Abstract
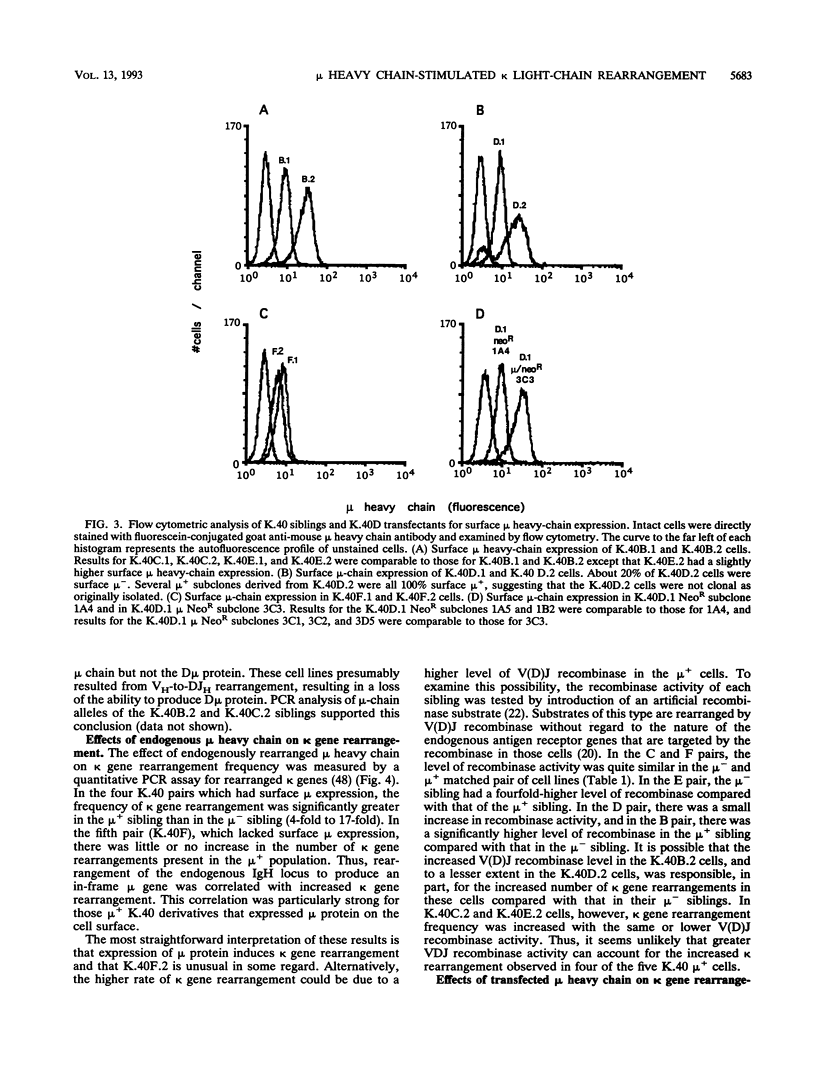
B-lymphocyte development exhibits a characteristic order of immunoglobulin gene rearrangements. Previous work has led to the hypothesis that expression of the immunoglobulin mu heavy chain induces rearrangement activity at the kappa light-chain locus. To examine this issue in more detail, we isolated five matched pairs of mu- and endogenously rearranged mu+ cell lines from the Abelson murine leukemia virus-transformed pro-B-cell line K.40. In four of the five mu+ cell lines, substantial expression of mu protein on the cell surface was observed, and this correlated with an enhanced frequency of kappa immunoglobulin gene rearrangement compared with that in the matched mu- cell lines. This increased kappa gene rearrangement frequency was not due to a general increase in the amount of V(D)J recombinase activity in the mu+ cells. Consistently, introduction of a functionally rearranged mu gene into one of the mu- pre-B-cell lines resulted in a fivefold increase in kappa gene rearrangements. In three of the four clonally matched pairs with increased kappa gene rearrangements, the increase in rearrangement frequency was not accompanied by a significant increase in germ line transcripts from the C kappa locus. However, in the fourth pair, K.40D, we observed an increase in germ line transcription of the kappa locus after expression of mu protein encoded by either an endogenously rearranged or a transfected functional heavy-chain allele. In these cells, the amount of the germ line C kappa transcript correlated with the measured frequency of rearranged kappa genes. These results support a regulated model of B-cell development in which mu protein expression in some way targets the V(D)J recombinase to the kappa gene locus.
