 0.04; Fig. 3D). TEA also prolonged the decay times of the transients. The prolongation was quite variable, and in the presence of TEA one cell displayed a fluorescence transient that developed into a plateau and did not decay back to baseline values over the duration of the recording. In the cells where the fluorescence transient returned to baseline, the decay time of the transients was increased [τControl: 316–674 ms (n = 4); τTEA: 464–4,800 ms (n = 3)]. The changes in the amplitude and duration of the Ca2+ transients in the proximal dendrite of hippocampal pyramidal neurons are comparable with the effect of TEA on the AP-induced Ca2+ transients in the dendrites of neocortical neurons (Markram et al. 1995).
Fig. 3.
Ca2+ transients induced by action potentials (APs) backpropagating to the proximal processes of hippocampal neurons. A: image of a cultured hippocampal neuron filled with fluo-4 (20 μM) and stimulated with a prolonged depolarization showing the Ca2+ accumulation within the cell. Two neuronal processes (p1 and p2) are indicated by arrows. Scale bar: 10 μm. B: short (10 ms) somatic depolarizing current injections elicited 2 APs (top) at 20 Hz. Line scans (middle) were recorded from the 2 processes (p1 and p2), at the position indicated by the dashed line in A. Line scans show an increase in fluorescence as a consequence of intracellular Ca2+ elevation. Scale bar: 250 ms. The corresponding relative changes in fluorescence (bottom) show a faster transient for the thinner process (p1) simply due to the different surface-to-volume ratio. For display purposes transients were digitally filtered off-line (adjacent-averaging routine, smoothing factor n = 10, Origin 7). C: effect of K+ channel blocker tetraethylammonium (TEA, 10 mM) on repolarization of the APs that induced the fluorescence transients displayed in D. Black, APs before TEA application (control); red, APs in the presence of TEA; blue, APs after washout of TEA. D: line scans recorded under control conditions, during application of 10 mM TEA, and after washout (top) and corresponding relative changes in fluorescence (bottom). Scale bar: 250 ms.
The effect of TEA on AP duration (Fig. 3C) and the amplitude and time course of the fluorescence transients was reversible (Fig. 3D). The reversibility of the effect of TEA demonstrates the stability of the signals observed under our recording and imaging conditions.
SK channels regulate action potential-induced Ca2+ influx.
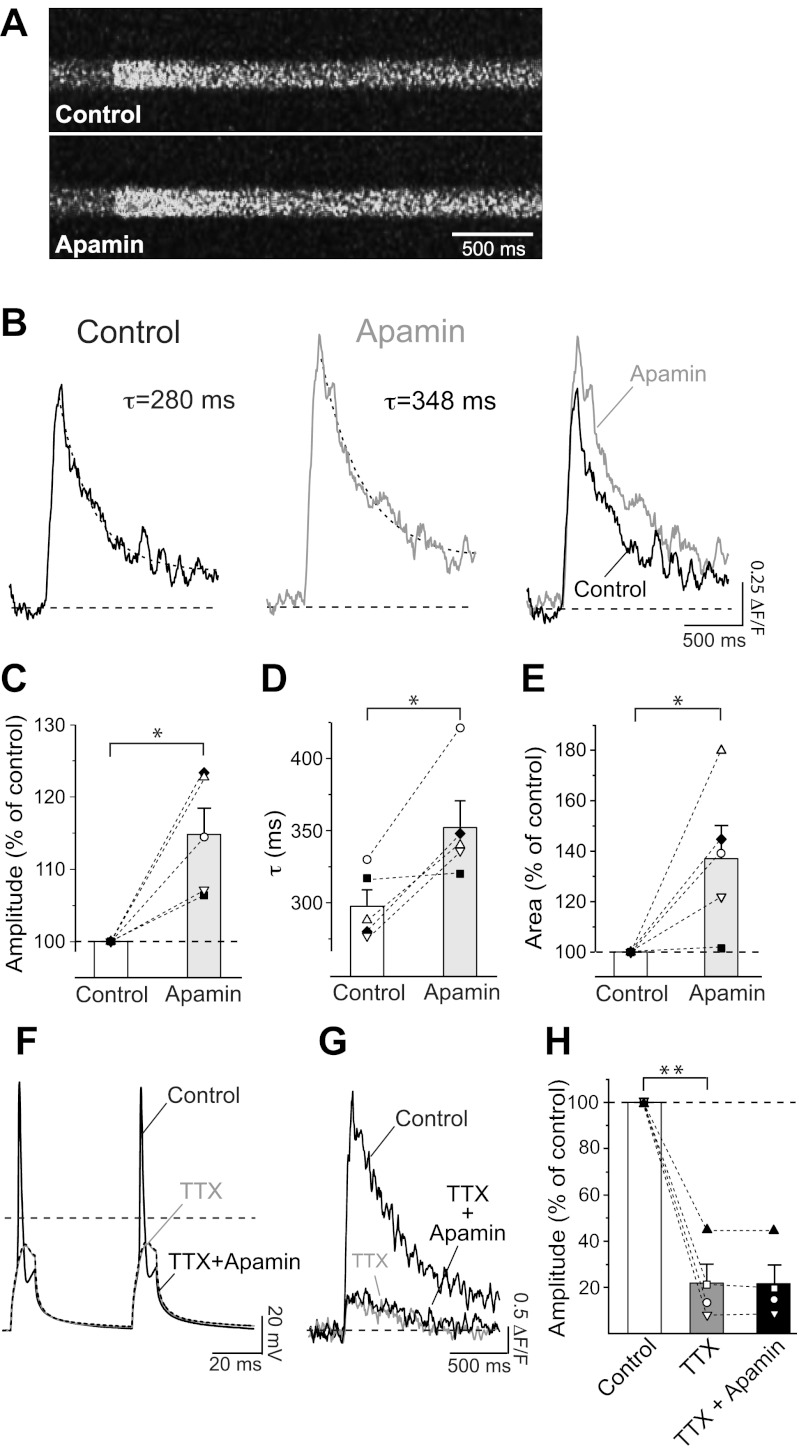
TEA at 10 mM blocks several voltage-gated K+ channels, including members of the Kv1, Kv2, Kv3, and Kv7 families, voltage- and Ca2+-activated large-conductance K+ (BK) channels (Coetzee et al. 1999), and, to some extent, SK channels (reviewed in Pedarzani and Stocker 2008). Consequently, the effect observed in the presence of TEA on the Ca2+ transient (Fig. 3, C and D) is due to the block of different types of K+ currents that contribute to the AP repolarization and afterhyperpolarization phases in hippocampal pyramidal neurons. SK channels have been shown to terminate glutamate-evoked Ca2+ plateau potentials in distal apical dendrites (Cai et al. 2004) and regulate the Ca2+ influx through NMDA receptors through a negative feedback mechanism in spines of hippocampal pyramidal neurons (Bloodgood and Sabatini 2007; Ngo-Anh et al. 2005). This makes SK channels good candidates to regulate AP-induced Ca2+ signals in proximal dendritic regions. To investigate whether and to what extent SK channels specifically contribute to the regulation of Ca2+ transients in the proximal dendrites of hippocampal neurons, SK channels were inhibited by apamin, a selective inhibitor. Apamin increased the amplitude of the fluorescence transient (Fig. 4, A and B) elicited by two APs at 20 Hz in the proximal dendrite to 115 ± 4% (n = 5, P < 0.05; Fig. 4C). Apamin also slowed the decay of the fluorescence transients by 18 ± 5% (Fig. 4, B and D; control: τ = 298 ± 11 ms, apamin: τ = 353 ± 18 ms; n = 5, P < 0.05). As a result of the increase in amplitude and prolongation of τ, the amount of Ca2+ entering the cell, characterized by the area under the curve of the fluorescence transient, was significantly increased in the presence of apamin (Fig. 4, B and E; 138 ± 13%; n = 5, P < 0.05).
