Abstract
Anterograde trafficking of newly synthesized G protein-coupled receptors (GPCRs) from the endoplasmic reticulum to the cell surface represents a crucial checkpoint in controlling the amount of the functional receptors at the cell surface and the strength of signaling initiated by the receptors. In contrast to the extensively studied, well-understood endocytic and recycling pathways, the molecular mechanisms underlying the cell-surface targeting of the receptors remain poorly defined. In this chapter, I will discuss current advances in understanding post-Golgi transport of GPCRs by focusing on specific motifs or sequences that may function as sorting signals regulating export from the Golgi and subsequent transport to the plasma membrane of GPCRs.
Keywords: Anterograde transport, Cell-surface targeting, G protein-coupled receptors, Post-Golgi transport, Small GTPases, Sorting motifs
5.1 Cell-Surface Transport of Nascent G Protein-Coupled Receptors (GPCRs)
Intracellular trafficking and precise targeting to the functional destinations of GPCRs plays a crucial role in controlling the physiological functions of the receptors. Similar to many other plasma membrane proteins, the life of GPCRs begins in the endoplasmic reticulum (ER) where they are synthesized. Once correctly folded and properly assembled, GPCRs are able to pass the ER quality-control system and to exit from the ER, beginning their long journey of intracellular trafficking. The nascent receptors then move through several successive intracellular compartments, which include the ER-Golgi intermediate compartment (ERGIC), the cis/medial/trans-Golgi apparatus, and the trans-Golgi network (TGN), en route to the cell surface where the receptors are able to bind to their cognate ligands, leading to the activation of downstream signaling molecules. Compared to the extensive efforts dedicated to understanding the events involved in the endocytic and recycling pathways over the past two to three decades (Hanyaloglu and von Zastrow 2008; Moore et al. 2007), the molecular mechanisms underlying export trafficking of the GPCR superfamily to the plasma membrane are relatively less well-defined.
It has been known that cell-surface targeting of GPCRs is coordinated by many regulatory factors. First, GPCR export to the cell surface is regulated by multiple proteins, such as receptor activity modifying proteins (RAMPs), ER chaperones, and accessory proteins. These proteins may stabilize receptor conformation, facilitate receptor maturation, and promote receptor delivery to the plasma membrane (Dong et al. 2007; Achour et al. 2008; Bouschet et al. 2008). Second, recent studies have indicated that the exit of GPCRs from the ER may be directed by specific motifs embedded within the receptors (Dong et al. 2007; Dong and Wu 2006; Zhang et al. 2011; Dong et al. 2012). Third, post-translational modifications, such as N-linked glycosylation, have long been proven to be involved in the delivery of some GPCRs to the cell surface (Rands et al. 1990). Fourth, GPCR cell-surface targeting depends on the microtubule networks (Saunders and Limbird 1997) and GPCRs may directly interact with tubulin to control their cell-surface movement (Duvernay et al. 2011). Fifth, GPCR dimerization may influence proper receptor folding/assembly and the ability of receptors to pass through the ER quality-control system (Terrillon and Bouvier 2004). In this chapter, I will review the regulation of post-Golgi transport of GPCRs by focusing on specific sequences that may function as sorting signals regulating export out of the Golgi and transport from the Golgi to the plasma membrane of GPCRs (Table 5.1).
Table 5.1.