Full text
PDF



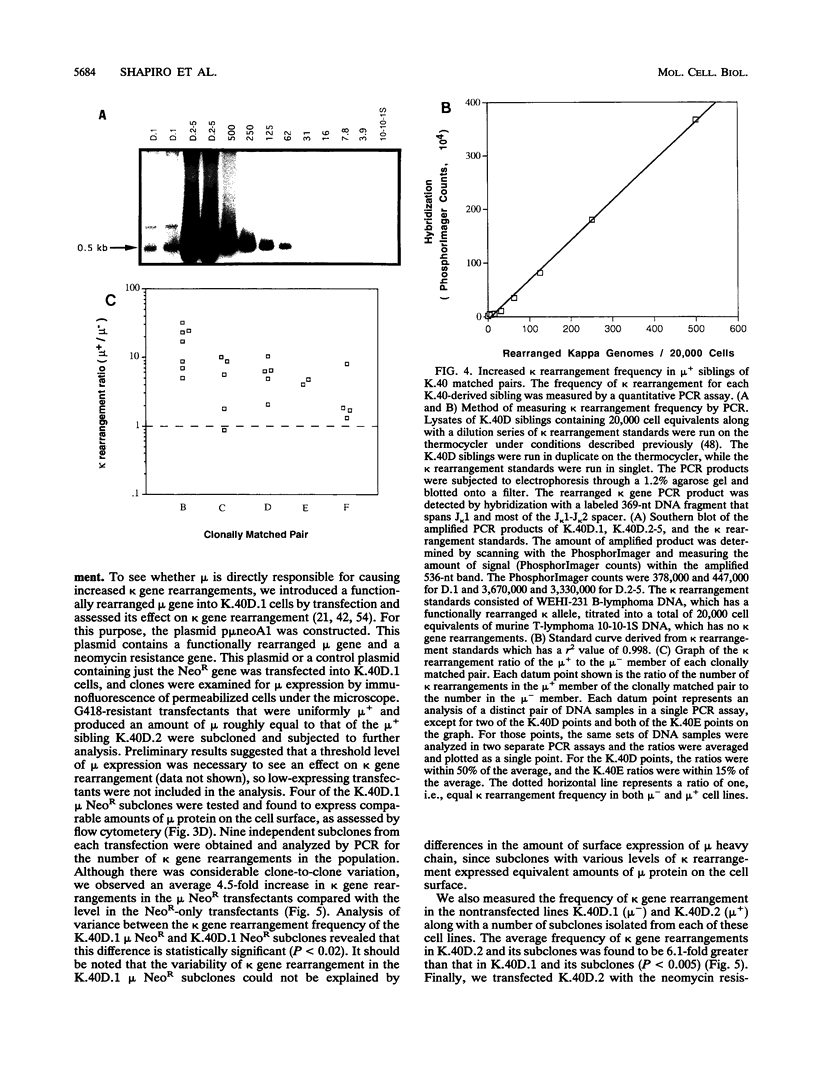
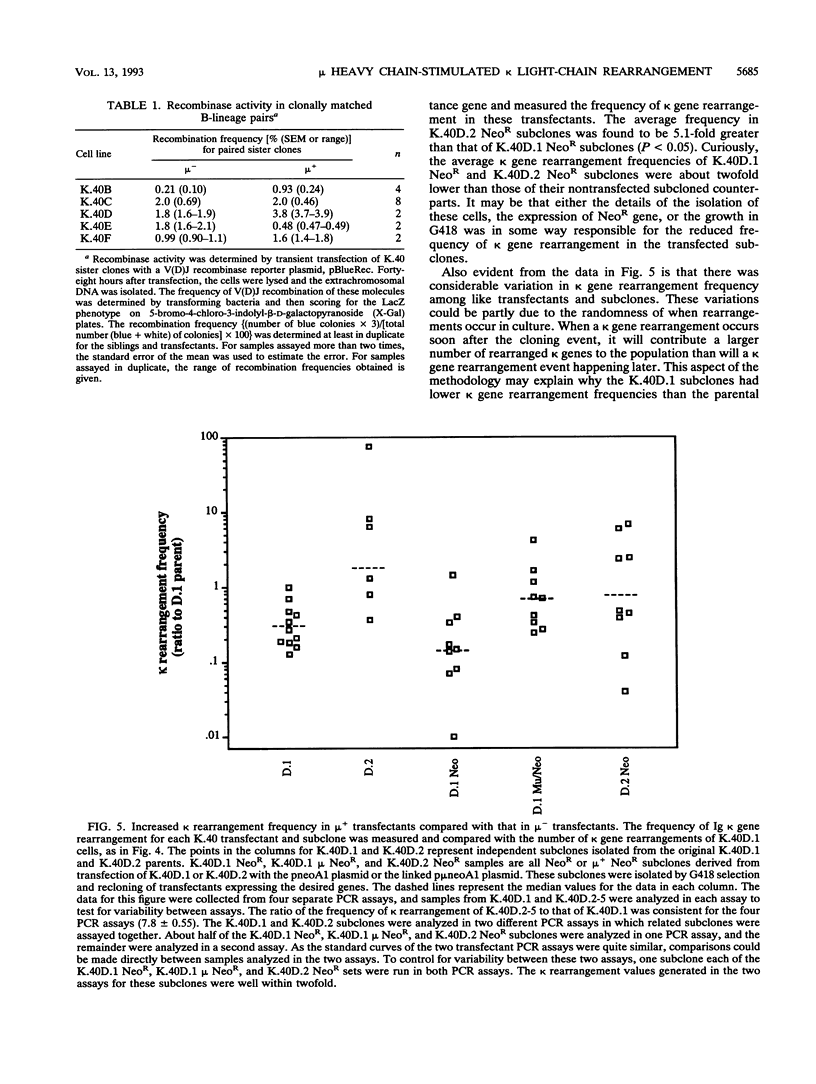
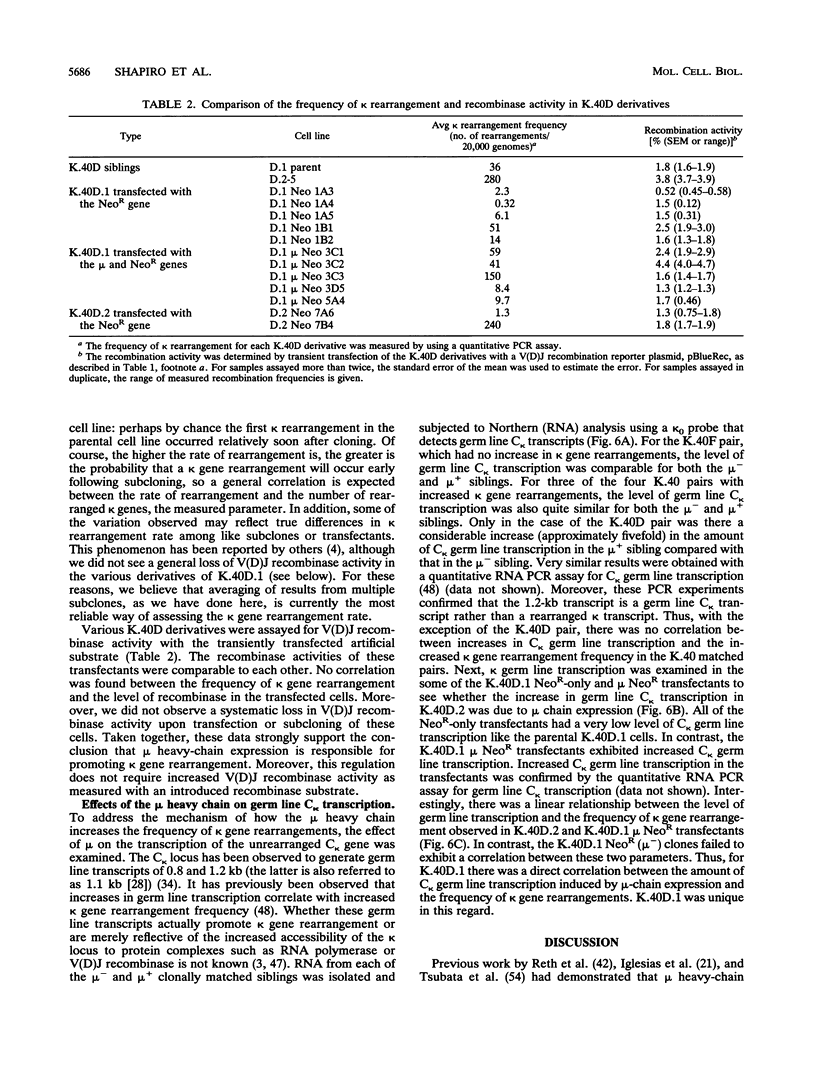
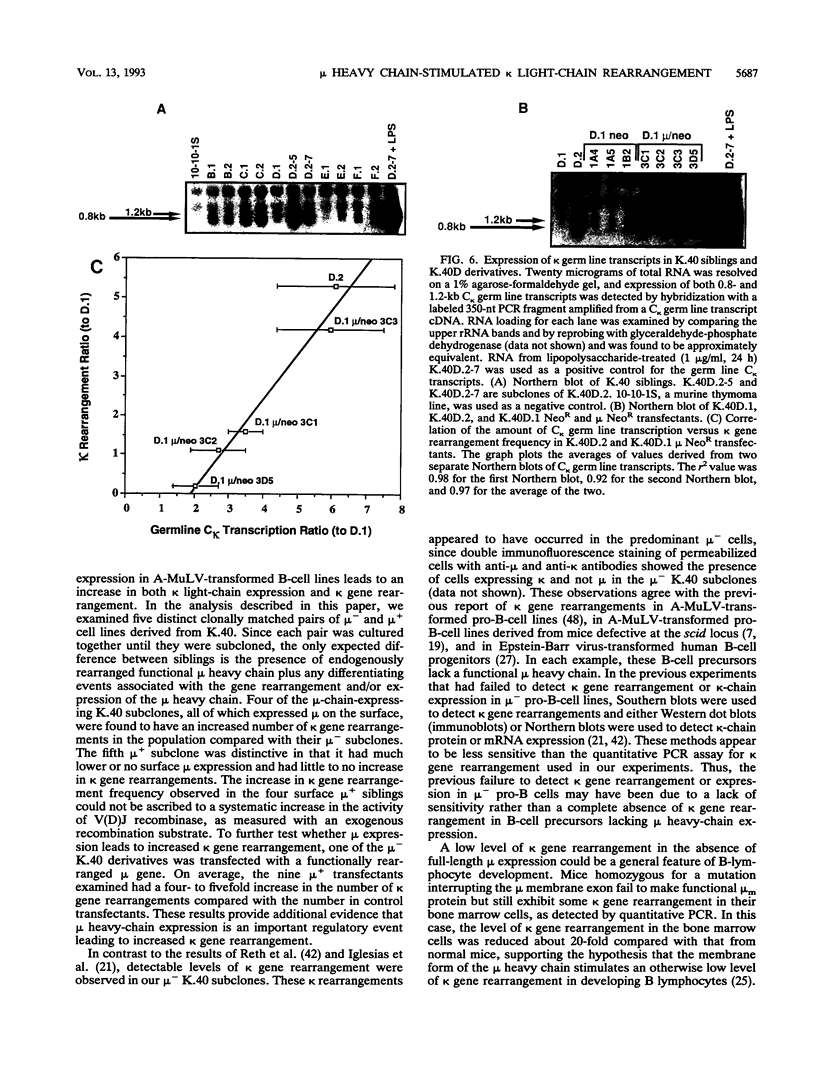



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Oltz E. M., Young F., Gorman J., Taccioli G., Chen J. VDJ recombination. Immunol Today. 1992 Aug;13(8):306–314. doi: 10.1016/0167-5699(92)90043-7. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Rathbun G., Oltz E., Taccioli G., Shinkai Y. Function and control of recombination-activating gene activity. Ann N Y Acad Sci. 1992 May 4;651:277–294. doi: 10.1111/j.1749-6632.1992.tb24626.x. [DOI] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Beck-Engeser G., Jäck H. M., Wabl M. Allelic inclusion in a pre-B-cell line that generates immunoglobulin heavy chain genes in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1060–1064. doi: 10.1073/pnas.84.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Alt F. W. Mechanism and developmental program of immunoglobulin gene rearrangement in mammals. Annu Rev Genet. 1989;23:605–636. doi: 10.1146/annurev.ge.23.120189.003133. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Malynn B. A., Pollock R. R., Ferrier P., Covey L. R., Fulop G. M., Phillips R. A., Yancopoulos G. D., Alt F. W. Isolation of scid pre-B cells that rearrange kappa light chain genes: formation of normal signal and abnormal coding joins. EMBO J. 1989 Mar;8(3):735–742. doi: 10.1002/j.1460-2075.1989.tb03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows P., LeJeune M., Kearney J. F. Evidence that murine pre-B cells synthesise mu heavy chains but no light chains. Nature. 1979 Aug 30;280(5725):838–840. doi: 10.1038/280838a0. [DOI] [PubMed] [Google Scholar]