Fig. 4.
Effect of apamin on Ca2+ transients recorded from proximal neuronal processes. A: line scans showing fluorescence changes triggered by 2 APs at 20 Hz in the proximal process of a hippocampal pyramidal neuron in the absence (Control) and in the presence of the SK channel blocker apamin (5 nM). B: relative change in fluorescence obtained from line scans in A. Transients were fitted with monoexponential functions, and time constant of decay τ is indicated. Right: fluorescence transients are superimposed to show the increase in amplitude and decay. C: a mean increase of 115 ± 4% (n = 5, *P < 0.05) in the amplitude of the relative change in fluorescence was observed in the presence of apamin. D: mean τ before (τ = 298 ± 11 ms) and after (τ = 353 ± 18 ms, n = 5) application of apamin. *P < 0.05. E: relative increase in area under the fluorescence transients in the absence (Control) and presence of apamin (138 ± 13%; n = 5, *P < 0.05). C–E: symbols represent individual cells. Bar diagrams show means ± SE. F: effect of Na+ channel blocker TTX (1 μM) on the APs that induced the fluorescence transient displayed in G. Black, APs before TTX application (Control); gray, membrane depolarization in the presence of TTX; dashed, membrane depolarization in the presence of TTX and apamin. G: fluorescence transients elicited by 2 depolarizing pulses under control conditions (i.e., in the presence of 2 APs, see F; black trace) and in the presence of TTX (gray) and TTX + apamin (black). H: summary bar diagram showing a mean decrease to 22 ± 8% (n = 4) in amplitude of the relative change in fluorescence in the presence of TTX. Apamin, when applied in the presence of TTX, did not cause any increase in the fluorescence transient (F3,2 = 27, **P < 0.001; TTX vs. control P < 0.05; TTX+apamin vs. control P < 0.05; TTX+apamin vs. TTX P > 0.05; 1-way repeated-measures ANOVA with Bonferroni post hoc test).
To test whether the observed effects were a consequence of apamin acting specifically on Ca2+ influx triggered by APs rather than caused by the direct action of the depolarizing current injection, we measured the impact of the SK channel inhibitor after TTX application. TTX strongly attenuated the fluorescent transients (Fig. 4, F–H; n = 4), confirming that, under our experimental conditions, Ca2+ signals in the proximal dendrite mainly arise from the backpropagation of APs. In the presence of TTX, apamin failed to increase Ca2+ influx (Fig. 4, F–H; F3,2 = 27, P = 0.001; TTX vs. control P < 0.05; TTX+apamin vs. control P < 0.05; TTX+apamin vs. TTX P > 0.05; 1-way repeated-measures ANOVA with Bonferroni post hoc test). This indicates that AP-induced activation of calcium channels is necessary for the SK-mediated modulation of Ca2+ signals.
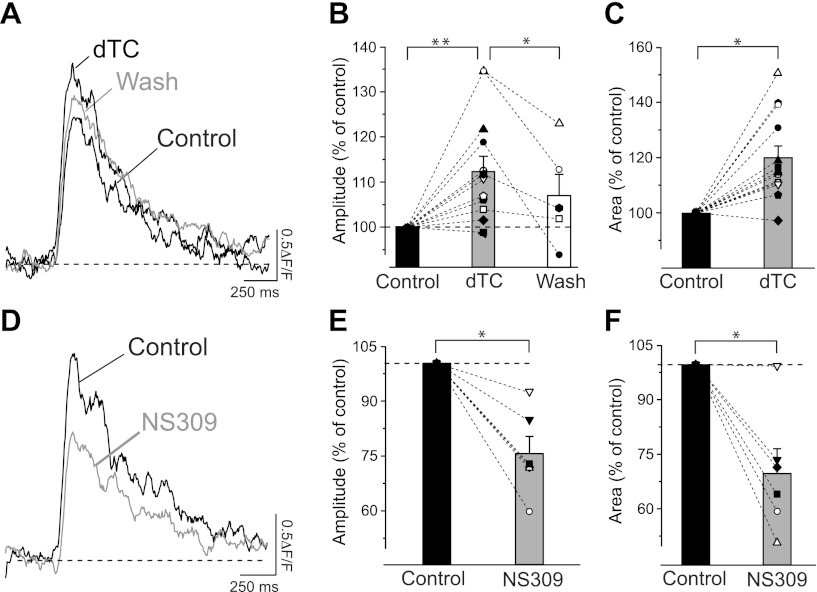
To corroborate the result obtained with apamin, we tested the effect of a structurally unrelated small organic SK channel blocker, dTC, on the Ca2+ transients in the proximal dendrite of hippocampal pyramidal neurons. Although less specific than apamin, dTC has the advantage that it blocks SK channels in a reversible manner. Application of 100 μM dTC led to an increase in the fluorescence transient amplitude to 113 ± 3% (Fig. 5, A and B), similar to the change observed with apamin. The effect of dTC on the amplitude of the fluorescence transients was reversed in five of six cells (Fig. 5, A and B). The reversibility of the dTC effect on the amplitude of the Ca2+ transients rules out the possibility that the observed increase might be a consequence of dye loading. Blocking SK channels with dTC also resulted in an increase of the area of the fluorescence transients to 120 ± 4% (Fig. 5, A and C) and in a prolongation of the time course of decay, which is reflected by an increase of τ by 20 ± 5%.