Specific motifs and their binding partners involved in the regulation of post-Golgi traffic of GPCRs
| GPCRs | Domains | Motifs | Binding partners | Refs |
|---|---|---|---|---|
| β2-AR | CT | LL | Rab8 | Dong et al. (2010a) |
| α2B-AR | CT | TVFN, PWTQTGW | Rab8 | Dong et al. (2010a) |
| α2B-AR | CT | Unknown | ARF1 | Dong et al. (2010b) |
| Rhodopsin | CT | VxPx | Tctex-1, ARF4 | Tai et al. (1999), Mazelova et al. (2009), Deretic et al. (2005) |
| P2Y4R, P2Y11R | ECL2 | Acidic residues | p23, p24 | Luo et al. (2007, 2011) |
| α2A-AR, α2B-AR | NT | YS | Unknown | Dong et al. (2007) |
CT, C-terminus; NT, N-terminus; ECL2, second extracellular loop
5.2 Sorting Signals for Vesicle-Mediated Post-Golgi Transport
Newly synthesized proteins are sorted at the Golgi/TGN compartments to be delivered to final cellular destinations, such as endosomes, lysosomes, and the plasma membrane. Therefore, the TGN is often referred to as the ‘sorting center’. Similar to the transport along the early secretory pathway which is mediated through COPI- and COPII-coated vesicles, post-Golgi transport can be mediated through clathrin-coated transport vesicles. Clathrin-coated transport vesicles are composed of clathrin and various adaptor proteins, including heterotetrameric adaptor protein complexes (APs), GGAs (Golgi-associated, γ-adaptin homologous, ARF-interacting proteins), and hepatocyte growth factor receptor substrate (Hrs). The formation of clathrin-coated vesicles involved in the post-Golgi traffic is highly regulated and complicated process. For example, the adaptor GGA proteins have three different isoforms: GGA1, 2 and 3, all with similar functions and structural features. Each GGA consists of four functional regions. The N-terminal VHS domain binds specific sorting signals in the cytoplasmic tail of cargo proteins. The GAT domain binds the activated form of ARF1 and this interaction is responsible for targeting GGAs onto the TGN membrane. The hinge region recruits clathrin and the C-terminal GAE domain interacts with several accessory proteins (Zhu et al. 2001) (Fig. 5.1). It is known that clathrin/GGA-coated vesicles are involved in the transport from the TGN to the lysosome of selective proteins, such as nascent lysosomal hydrolases (Bonifacino and Traub 2003), whereas clathrin/AP-coated vesicles mediate endocytosis of transmembrane receptors from the plasma membrane to the endosomal compartment. Interestingly, several studies have suggested that transport from the TGN to the plasma membrane is mediated by large pleiomorphic carriers rather than small vesicles (Bonifacino and Lippincott-Schwartz 2003).
Fig. 5.1.
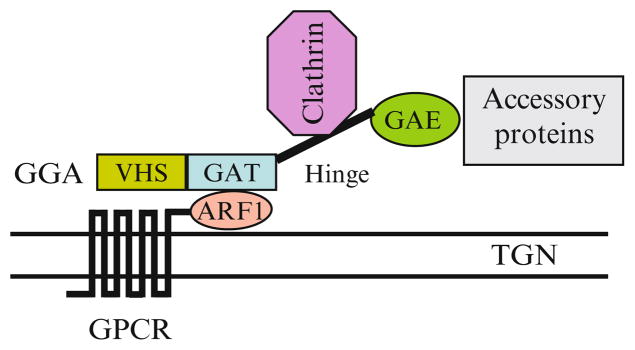
The formation of clathrin- and GGA-coated vesicles on the TGN membrane and the possible function of the interaction of GPCRs, via their C-termini, with ARF1 in facilitating receptor recruitment onto the vesicles
Recent studies have demonstrated that, similar to exit from the ER directed by ER export signals such as the di-acidic motifs (Sevier et al. 2000; Ma et al. 2001; Nishimura and Balch 1997; Nishimura et al. 1999; Zuzarte et al. 2007; Wang et al. 2004), protein export from the Golgi/TGN compartments can be dictated by specific motifs. For examples, there are several well-defined endosomal sorting signals, such as tyrosine-based motifs (NPxY and YxxØ, where x can be any residue and Ø is a hydrophobic residue) and di-leucine-based motifs ([D/E]xxxL[L/I] and DxxLL). Whereas YxxØ and [D/E]xxxL[L/I] motifs are recognized by the AP complexes, DxxLL is recognized by GGAs (Puertollano et al. 2001a, b; Hirst et al. 2000). These motifs function to sort protein transport from the TGN to the endosomal compartment (Chen et al. 1997; Lori et al. 2007; Johnson and Kornfeld 1992; Hou et al. 2006; Boucher et al. 2008). Although these endosomal sorting motifs are also found in several GPCRs (Marchese et al. 2008), their exact functions remain to be elucidated. It is interesting to note that β2-AR has an ExxLL motif within the C-terminal sequence EKENKLL and this motif has been demonstrated to be involved in the regulation of recycling of internalized β2-AR from endosomes to the plasma membrane (Hanyaloglu and von Zastrow 2007).
It has been well demonstrated that clathrin/AP-coated vesicles mediate endocytosis of agonist-occupied GPCRs from the plasma membrane to endosomes. In contrast, the vesicles involved in the transport of newly synthesized GPCRs from the Golgi/TGN to the plasma membrane remain unknown. However, several proteins, including ARF4, Rab11, the Rab11/ARF effector FIP3, and the ARF GAP ASAP1, may form a complex on the TGN to regulate ciliary targeting of rhodopsin, a photoactivated GPCR in the retina (Mazelova et al. 2009). We have recently demonstrated that expression of GTP-bound mutant of the small GTPase ARF1, which regulates the formation of clathrin-coated vesicles from the TGN, arrested GPCRs in the Golgi compartments (Dong et al. 2010b), suggesting that ARF1-coordinated vesicles may play a role in mediating GPCR export from the Golgi.