- Cherayil B. J., Pillai S. The omega/lambda 5 surrogate immunoglobulin light chain is expressed on the surface of transitional B lymphocytes in murine bone marrow. J Exp Med. 1991 Jan 1;173(1):111–116. doi: 10.1084/jem.173.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeFranco A. L. Tyrosine phosphorylation and the mechanism of signal transduction by the B-lymphocyte antigen receptor. Eur J Biochem. 1992 Dec 1;210(2):381–388. doi: 10.1111/j.1432-1033.1992.tb17432.x. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Hamblin T. J., Smith J. L., Stevenson F. K., Stevenson G. T. A human b-cell lymphoma synthesizing and expressing surface mu-chain in the absence of detectable light chain. Blood. 1981 Sep;58(3):552–556. [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Gu H., Kitamura D., Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991 Apr 5;65(1):47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- Hendershot L., Levitt D. Analysis of surface mu-chain expression in human lymphoblastoid cell lines that do not produce light chains. J Immunol. 1984 Jan;132(1):502–509. [PubMed] [Google Scholar]
- Hendrickson E. A., Schlissel M. S., Weaver D. T. Wild-type V(D)J recombination in scid pre-B cells. Mol Cell Biol. 1990 Oct;10(10):5397–5407. doi: 10.1128/mcb.10.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Iglesias A., Kopf M., Williams G. S., Bühler B., Köhler G. Molecular requirements for the mu-induced light chain gene rearrangement in pre-B cells. EMBO J. 1991 Aug;10(8):2147–2155. doi: 10.1002/j.1460-2075.1991.tb07749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach S., Goodhardt M., Rougeon F. A rapid test for V(D)J recombinase activity. Nucleic Acids Res. 1990 Nov 25;18(22):6730–6730. doi: 10.1093/nar/18.22.