Fig. 5.
Modulation of AP-induced Ca2+ transients by dTC and NS309. A: representative fluorescence transients elicited by 2 APs at 20 Hz in the proximal dendrite, showing an increase in amplitude and area under the curve caused by the SK channel blocker dTC (100 μM). This effect was partially reversed (Wash). B: increase of mean relative amplitude of fluorescence transients in the presence of dTC to 113 ± 3% of control (n = 13; **P < 0.01). The effect of dTC on the amplitude of the fluorescence transients was reversed in 5 of 6 cells (Wash, 107 ± 5% of control; n = 5; *P < 0.05). Only cells with a current amplitude >90% after washout were analyzed and included. C: normalized area under the curve of the fluorescence transients after dTC application increased to 120 ± 4% of the control value (n = 13; *P < 0.01). D: SK channel enhancer NS309 (5 μM) caused a reduction of the fluorescence transient under the same recording conditions. E: reduction by 25 ± 5% (n = 6; *P < 0.05) of the mean relative amplitude of fluorescence transients in the presence of NS309. F: with respect to control, normalized area under the curve of the fluorescence transients was reduced by 30 ± 6% (n = 6; *P < 0.05) after application of NS309 (5 μM). Symbols in B, C, E, and F represent individual cells. Bar diagrams show means ± SE.
Several SK channel enhancers have recently been characterized (reviewed in Pedarzani and Stocker 2008) and act by increasing the apparent Ca2+ sensitivity of SK channels (Pedarzani et al. 2001). If the increase in the Ca2+ transients observed upon application of apamin and dTC is due to the inhibition of SK channels acting as negative feedback regulators of Ca2+ influx triggered by APs, then enhancement of SK channel activity should lead to a reduction in the Ca2+ transients. To test this hypothesis the SK channel enhancer NS309 was used (Pedarzani et al. 2005). NS309 (5 μM) reduced both the amplitude of the fluorescence transients by 25 ± 5% (Fig. 5, D and E) and their area by 30 ± 6% (Fig. 5, D and F) in all cells tested (n = 6). In the presence of NS309 the time constant of decay of the fluorescence transients was also shortened by 21 ± 6% (n = 6, Fig. 5D). The overall reduction of the Ca2+ transients observed in the presence of NS309 and the observed opposite effect obtained with SK channel blockers further support the hypothesis that SK channels regulate Ca2+ transients elicited by APs in the proximal dendrite of hippocampal pyramidal neurons by a negative feedback mechanism.
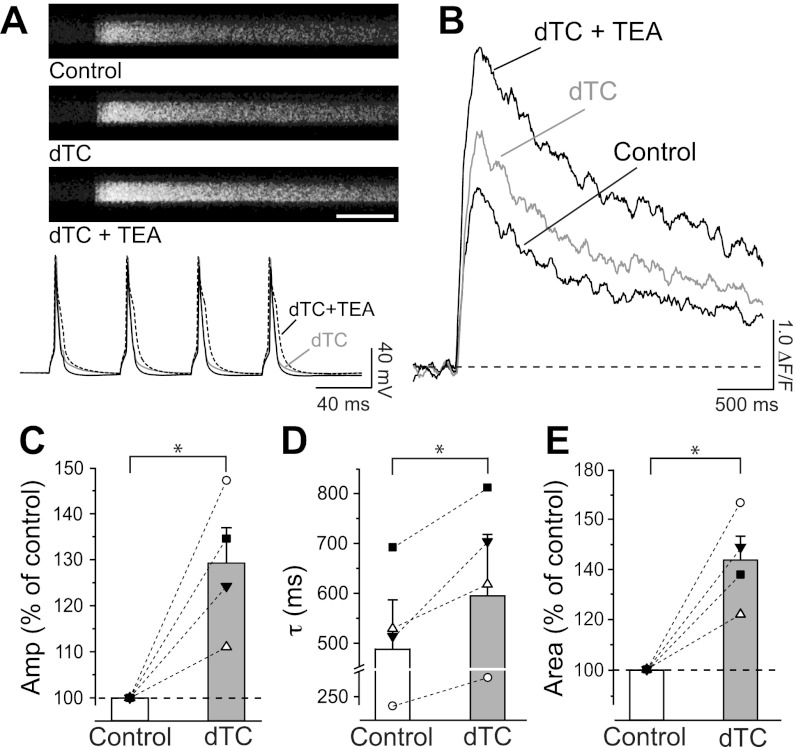
If SK channels, activated by Ca2+ entering through voltage-gated Ca2+ channels, modulate the Ca2+ transients generated by APs, then increasing the number of APs should lead to an enhanced Ca2+ influx, and therefore a stronger SK channel recruitment and a greater effect on the Ca2+ transients. The fluorescence transients measured in the proximal dendrites of pyramidal neurons in response to four APs were 21% larger than those observed in response to two APs at 20 Hz (4 APs: Fig. 6, A and B, ΔF/F = 1.72 ± 0.11, n = 18; 2 APs: Figs. 3–5, ΔF/F = 1.42 ± 0.12, n = 28; P = 0.02, Mann-Whitney test). The inhibition of SK channels by dTC (100 μM) resulted in an increase of amplitude of the fluorescence transients to 130 ± 8% (Fig. 6, A–C) and of area to 144 ± 9% (Fig. 6, B and E; n = 4, P < 0.05). Additionally, dTC caused a prolongation of the fluorescence transients by 21 ± 5% (Fig. 6D; n = 4, P < 0.05). The relative increases in amplitude and area of the fluorescence transients were significantly larger than those observed with two APs after application of dTC (compare Fig. 5, B and C, with Fig. 6, C and E; P < 0.05). To test whether the relative increase in the amplitude of Ca2+ transients observed in response to four APs in the presence of dTC was limited by dye saturation, we applied 10 mM TEA in the presence of dTC. As expected (see also Fig. 3, C and D), TEA substantially increased the influx of Ca2+ by prolonging the duration of the APs (Fig. 6A, bottom), thereby causing a further large increase of the fluorescence transients (Fig. 6B; amplitude 149 ± 15%, area 213 ± 22%; n = 3). The effect of TEA in this context confirms that the increase of the fluorescence transients observed after dTC application was not limited by fluo-4 saturation following four APs at 20 Hz.