5.3 Regulation of Post-Golgi Traffic of GPCRs by Specific Motifs and Possible Mechanisms
5.3.1 The LL Motif and Its Interaction with Rab8 GTPase in Post-Golgi Transport of β2-AR
Rab GTPases are the largest branch of the Ras-related GTPase superfamily, consisting of more than 60 members in mammals and 11 in yeast, and are involved in almost every step of vesicle-mediated protein transport, particularly the targeting, tethering, and fusion of transport vesicles with the appropriate acceptor membrane (Grosshans et al. 2006). Each Rab GTPase has a distinct subcellular localization pattern that correlates with the compartments between which it coordinates transport. Several recent studies have determined the roles of Rab GTPases in the anterograde transport of newly synthesized GPCRs. Specifically, Rab1, Rab2, and Rab6 are involved in GPCR transport along the early secretory pathway (Wu et al. 2003; Filipeanu et al. 2004, 2006; Zhang et al. 2009; Dong and Wu 2007; Wang and Wu 2012) and Rab8 GTPase regulates the post-Golgi transport of GPCRs (Dong et al. 2010a).
Rab8 has been extensively investigated in protein transport from the TGN to the apical/basolateral membrane under polarized conditions (Sato et al. 2007; Moritz et al. 2001; Nachury et al. 2007). Similar to Rab11 function in Drosophila (Li et al. 2007; Satoh et al. 2005), Rab8 modulates the post-Golgi transport of rhodopsin in Xenopus (Deretic et al. 1995; Moritz et al. 2001). We have recently determined the role of Rab8 in the cell-surface targeting of α2B-AR and β2-AR in several cell lines and primary neurons (Dong et al. 2010a). Our studies have shown that attenuation of Rab8 function via expressing Rab8 mutants and shRNA reduced the cell-surface expression of α2B-AR and β2-AR and arrested the receptors in the TGN compartment. These studies demonstrate that Rab8 is required for post-Golgi traffic of some GPCRs.
We have also demonstrated that Rab8 physically associated with both α2B-AR and β2-AR, suggesting that the cargo GPCRs may interact with Rab GTPases to coordinate their post-Golgi traffic. More interestingly, distinct motifs in the C-termini of α2B-AR and β2-AR were identified to interact with Rab8 and these motifs probably dictated differential regulation of these two receptors by Rab8 (Dong et al. 2010a). The di-leucine (LL) motif was shown to be required for β2-AR interaction with Rab8, whereas multiple residues scattered in the C-terminus were identified to modulate α2B-AR interaction with Rab8.
The LL motif has been described to function as a sorting signal at the TGN for basolateral cell-surface transport and at the plasma membrane for endocytosis in clathrin-coated vesicles (Heilker et al. 1996; Hunziker and Fumey 1994; Bonifacino and Traub 2003). We and others have recently demonstrated that the LL motif is involved in the cell-surface transport of several GPCRs, including α2B-AR, α1B-AR, β2-AR, angiotensin II type 1 receptor (AT1R), serotonin 5HT1A and 5-HT1B receptors, vasopressin V2 receptor, and M1-muscarinc receptor (M1-MR) (Duvernay et al. 2004; Schulein et al. 1998; Carrel et al. 2006; Sawyer et al. 2010). The LL motif has been postulated to modulate receptor transport from the ER as its mutants were unable to exit from the ER. Importantly, the LL motif is highly conserved in the membrane-proximal C-termini of the family A GPCRs (Duvernay et al. 2004) and therefore, it may provide a common mechanism for GPCR export from the ER compartment. The fact that mutation of the LL motif influences the interaction with Rab8 of β2-AR, but not α2B-AR (Dong et al. 2010a), suggests that it may have different roles in the regulation of post-Golgi transport of distinct GPCRs. It is possible that, similar to the function of the ExD motif in VSVG transport (Nishimura et al. 2002), a single LL motif may modulate export trafficking of some, but not all, GPCRs (e.g. β2-AR) at multiple intracellular compartments. In addition to regulating ER export, it may also coordinate GPCR exit from the Golgi/TGN.