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Kudo A., Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990 Sep 1;172(3):969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992 Mar 12;356(6365):154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Kubagawa H., Cooper M. D., Carroll A. J., Burrows P. D. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2356–2360. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq L., Butkeraitis P., Reth M. A novel germ-line JK transcript starting immediately upstream of JK1. Nucleic Acids Res. 1989 Sep 12;17(17):6809–6819. doi: 10.1093/nar/17.17.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Maki R., Kearney J., Paige C., Tonegawa S. Immunoglobulin gene rearrangement in immature B cells. Science. 1980 Sep 19;209(4463):1366–1369. doi: 10.1126/science.6774416. [DOI] [PubMed] [Google Scholar]
- Manz J., Denis K., Witte O., Brinster R., Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane mu, but not by secreted mu heavy chains. J Exp Med. 1988 Oct 1;168(4):1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. J., van Ness B. G. Initiation and processing of two kappa immunoglobulin germ line transcripts in mouse B cells. Mol Cell Biol. 1990 May;10(5):1950–1958. doi: 10.1128/mcb.10.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M., Fisher A. G. CD45 isoform switching precedes the activation-driven death of human thymocytes by apoptosis. Int Immunol. 1991 Jan;3(1):1–7. doi: 10.1093/intimm/3.1.1. [DOI] [PubMed] [Google Scholar]