Fig. 6.
Effects of dTC and TEA on Ca2+ transients induced by a train of 4 APs. A: line scans (top) and APs (20 Hz; bottom) triggered by somatic current injections in control and after application of dTC (100 μM) and further addition of TEA (10 mM). Scale bar: 500 ms. B: blocking of SK channels by dTC resulted in an increase of the relative fluorescence signal in the proximal dendritic process. This effect was further enhanced by TEA application. C: increase of mean relative amplitude of fluorescence transients in presence of dTC to 130 ± 8% of control (n = 4; *P < 0.05). D: mean τ, obtained from monoexponential fits to the fluorescent transients, increased from τ = 486 ± 99 ms before to τ = 593 ± 123 ms after application of dTC (n = 4; *P < 0.05). E: application of dTC resulted in a mean increase in area under the curve of the fluorescence transients to 144 ± 9% (n = 4; *P < 0.05) with respect to control. Symbols in C–E represent individual cells. Bar diagrams show means ± SE.
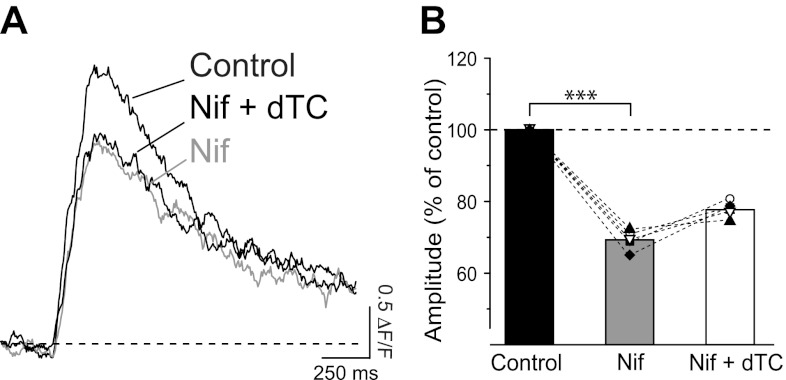
In the proximal dendrites of CA1 neurons, different subtypes of voltage-gated Ca2+ channels are activated by the backpropagation of APs and contribute to local Ca2+ elevations, with a predominant role played by Cav1 (L type) channels (Christie et al. 1995). Moreover, L-type Ca2+ channels have been shown to be physically colocalized and selectively coupled to the activation of small-conductance Ca2+-activated K+ channels in somatic patches from acutely dissociated CA1 pyramidal neurons (Marrion and Tavalin 1998). L-type Ca2+ channels were shown to be the main contributors to the activation of the SK-mediated IAHP also in whole cell recordings from CA1 neurons, with a reduction of IAHP by ∼48% upon application of the L-type Ca2+ channel blocker nifedipine (Bosurgi and Pedarzani 2006). We therefore tested whether the regulatory effect of SK channels on AP-induced Ca2+ transients is triggered by Ca2+ influx through L-type Ca2+ channels. First the contribution of L-type Ca2+ channels to the Ca2+ transients in the proximal dendrite was assessed. In all cells tested, application of the L-type channel blocker nifedipine at 10 μM markedly decreased the amplitude and the area of the fluorescence transients induced by four APs at 20 Hz (Fig. 7; n = 5). As expected, τ of the fluorescence transients was not affected by nifedipine (Fig. 7A; τcontrol = 414 ± 39 ms, τNifedipine = 423 ± 64 ms; n = 5), because τ is mainly determined by Ca2+ extrusion. The subsequent application of dTC in the presence of nifedipine did not increase the amplitude of the fluorescence transients significantly (Fig. 7; F4,2 = 61, P < 0.0001; nifedipine vs. control 69.5 ± 1.2%, P < 0.05; nifedipine+dTC vs. control 77.9 ± 1%, P < 0.05; nifedipine vs. nifedipine+dTC, P > 0.05; 1-way repeated-measures ANOVA with Bonferroni post hoc test), and similar results were obtained for the area of the Ca2+ transients (F4,2 = 18.47, P < 0.001; nifedipine vs. control 81 ± 2%, P < 0.01; nifedipine+dTC vs. control 85.2 ± 4.1%, P < 0.01; nifedipine vs. nifedipine+dTC, P > 0.05; 1-way repeated-measures ANOVA with Bonferroni post hoc test). Similarly, no significant difference for the time constant of decay of the fluorescence transients was observed when comparing controls, nifedipine alone, and the dTC-nifedipine coapplication (Fig. 7A; F4,2 = 0.14, P = 0.9; 1-way repeated-measures ANOVA). When applied to neurons in the presence of nifedipine, the effect of dTC on both the amplitude and the duration of the fluorescence transients was therefore strongly attenuated compared with the results obtained in the absence of the L-type Ca2+ channel blocker (Fig. 6, B–D, and Fig. 7A). We performed additional experiments by first applying dTC, followed by the addition of nifedipine. When applied in the presence of dTC, nifedipine reduced the amplitude (F14,2 = 22, P < 0.0001; dTC vs. control 123 ± 5%, P < 0.01; dTC+nifedipine vs. control 78 ± 7%, P < 0.05; dTC vs. dTC+nifedipine, P < 0.001; 1-way ANOVA with Bonferroni post hoc test) and area (F14,2 = 21, P < 0.0001; dTC vs. control 133 ± 7%, P < 0.01; dTC+nifedipine vs. control 76 ± 6%, P > 0.05; dTC vs. dTC+nifedipine, P < 0.001; 1-way ANOVA with Bonferroni post hoc test) of the Ca2+ transients to values similar or below the control values. These results suggest that the activation of SK channels by APs is at least in part due to the activation of L-type Ca2+ channels and in turn regulates Ca2+ influx in the proximal dendrite.