5.3.2 The C-Terminal Tails and ARF1 GTPase in Post-Golgi Transport of GPCRs
Based on their amino acid sequence homology and gene organization, five ARF GTPases identified in human are divided into three classes: class I (ARF1/3), class II (ARF4/5) and class III (ARF6). ARF1 plays a crucial role in both anterograde and retrograde trafficking and its traffic function is mediated through regulating the formation of transport vesicles on different intracellular compartments. In the early secretory pathway, ARF1 recruits a complex of cytosolic proteins, collectively known as coatomers and this leads to the formation of COPI-coated vesicles that mediate cargo transport from the Golgi to the ER, from the ERGIC to the Golgi, and between Golgi cisternae (Spang 2002). In the post-Golgi transport, ARF1 directly interacts with APs and GGAs (Fig. 5.1), initiating the formation of the clathrin-coated vesicles.
Consistent with its well-established trafficking function, ARF1 is involved in the cell-surface transport of newly synthesized GPCRs. We have recently demonstrated that expression of ARF1 mutants dramatically inhibited the cell-surface transport of all GPCRs tested, including α2B-AR, β2-AR, AT1R, C-X-C chemokine receptor type 4 (CXCR4), and M3-MR (Dong et al. 2010b). Similarly, ARF3 mutants attenuated the cell-surface expression of α2B-AR (Dong et al. 2010b), suggesting that the class I ARF GTPases, ARF1 and ARF3, play an important role in regulating the movement of GPCRs to the cell surface. More interestingly, expression of the GDP- and GTP-bound ARF1 mutants arrested the receptors in different intracellular compartments. Whereas the GDP-bound mutant ARF1T31N strongly blocked receptor export from the ER, the GTP-bound mutant ARF1Q71L inhibited receptor export out of the Golgi. These data are consistent with other studies showing that expression of different ARF1 mutants disrupted protein transport at different compartments (Ward et al. 2001). These data suggest that ARF1 may modulate GPCR transport at multiple transport steps and that GTP-hydrolysis of GTP-bound ARF1 GTPase plays a critical role in the post-Golgi transport of GPCRs.
Furthermore, we found that the C-terminus of α2B-AR physically interacted with ARF1. As purified His-tagged ARF1 and GST-tagged C-terminus were still able to form a complex, the interaction between the receptor and ARF1 is likely direct. It is possible that the cargo GPCRs, via their C-termini, directly interact with the small GTPase ARF1 and this interaction facilitates receptor recruitment onto the ARF1-coordinated, Golgi/TGN-derived transport vesicles (Fig. 5.1).
5.3.3 The VxPx Motif and Its Interaction with ARF4 GTPase in Post-Golgi Transport of Rhodopsin
It has been known that the four highly conserved amino acids located in the C-terminus of rhodopsin plays an important role in the regulation of intracellular targeting and function of the receptor. Indeed, mutation of the C-terminal VxPx motif is clearly linked to the pathogenesis of neurodegenerative diseases, particularly the severe forms such as autosomal dominant retinitis pigmentosa. The function of the VxPx motif in regulating rhodopsin trafficking has been suggested to be mediated through interacting with Tctex-1, a light chain of cytoplasmic dynein that translocates rhodopsin-bearing vesicles along microtubules (Tai et al. 1999).
Deretic et al. have demonstrated that the VxPx motif mediates rhodopsin interaction with the small GTPase ARF4 (Deretic et al. 2005) and this interaction plays an important role in receptor sorting at the TGN into membrane carriers which transport to the rod outer segments (Deretic et al. 2005; Mazelova et al. 2009). They have also identified a complex containing ARF4 that is formed on the TGN and regulates post-Golgi transport of rhodopsin (Mazelova et al. 2009). These studies have demonstrated that the C-terminal sequence through physically association with transport machinery proteins may provide an efficient transport system for the vectorial delivery of large numbers of nascent rhodopsin. In addition, mutation of the glutamic acid residue at the position 150 in the second intracellular loop of rhodopsin (E150K) induced an accumulation of the receptor in the cis/medial Golgi compartments and this mutation is linked to the development of autosomal recessive retinitis pigmentosa (Zhu et al. 2006), further suggesting that rhodopsin export from the Golgi and subsequent transport to the functional destination is a regulated process. These data also suggest that defective post-Golgi traffic may be the molecular basis of retinitis pigmentosa.