- Nishimoto N., Kubagawa H., Ohno T., Gartland G. L., Stankovic A. K., Cooper M. D. Normal pre-B cells express a receptor complex of mu heavy chains and surrogate light-chain proteins. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6284–6288. doi: 10.1073/pnas.88.14.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Independent control of immunoglobulin heavy and light chain expression in a murine pre-B-cell line. Nature. 1981 Aug 13;292(5824):631–633. doi: 10.1038/292631a0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. The omega and iota surrogate immunoglobulin light chains. Curr Top Microbiol Immunol. 1988;137:136–139. doi: 10.1007/978-3-642-50059-6_20. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Reth M., Petrac E., Wiese P., Lobel L., Alt F. W. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO J. 1987 Nov;6(11):3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. Regulation of B-cell development by pre-B-cell receptors. Curr Biol. 1991 Jun;1(3):198–199. doi: 10.1016/0960-9822(91)90233-m. [DOI] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Köhler G. Transmission and expression of a specific pair of rearranged immunoglobulin mu and kappa genes in a transgenic mouse line. 1985 Mar 28-Apr 3Nature. 314(6009):330–334. doi: 10.1038/314330a0. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Schlissel M. S. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Corcoran L. M., Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J Exp Med. 1991 Mar 1;173(3):711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman C., Potash M. J., Köhler G. Roles of protein and carbohydrate in glycoprotein processing and secretion. Studies using mutants expressing altered IgM mu chains. J Biol Chem. 1981 Dec 25;256(24):13180–13187. [PubMed] [Google Scholar]