Fig. 7.
Inhibition of L-type voltage-gated Ca2+ channels prevents the dTC-mediated effect on Ca2+ transients induced by a train of 4 APs. A: application of L-type Ca2+ channel blocker nifedipine (Nif, 10 μM) significantly decreased the fluorescent transient induced by a train of 4 APs. Subsequent coapplication of dTC (100 μM) caused only a small, nonsignificant increase in the fluorescence transient (Nif+dTC). B: decrease in mean amplitude of relative changes in fluorescence to 69.5 ± 1.2% (n = 5) in the presence of Nif with respect to control. When dTC was subsequently applied in the presence of Nif, amplitude of the fluorescence transients was not significantly changed (F4,2 = 61, ***P < 0.0001; Nif vs. control P < 0.05; Nif+dTC vs. control P < 0.05; Nif vs. Nif+dTC P > 0.05; 1-way repeated-measures ANOVA with Bonferroni post hoc test). Symbols represent individual cells. Bar diagram shows means ± SE.
DISCUSSION
The dynamic response of pyramidal neurons in the hippocampus is modulated by Ca2+ transients that result from influx through voltage-gated Ca2+ channels. In particular, Ca2+ elevations triggered by backpropagating APs show a maximal amplitude in the proximal dendrites and decrease rapidly with distance from the soma (Callaway and Ross 1995; Christie et al. 1995; Jaffe et al. 1992; Regehr et al. 1989; Regehr and Tank 1994; Spruston et al. 1995). In this study, we have investigated how the activation of SK channels affects AP-induced changes in intracellular Ca2+ levels in proximal processes of hippocampal pyramidal neurons. We have found that in this cellular compartment SK channels limit the amplitude and duration of AP-induced Ca2+ transients.
SK channels have been shown to modulate the amplitude and duration of intracellular Ca2+ signals by feedback regulation of the relevant Ca2+ sources in different dendritic compartments, thus affecting dendritic signal integration and synaptic plasticity. In organotypic hippocampal cultures, SK channels are responsible for the repolarization of local dendritic plateau potentials triggered by focal glutamate application to distal apical dendrites of CA1 pyramidal neurons (Cai et al. 2004). In acute hippocampal slices, synaptic stimulation activates glutamate receptors, leading to the activation of SK channels located on the spine heads, which in turn reduces Ca2+ influx through the NMDA receptors (Bloodgood and Sabatini 2007; Lujan et al. 2009; Ngo-Anh et al. 2005). Both types of feedback regulation were shown to occur in response to synaptically evoked processes and on dendritic compartments receiving primarily glutamatergic excitatory synaptic inputs. The physiological role of the second- and higher-order dendrites, where the excitatory inputs predominate, is fundamentally different from that of the proximal apical dendrite, which mainly receives inhibitory synaptic inputs from GABAergic interneurons (Papp et al. 2001). In the proximal dendrite our results show feedback regulation of the amplitude and duration of AP-induced Ca2+ transients by SK channels. This modulation of transient Ca2+ elevations by SK channels could affect the communication between the soma and the distal apical dendritic tree at the single-cell level and shift the balance between excitation and inhibition at the network level.
Given their high sensitivity to Ca2+ (EC50 ∼300 nM; Xia et al. 1998) and relatively fast time constant of activation (∼5 ms at saturating Ca2+ concentrations; Pedarzani et al. 2001; Xia et al. 1998), SK channels are well suited to take part in a feedback loop to regulate Ca2+ influx in proximal apical dendrites of CA1 neurons, where a single AP leads to Ca2+ elevations on the order of ∼300 nM lasting 70–90 ms, while higher and longer-lasting free Ca2+ concentrations are reached in response to trains of APs (Helmchen et al. 1996; Maravall et al. 2000).
Our results suggest that SK channels act in a negative feedback loop by reducing Ca2+ influx through the Ca2+ channels that activate them after APs. This role of SK channels is supported by the effects of specific SK channel blockers (apamin, dTC) and enhancers (NS309) on the magnitude of Ca2+ transients. The presence of apamin or dTC boosted the Ca2+ influx induced by a train of two APs. Consistent with a negative feedback mechanism, application of the SK channel enhancer NS309 resulted in a reduction of the Ca2+ transient.
The regulatory effect of SK channels was particularly evident when a train of four APs was used to elicit a larger Ca2+ influx, leading to a stronger recruitment of SK channels. The increases in the amplitude of Ca2+ transients following application of SK channel blockers were larger compared with the stimulation with two APs and consistently observed in every cell tested.
Ca2+ buffering by Ca2+-sensitive dyes could in principle mimic the effect of SK channel inhibition. However, this is unlikely in our case because we used a low concentration of Ca2+-sensitive dye and did not observe significant changes in the baseline fluorescence over the course of our experiments. If anything, the added buffer capacity would result in gradual decrease in the Ca2+ transients (Helmchen et al. 1996; Maravall et al. 2000) and lead to an underestimation of the effects of SK channel inhibitors on the amplitude of Ca2+ transients. The validity of the increase of the Ca2+ transients induced by SK blockers is further supported by the reversibility achieved on washout of dTC.