5.3.4 The Acidic Motifs and Their Interaction with the p24 Family Proteins in the Post-Golgi Transport of GPCRs
The di-acidic sequences have been demonstrated to function as ER export motifs in several membrane proteins (Sevier et al. 2000; Ma et al. 2001; Nishimura and Balch 1997; Nishimura et al. 1999; Zuzarte et al. 2007; Wang et al. 2004). Their ER export functions are mediated through directly interacting with Sec24, a component of ER-derived COPII vesicles that mediate cargo transport exclusively from the ER. This interaction enhances the recruitment and concentration of the cargo carrying the di-acidic motifs into the COPII vesicles. The di-acidic motifs in the membrane-proximal C-termini have been also demonstrated to regulate the transport of AT1R and AT2R, but not β2-AR and α1B-AR, from the ER (Zhang et al. 2011 ). In addition to regulating ER export, the di-acidic motifs are also involved in post-Golgi trafficking through interacting with the adaptor protein AP3 (Nishimura et al. 2002).
Luo et al. have demonstrated that protease-activated receptor-2 (PAR2) interacted with p24A, a type 1 transmembrane protein. This interaction is regulated by the small GTPase ARF1 and plays an important role in the transport of PAR-2 from the Golgi to the plasma membrane (Luo et al. 2007). Subsequently, this group demonstrates that p24 interacted with several other GPCRs, including PAR-1, μ-opioid receptor 1B, and the nucleotide receptors (P2Y1R, P2Y2R, P2Y4R, and P2Y11R) (Luo et al. 2011). In addition to p24, p23, another member of the p24 family protein, is also able to bind to these GPCRs. More interestingly, the conserved acidic residues located in the second extracellular loop are required for the interaction of P2Y4R and P2Y11R with p24 (Luo et al. 2011). These data strongly imply that GPCRs, via the acidic domains, may interact with Golgi-localized proteins, such as the p24 family proteins p23 and p24, to modulate their post-Golgi trafficking along the biosynthetic pathway.
5.3.5 The YS Motif in Post-Golgi Traffic of α2-AR
As described above the C-termini of GPCRs contain signals directing the post-Golgi transport of GPCRs and their function can be mediated by physically associating with components of the transport machinery, such as small GTPases. Similar to the C-termini, the N-termini are also involved in the regulation of anterograde trafficking of newly synthesized GPCRs. For example, the deletion of the N-termini facilitated the cell-surface export of α1D-AR and α2C-AR, suggesting that the N-termini may contain signals retaining the receptors in the ER (Angelotti et al. 2010; Hague et al. 2004). Indeed, a hydrophobic sequence in the C-terminus of α2C-AR was proven to function as an ER retention motif (Angelotti et al. 2010), which provides a mechanism responsible for the intra-cellular accumulation of α2C-AR in some types of cells.
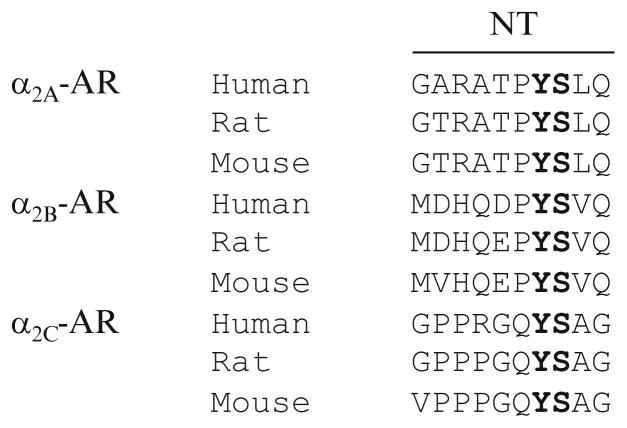
We have found that the N-termini may play an important role in dictating GPCR export from the Golgi. We have demonstrated that single and double substitution of Y12 and S13 residues in the membrane-proximal N-terminal region significantly reduced the cell-surface expression of α2B-AR (Dong and Wu 2006) and the Y12/S13 motif mutants were retained in the Golgi apparatus (Dong and Wu 2006). These data suggest that the Y12/S13 motif mediates α2B-AR export at the level of the Golgi. The YS motif only exists in three α2-AR family members (Dong and Wu 2006) (Fig. 5.2) and indeed, it exerts a similar function on α2A-AR trafficking (Dong and Wu 2006). Therefore, the YS motif may function as an export signal specifically modulating the Golgi export of the members of α2-AR subfamily. It should be pointed out that the N-terminal tails are positioned towards the lumen of the ER and the Golgi during the export process and the YS motif is not able to directly interact with components of transport machinery in the cytoplasm. Further investigation is needed to clarify the molecular mechanism underlying the function of YS motif in the regulation of receptor export from the Golgi.