- Storb U. Transgenic mice with immunoglobulin genes. Annu Rev Immunol. 1987;5:151–174. doi: 10.1146/annurev.iy.05.040187.001055. [DOI] [PubMed] [Google Scholar]
- Takemori T., Mizuguchi J., Miyazoe I., Nakanishi M., Shigemoto K., Kimoto H., Shirasawa T., Maruyama N., Taniguchi M. Two types of mu chain complexes are expressed during differentiation from pre-B to mature B cells. EMBO J. 1990 Aug;9(8):2493–2500. doi: 10.1002/j.1460-2075.1990.tb07428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Tsubata R., Reth M. Crosslinking of the cell surface immunoglobulin (mu-surrogate light chains complex) on pre-B cells induces activation of V gene rearrangements at the immunoglobulin kappa locus. Int Immunol. 1992 Jun;4(6):637–641. doi: 10.1093/intimm/4.6.637. [DOI] [PubMed] [Google Scholar]
- Tsutsumi A., Terajima J., Jung W., Ransom J. Surface mu heavy chain expressed on pre-B lymphomas transduces Ca2+ signals but fails to cause growth arrest of pre-B lymphomas. Cell Immunol. 1992 Jan;139(1):44–57. doi: 10.1016/0008-8749(92)90098-a. [DOI] [PubMed] [Google Scholar]
- Warner N. L., Daley M. J., Richey J., Spellman C. Flow cytometry analysis of murine B cell lymphoma differentiation. Immunol Rev. 1979;48:197–243. doi: 10.1111/j.1600-065x.1979.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Weaver D., Costantini F., Imanishi-Kari T., Baltimore D. A transgenic immunoglobulin mu gene prevents rearrangement of endogenous genes. Cell. 1985 Aug;42(1):117–127. doi: 10.1016/s0092-8674(85)80107-0. [DOI] [PubMed] [Google Scholar]