In CA1 pyramidal neurons, APs induce Ca2+ transients that are largest in the proximal dendrites (Callaway and Ross 1995; Christie et al. 1995; Spruston et al. 1995), where they are mediated by different subtypes of high-voltage-activated Ca2+ channels (Bloodgood and Sabatini 2007; Christie et al. 1995, 1996). In particular, L-type Ca2+ channels are highly expressed in the somato-dendritic compartment of pyramidal cells in sections (Leitch et al. 2009; Tippens et al. 2008; Westenbroek et al. 1990) and primary cultures (Pravettoni et al. 2000). Additionally, experiments on the specific high-voltage-activated Ca2+ channel subtypes coupled to the activation of the SK-mediated IAHP in hippocampal pyramidal neurons have revealed that L-type Ca2+ channels are important contributors to the activation of IAHP, which was reduced by ∼48% by the L-type Ca2+ channel blocker nifedipine (Bosurgi and Pedarzani 2006). Application of nifedipine showed a contribution of ∼30% by L-type Ca2+ channels to the total AP-induced Ca2+ elevation in the proximal dendrites of cultured hippocampal pyramidal neurons (Fig. 7). This is in line with a previous report on the relative contributions of different voltage-gated Ca2+ channel subtypes to spike-induced Ca2+ influx in hippocampal pyramidal neurons in brain slices (Christie et al. 1995). L-type Ca2+ channel inhibition prevents the increase in the amplitude of the Ca2+ transients by SK channel blockers (Fig. 7) or reverses it back to control values or below when nifedipine is applied after dTC. This is evidence that L-type Ca2+ channels are implicated in the AP-induced Ca2+ influx leading to SK channel activation in the proximal dendrite of hippocampal pyramidal neurons. We cannot, however, exclude the contribution of other Ca2+ channel subtypes (see also Jones and Stuart 2012).
How do SK channels regulate transient Ca2+ elevations triggered by APs in dendrites? Voltage-gated Ca2+ channels open during the repolarizing phase of APs. While inhibition of BK and voltage-dependent K+ channels by TEA leads to broader APs and increased Ca2+ influx (Fig. 3, C and D), SK channel inhibition does not affect the duration of somatic APs (Fig. 6A). However, we cannot exclude the possibility that SK channels might contribute to shaping the waveform of dendritic APs, which have a lower amplitude and a longer duration in CA1 dendrites (Johnston et al. 2000; Spruston et al. 1995). While the waveform of the somatic AP is not directly affected by SK channel activation, the SK channel effect on AP-induced Ca2+ entry in the proximal dendrite might result from the functional interaction of these channels with other dendritic conductances. Thus two K+ currents, IA and ID, are expressed in CA1 dendrites (Golding et al. 1999; Hoffman et al. 1997) and are inactivated at depolarized potentials. The voltage-dependent inactivation properties of A-type K+ channels enable modest levels of membrane depolarization to decrease the size of the available A channel population and likewise increase dendritic AP amplitude and duration (Hoffman et al. 1997). By hyperpolarizing the membrane potential, SK channels could affect the availability and activation state of these conductances in AP trains, with SK channel inhibition and corresponding membrane depolarization favoring the inactivation of IA and ID. Upon inhibition of IA and ID, large-amplitude, backpropagating APs have been shown to activate dendritic Ca2+ channels or favor the dendritic initiation of Ca2+-dependent potentials, resulting in a massive influx of Ca2+ into the dendrites (Golding et al. 1999; Hoffman et al. 1997; Magee and Carruth 1999). This supports the possibility of a potential interaction between SK channels and A- and/or D-type K+ channels that may underlie the increase in Ca2+ influx observed upon SK channel inhibitions in our recordings. The relatively small, albeit significant, effect exerted by SK channel inhibition on Ca2+ influx in proximal hippocampal pyramidal cell dendrites might well match the gradient of A-type K+ channel density, with fewer channels in the proximal compared with the distal dendritic compartment.
A second potential mechanism for the SK-mediated enhancement of Ca2+ transients in the proximal dendritic compartment is linked to the coexistence of L-type Ca2+ channels with different gating behaviors in neurons, which are thought to give rise to distinct intracellular calcium signals in response to neuronal activity (Forti and Pietrobon 1993; Kavalali and Plummer 1994; Koschak et al. 2007). Thus, in addition to cardiac-like L-type channels, hippocampal neurons display L-type Ca2+ channels with anomalous gating properties, characterized by long channel reopenings after repolarization following strong depolarizations, such as bursts or trains of APs (Kavalali and Plummer 1994; Schjott and Plummer 2000). One potential mechanism by which SK channels might modulate Ca2+ influx would therefore be by reducing the activity of L-type Ca2+ channels in their “anomalous gating” phase. This hypothesis is supported by our results showing that strong depolarization caused by APs is essential to generate the SK-mediated feedback on Ca2+ influx, because this was absent in response to pure electrotonic spread when Na+ channels were blocked by TTX (Fig. 4, F–H).
In addition to anomalous gating properties, L-type Ca2+ channels are subject to various mechanisms of channel inactivation that contribute to the control of Ca2+ entry during ongoing neuronal electrical activity. These include Ca2+-dependent inactivation and fast and slow voltage-dependent inactivation (Budde et al. 2002). The inactivation kinetics of L-type Ca2+ channels are generally described as slow, but they vary in different cell types, possibly because of molecular diversity of the channels (splice variants of the pore-forming subunit; interaction with other Ca2+ channel subunits and modulatory proteins) (Budde et al. 2002). The inactivation profile of L-type Ca2+ channels in hippocampal pyramidal neurons has not been specifically characterized. We cannot exclude that SK channels could interfere in some indirect manner with the inactivation process of Ca2+ channels in proximal dendritic processes, contributing to the increase in Ca2+ influx we have observed on SK channel inhibition.