Fig. 5.2.
The conserved YS motif in the N-termini (NT) of three α2-AR subtypes
5.4 Concluding Remarks
It has become increasingly apparent that cell-surface targeting of GPCRs is one of the important factors determining the functionality of the receptors and is directly linked to the pathogenesis of several human diseases that involve the presence of greater or fewer functional receptors at the cell surface. In particular, many GPCR trafficking-related diseases are caused by naturally occurring mutations and truncations in the receptors which lead to retention of the receptors in the intracellular compartments (Conn et al. 2007). As discussed above, retinitis pigmentosa is clearly linked to mutations of rhodopsin which cause the accumulation of the receptor in the Golgi compartment. Therefore, to thoroughly understand the mechanism underlying export trafficking of GPCRs will provide a foundation for the development of therapeutic strategies targeting on specific components of the transport pathway.
Acknowledgments
This work was supported by National Institutes of Health Grant R01GM076167 (to G Wu).
References
- Achour L, Labbe-Jullie C, Scott MG, Marullo S. An escort for GPCRs: implications for regulation of receptor density at the cell surface. Trends Pharmacol Sci. 2008;29(10):528–535. doi: 10.1016/j.tips.2008.07.009. pii:S0165-6147(08)00169-7. [DOI] [PubMed] [Google Scholar]
- Angelotti T, Daunt D, Shcherbakova OG, Kobilka B, Hurt CM. Regulation of G-protein coupled receptor traffic by an evolutionary conserved hydrophobic signal. Traffic. 2010;11(4): 560–578. doi: 10.1111/j.1600-0854.2010.01033.x. pii:TRA1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4(5):409–414. doi: 10.1038/nrm1099. pii:nrm1099. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. pii:121801.161800. [DOI] [PubMed] [Google Scholar]
- Boucher R, Larkin H, Brodeur J, Gagnon H, Theriault C, Lavoie C. Intracellular trafficking of LRP9 is dependent on two acidic cluster/dileucine motifs. Histochem Cell Biol. 2008;130(2):315–327. doi: 10.1007/s00418-008-0436-5. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Martin S, Henley JM. Regulation of calcium-sensing-receptor trafficking and cell-surface expression by GPCRs and RAMPs. Trends Pharmacol Sci. 2008;29(12):633–639. doi: 10.1016/j.tips.2008.09.002. pii:S0165-6147(08)00208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel D, Hamon M, Darmon M. Role of the C-terminal di-leucine motif of 5-HT1A and 5-HT1B serotonin receptors in plasma membrane targeting. J Cell Sci. 2006;119(Pt 20):4276–4284. doi: 10.1242/jcs.03189. pii:jcs.03189. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Yuan J, Lobel P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J Biol Chem. 1997;272(11):7003–7012. doi: 10.1074/jbc.272.11.7003. [DOI] [PubMed] [Google Scholar]
- Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59(3):225–250. doi: 10.1124/pr.59.3.2. pii:59/3/225. [DOI] [PubMed] [Google Scholar]
- Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. Rab8 In retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108(Pt 1):215–224. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc Natl Acad Sci U S A. 2005;102(9):3301–3306. doi: 10.1073/pnas.0500095102. pii:0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Wu G. Regulation of anterograde transport of alpha2-adrenergic receptors by the N termini at multiple intracellular compartments. J Biol Chem. 2006;281(50):38543–38554. doi: 10.1074/jbc.M605734200. pii:M605734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Wu G. Regulation of anterograde transport of adrenergic and angiotensin II receptors by Rab2 and Rab6 GTPases. Cell Signal. 2007;19(11):2388–2399. doi: 10.1016/j.cellsig.2007.07.017. pii:S0898-6568(07)00234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768(4):853–870. doi: 10.1016/j.bbamem.2006.09.008. pii:S0005-2736(06)00351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Yang L, Zhang X, Gu H, Lam ML, Claycomb WC, Xia H, Wu G. Rab8 Interacts with distinct motifs in {alpha}2B- and {beta}2-adrenergic receptors and differentially modulates their transport. J Biol Chem. 2010a;285(26):20369–20380. doi: 10.1074/jbc.M109.081521. pii:M109.081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Zhang X, Zhou F, Dou H, Duvernay MT, Zhang P, Wu G. ADP-ribosylation factors modulate the cell surface transport of G protein-coupled receptors. J Pharmacol Exp Ther. 2010b;333(1):174–183. doi: 10.1124/jpet.109.161489. pii:jpet.109.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Nichols CD, Guo J, Huang W, Lambert NA, Wu G. A triple Arg motif modulates α2B-adrenergic receptor interaction with sec 24C/D and export. Traffic. 2012;13(6):857–868. doi: 10.1111/j.1600-0854.2012.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J Biol Chem. 2004;279(29):30741–30750. doi: 10.1074/jbc.M313881200. pii:M313881200. [DOI] [PubMed] [Google Scholar]