The pharmacological manipulation of SK channel activity not only increased or decreased the amplitude but also affected the area under the curve of the Ca2+ transients elicited by APs in the proximal dendrite of hippocampal pyramidal neurons (Figs. 4–6). SK channel inhibition led also to a significant prolongation of the Ca2+ transients (Figs. 4–6). Interestingly, the L-type Ca2+ channel blocker nifedipine prevented the effect of SK channel inhibitors on the time constant of decay of Ca2+ transients (Fig. 7). The time course of decay of AP-induced Ca2+ transients in dendrites directly reflects the rate of Ca2+ clearance (Scheuss et al. 2006). Sarco(endo)plasmic reticulum Ca2+-ATPases (Mainen et al. 1999; Majewska et al. 2000; Sabatini et al. 2002), plasma membrane Ca2+-ATPases, and Na+/Ca2+ exchangers (Lorincz et al. 2007; Scheuss et al. 2006) are responsible for the Ca2+ clearance from the cytosol of dendrites and spines in CA1 pyramidal neurons. Notably, both plasma membrane Ca2+-ATPases and Na+/Ca2+ exchangers are expressed in the dendrites of primary hippocampal neurons (Kiedrowski 2004; Kip et al. 2006). The increased duration of Ca2+ signals might simply reflect the longer time needed to clear the augmented Ca2+ after SK channel inhibition (Regehr and Tank 1992). Alternatively, SK channels might affect extrusion mechanisms in different ways. In the thalamus, for example, SK channels and sarco(endo)plasmic reticulum Ca2+-ATPases compete for available Ca2+ and shape Ca2+ transients in an interactive manner (Cueni et al. 2008). Another possibility is that the larger Ca2+ accumulations due to SK channel inhibition attenuated Ca2+ extrusion by plasma membrane Ca2+ ATPases and Na+/Ca2+ exchangers, whose function is reduced in a Ca2+-dependent manner (Scheuss et al. 2006), thereby leading to the observed prolongation of the duration of Ca2+ signals.
Our results demonstrate for the first time that the activity of SK channels can regulate the duration of Ca2+ transient decays in the proximal dendrite of hippocampal neurons. This may affect temporal summation of Ca2+ signals, potentially leading to changes in spike timing-dependent plasticity (Caporale and Dan 2008), as we have recently shown in another brain region, the striatum (Nazzaro et al. 2012). Here SK channels take part in the regulation of Ca2+-dependent release of endocannabinoids and plasticity, through a functional coupling with L-type voltage-gated Ca2+ channels activated by trains of APs (Nazzaro et al. 2012). SK-mediated modulation of intracellular Ca2+ dynamics may similarly be relevant for the activation of Ca2+-dependent signaling cascades to induce different forms of plasticity also in the hippocampal region (Cummings et al. 1996).
GRANTS
This work was supported by an EMBO short-term fellowship (EMBO ASTF 137.00-03) and a Human Frontier Science Program (HFSP) short-term fellowship (ST00323/2002-C) to R. Tonini; a Career Establishment Grant from the UK Medical Research Council to P. Pedarzani (CEG G0100066); a Wellcome Trust Student Prize fellowship to T. Ferraro (068583/Z/02/Z); an MRC-DTA PhD fellowship to M. Sampedro-Castañeda; and a Wellcome Trust Senior Research fellowship to M. Stocker (061198/Z/00A). P. Pedarzani and M. Stocker acknowledge support by the ENI-Net. P. Pedarzani acknowledges support by the HFSP (RGP0013/2010).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.T., T.F., M.S.-C., A.C., C.D.R., and P.P. performed experiments; R.T., T.F., M.S.-C., A.C., and C.D.R. analyzed data; R.T., T.F., M.S., C.D.R., and P.P. interpreted results of experiments; R.T., T.F., and M.S. prepared figures; R.T., T.F., M.S.-C., M.S., C.D.R., and P.P. edited and revised manuscript; R.T., M.S., C.D.R., and P.P. approved final version of manuscript; M.S., C.D.R., and P.P. conception and design of research; P.P. drafted manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge J. Dempster for supplying the Strathclyde Electrophysiology Software and Dr. D. DiGregorio for useful advice and discussion.
REFERENCES
- Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74: 245–269, 2012 [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV2.3 voltage-sensitive calcium channels located in dendritic spines. Neuron 53: 249–260, 2007 [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 24: 5301–5306, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosurgi R, Pedarzani P. High voltage-activated calcium channel types responsible for the activation of the SK-mediated IAHP in rat hippocampal CA1 pyramidal neurons (Abstract). FENS Forum Abstr 3: A084.5, 2006 [Google Scholar]
- Budde T, Meuth S, Pape HC. Calcium-dependent inactivation of neuronal calcium channels. Nat Rev Neurosci 3: 873–883, 2002 [DOI] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44: 351–364, 2004 [DOI] [PubMed] [Google Scholar]
- Cai X, Wei DS, Gallagher SE, Bagal A, Mei YA, Kao JP, Thompson SM, Tang CM. Hyperexcitability of distal dendrites in hippocampal pyramidal cells after chronic partial deafferentation. J Neurosci 27: 59–68, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway JC, Ross WN. Frequency-dependent propagation of sodium action potentials in dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol 74: 1395–1403, 1995 [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci 31: 25–46, 2008 [DOI] [PubMed] [Google Scholar]
- Christie BR, Eliot LS, Ito K, Miyakawa H, Johnston D. Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. J Neurophysiol 73: 2553–2557, 1995 [DOI] [PubMed] [Google Scholar]
- Christie BR, Magee JC, Johnston D. Dendritic calcium channels and hippocampal long-term depression. Hippocampus 6: 17–23, 1996 [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Gymnopoulos M, Boccaccio A, Stocker M, Pedarzani P. Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons. J Neurosci 22: 4456–4467, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci 868: 233–285, 1999 [DOI] [PubMed] [Google Scholar]
- Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, Franken P, Adelman JP, Luthi A. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci 11: 683–692, 2008 [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron 16: 825–833, 1996 [DOI] [PubMed] [Google Scholar]
- Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 50: 145–157, 2006 [DOI] [PubMed] [Google Scholar]
- Empson RM, Jefferys JG. Ca2+ entry through L-type Ca2+ channels helps terminate epileptiform activity by activation of a Ca2+ dependent afterhyperpolarisation in hippocampal CA3. Neuroscience 102: 297–306, 2001 [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 8: 635–641, 2005 [DOI] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of KCa channels by calcium nano/microdomains. Neuron 59: 873–881, 2008 [DOI] [PubMed] [Google Scholar]
- Fernandez de Sevilla D, Garduno J, Galvan E, Buno W. Calcium-activated afterhyperpolarizations regulate synchronization and timing of epileptiform bursts in hippocampal CA3 pyramidal neurons. J Neurophysiol 96: 3028–3041, 2006 [DOI] [PubMed] [Google Scholar]
- Forti L, Pietrobon D. Functional diversity of L-type calcium channels in rat cerebellar neurons. Neuron 10: 437–450, 1993 [DOI] [PubMed] [Google Scholar]
- Golding NL, Jung HY, Mickus T, Spruston N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. J Neurosci 19: 8789–8798, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker G. Rat hippocampal neurons in low-density culture. In: Culturing Nerve Cells, edited by Banker G, Goslin K. Cambridge, MA: MIT Press, 1991, p. 251–281 [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol 566: 689–715, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Imoto K, Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys J 70: 1069–1081, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13: 958–966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387: 869–875, 1997 [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Johnston D, Lasser-Ross N, Lisman JE, Miyakawa H, Ross WN. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature 357: 244–246, 1992 [DOI] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM, Migliore M. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol 525: 75–81, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Stuart GJ. Different calcium sources control somatic and dendritic SK channel activation in cortical pyramidal neurons (Abstract). FENS Forum Abstr 6: P030.12, 2012 [Google Scholar]
- Kaczorowski CC, Disterhoft J, Spruston N. Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol 578: 799–818, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Plummer MR. Selective potentiation of a novel calcium channel in rat hippocampal neurones. J Physiol 480: 475–484, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski L. High activity of K+-dependent plasmalemmal Na+/Ca2+ exchangers in hippocampal CA1 neurons. Neuroreport 15: 2113–2116, 2004 [DOI] [PubMed] [Google Scholar]
- Kip SN, Gray NW, Burette A, Canbay A, Weinberg RJ, Strehler EE. Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons. Hippocampus 16: 20–34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Obermair GJ, Pivotto F, Sinnegger-Brauns MJ, Striessnig J, Pietrobon D. Molecular nature of anomalous L-type calcium channels in mouse cerebellar granule cells. J Neurosci 27: 3855–3863, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci 24: 5151–5161, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch B, Szostek A, Lin R, Shevtsova O. Subcellular distribution of L-type calcium channel subtypes in rat hippocampal neurons. Neuroscience 164: 641–657, 2009 [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci 11: 170–177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Rozsa B, Katona G, Vizi ES, Tamas G. Differential distribution of NCX1 contributes to spine-dendrite compartmentalization in CA1 pyramidal cells. Proc Natl Acad Sci USA 104: 1033–1038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Maylie J, Adelman JP. New sites of action for GIRK and SK channels. Nat Rev Neurosci 10: 475–480, 2009 [DOI] [PubMed] [Google Scholar]
- Magee JC, Carruth M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J Neurophysiol 82: 1895–1901, 1999 [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature 399: 151–155, 1999 [DOI] [PubMed] [Google Scholar]
- Majewska A, Brown E, Ross J, Yuste R. Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J Neurosci 20: 1722–1734, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J 78: 2655–2667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Helm PJ, Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol 485: 1–20, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature 395: 900–905, 1998 [DOI] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, Parolaro D, Tkatch T, Benfenati F, Pedarzani P, Tonini R. SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat Neurosci 15: 284–293, 2012 [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34: 171–175, 1971 [DOI] [PubMed] [Google Scholar]
- Oh MM, Power JM, Thompson LT, Disterhoft JF. Apamin increases excitability of CA1 hippocampal pyramidal neurons. Neurosci Res Commun 27: 135–142, 2000 [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 26: 595–601, 2000 [DOI] [PubMed] [Google Scholar]
- Papp E, Leinekugel X, Henze DA, Lee J, Buzsaki G. The apical shaft of CA1 pyramidal cells is under GABAergic interneuronal control. Neuroscience 102: 715–721, 2001 [DOI] [PubMed] [Google Scholar]
- Parvez S, Ramachandran B, Frey JU. Functional differences between and across different regions of the apical branch of hippocampal CA1 dendrites with respect to long-term depression induction and synaptic cross-tagging. J Neurosci 30: 5118–5123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, Strobaek D, Stocker M. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current IAHP and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem 280: 41404–41411, 2005 [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem 276: 9762–9769, 2001 [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Stocker M. Molecular and cellular basis of small- and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci 65: 3196–3217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravettoni E, Bacci A, Coco S, Forbicini P, Matteoli M, Verderio C. Different localizations and functions of L-type and N-type calcium channels during development of hippocampal neurons. Dev Biol 227: 581–594, 2000 [DOI] [PubMed] [Google Scholar]
- Regehr WG, Connor JA, Tank DW. Optical imaging of calcium accumulation in hippocampal pyramidal cells during synaptic activation. Nature 341: 533–536, 1989 [DOI] [PubMed] [Google Scholar]
- Regehr WG, Tank DW. Calcium concentration dynamics produced by synaptic activation of CA1 hippocampal pyramidal cells. J Neurosci 12: 4202–4223, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Tank DW. Dendritic calcium dynamics. Curr Opin Neurobiol 4: 373–382, 1994 [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca2+ ions in dendritic spines. Neuron 33: 439–452, 2002 [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci 22: 9698–9707, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuss V, Yasuda R, Sobczyk A, Svoboda K. Nonlinear Ca2+ signaling in dendrites and spines caused by activity-dependent depression of Ca2+ extrusion. J Neurosci 26: 8183–8194, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjott JM, Plummer MR. Sustained activation of hippocampal Lp-type voltage-gated calcium channels by tetanic stimulation. J Neurosci 20: 4786–4797, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Javadzadeh-Tabatabaie M, Benton DC, Ganellin CR, Haylett DG. Enhancement of hippocampal pyramidal cell excitability by the novel selective slow-afterhyperpolarization channel blocker 3-(triphenylmethylaminomethyl)pyridine (UCL2077). Mol Pharmacol 70: 1494–1502, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science 268: 297–300, 1995 [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 96: 4662–4667, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippens AL, Pare JF, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A. Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. J Comp Neurol 506: 569–583, 2008 [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature 347: 281–284, 1990 [DOI] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507, 1998 [DOI] [PubMed] [Google Scholar]
- Yasuda R, Nimchinsky EA, Scheuss V, Pologruto TA, Oertner TG, Sabatini BL, Svoboda K. Imaging calcium concentration dynamics in small neuronal compartments. Sci STKE 219: pl5, 2004 [DOI] [PubMed] [Google Scholar]