- Duvernay MT, Wang H, Dong C, Guidry JJ, Sackett DL, Wu G. {Alpha}2B-adrenergic receptor interaction with tubulin controls its transport from the endoplasmic reticulum to the cell surface. J Biol Chem. 2011;286(16):14080–14089. doi: 10.1074/jbc.M111.222323. pii:M111.222323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem. 2004;279(39):41077–41084. doi: 10.1074/jbc.M405988200. pii:M405988200. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Fugetta EK, Wu G. Differential regulation of the cell-surface targeting and function of beta- and alpha1-adrenergic receptors by Rab1 GTPase in cardiac myocytes. Mol Pharmacol. 2006;69(5):1571–1578. doi: 10.1124/mol.105.019984. pii:mol.105.019984. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. pii:0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague C, Chen Z, Pupo AS, Schulte NA, Toews ML, Minneman KP. The N terminus of the human alpha1D-adrenergic receptor prevents cell surface expression. J Pharmacol Exp Ther. 2004;309(1):388–397. doi: 10.1124/jpet.103.060509. pii:jpet.103.060509. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. A novel sorting sequence in the beta2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. J Biol Chem. 2007;282(5):3095–3104. doi: 10.1074/jbc.M605398200. pii:M605398200. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev. pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Heilker R, Manning-Krieg U, Zuber JF, Spiess M. In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO J. 1996;15(11):2893–2899. [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lui WWY, Bright NA, Totty N, Seaman MNJ, Robinson MS. A family of proteins with {gamma}-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J Cell Biol. 2000;149(1):67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JC, Suzuki N, Pessin JE, Watson RT. A specific dileucine motif is required for the GGA-dependententry of newly synthesized insulin-responsive aminopeptidase into the insulin-responsive compartment. J Biol Chem. 2006;281(44):33457–33466. doi: 10.1074/jbc.M601583200. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13(13):2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KF, Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol. 1992;119(2):249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing drosophila photoreceptors. J Cell Biol. 2007;177(4):659–669. doi: 10.1083/jcb.200610157. pii:jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori LT, Florencia BS, Frederick RM. Role of an acidic cluster/dileucine motif in cation-independent mannose 6-phosphate receptor traffic. Traffic. 2007;8(4):402–413. doi: 10.1111/j.1600-0854.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- Luo W, Wang Y, Reiser G. p24A, A type I transmembrane protein, controls ARF1-dependent resensitization of protease-activated receptor-2 by influence on receptor trafficking. J Biol Chem. 2007;282(41):30246–30255. doi: 10.1074/jbc.M703205200. pii:M703205200. [DOI] [PubMed] [Google Scholar]
- Luo W, Wang Y, Reiser G. Proteinase-activated receptors, nucleotide P2Y receptors, and mu-opioid receptor-1B are under the control of the type I transmembrane proteins p23 and p24A in post-Golgi trafficking. J Neurochem. 2011;117(1):71–81. doi: 10.1111/j.1471-4159.2011.07173.x. [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. Role of ER export signals in controlling surface potassium channel numbers. Science. 2001;291(5502):316–319. doi: 10.1126/science.291.5502.316. pii:291/5502/316. [DOI] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BRS, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48(1):601–629. doi: 10.1146/annurev. pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28(3):183–192. doi: 10.1038/emboj.2008.267. pii:emboj2008267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic xenopus rods. Mol Biol Cell. 2001;12(8):2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. pii:S0092-8674(07)00534-X. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277(5325):556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Bannykh S, Slabough S, Matteson J, Altschuler Y, Hahn K, Balch WE. A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J Biol Chem. 1999;274(22):15937–15946. doi: 10.1074/jbc.274.22.15937. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Plutner H, Hahn K, Balch WE. The delta subunit of AP-3 is required for efficient transport of VSV-G from the trans-Golgi network to the cell surface. Proc Natl Acad Sci U S A. 2002;99(10):6755–6760. doi: 10.1073/pnas.092150699. pii:092150699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001a;292(5522):1712–1716. doi: 10.1126/science.1060750. pii:292/5522/1712. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001b;105(1):93–102. doi: 10.1016/s0092-8674(01)00299-9. pii:S0092-8674(01) 00299-9. [DOI] [PubMed] [Google Scholar]
- Rands E, Candelore MR, Cheung AH, Hill WS, Strader CD, Dixon RA. Mutational analysis of beta-adrenergic receptor glycosylation. J Biol Chem. 1990;265(18):10759–10764. [PubMed] [Google Scholar]
- Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, Harada R, Harada A. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448(7151):366–369. doi: 10.1038/nature05929. pii:nature05929. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O’Tousa JE, Ozaki K, Ready DF. Rab11 Mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of drosophila photoreceptors. Development. 2005;132(7):1487–1497. doi: 10.1242/dev.01704. pii:dev.01704. [DOI] [PubMed] [Google Scholar]
- Saunders C, Limbird LE. Disruption of microtubules reveals two independent apical targeting mechanisms for G-protein-coupled receptors in polarized renal epithelial cells. J Biol Chem. 1997;272(30):19035–19045. doi: 10.1074/jbc.272.30.19035. [DOI] [PubMed] [Google Scholar]
- Sawyer GW, Ehlert FJ, Shults CA. A conserved motif in the membrane proximal C-terminal tail of human muscarinic m1 acetylcholine receptors affects plasma membrane expression. J Pharmacol Exp Ther. 2010;332(1):76–86. doi: 10.1124/jpet.109.160986. pii:jpet.109.160986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulein R, Hermosilla R, Oksche A, Dehe M, Wiesner B, Krause G, Rosenthal W. A dileucine sequence and an upstream glutamate residue in the intracellular carboxyl terminus of the vasopressin V2 receptor are essential for cell surface transport in COS.M6 Cells. Mol Pharmacol. 1998;54(3):525–535. doi: 10.1124/mol.54.3.525. [DOI] [PubMed] [Google Scholar]
- Sevier CS, Weisz OA, Davis M, Machamer CE. Efficient export of the vesicular stomatitis virus G protein from the endoplasmic reticulum requires a signal in the cytoplasmic tail that includes both tyrosine-based and di-acidic motifs. Mol Biol Cell. 2000;11(1):13–22. doi: 10.1091/mbc.11.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A. ARF1 Regulatory factors and COPI vesicle formation. Curr Opin Cell Biol. 2002;14(4):423–427. doi: 10.1016/s0955-0674(02)00346-0. pii:S0955067402003460. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain tctex-1. Cell. 1999;97(7):877–887. doi: 10.1016/s0092-8674(00)80800-4. pii:S0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5(1):30–34. doi: 10.1038/sj.embor.7400052. pii:7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Matteson J, An Y, Moyer B, Yoo JS, Bannykh S, Wilson IA, Riordan JR, Balch WE. COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a di-acidic exit code. J Cell Biol. 2004;167(1):65–74. doi: 10.1083/jcb.200401035. pii:jcb.200401035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wu G. Small GTPase regulation of GPCR anterograde trafficking. Trends Pharmacol Sci. 2012;33(1):28–34. doi: 10.1016/j.tips.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol. 2001;155(4):557–570. doi: 10.1083/jcb.200107045. pii:jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G protein-coupled receptor. J Biol Chem. 2003;278(47):47062–47069. doi: 10.1074/jbc.M305707200. pii:M305707200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong C, Wu QJ, Balch WE, Wu G. Di-acidic motifs in the membrane-distal C termini modulate the transport of angiotensin II receptors from the endoplasmic reticulum to the cell surface. J Biol Chem. 2011;286(23):20525–20535. doi: 10.1074/jbc.M111.222034. pii:M111.222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang G, Dupre DJ, Feng Y, Robitaille M, Lazartigues E, Feng Y-H, Hebert TE, Wu G. Rab1 GTPase and dimerization in the cell surface expression of angiotensin II type 2 receptor. J Pharmacol Exp Ther. 2009;330(1):109–117. doi: 10.1124/jpet.109.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Imanishi Y, Filipek S, Alekseev A, Jastrzebska B, Sun W, Saperstein DA, Palczewski K. Autosomal recessive retinitis pigmentosa and E150K mutation in the opsin gene. J Biol Chem. 2006;281(31):22289–22298. doi: 10.1074/jbc.M602664200. pii:M602664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Doray B, Poussu A, Lehto VP, Kornfeld S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292(5522):1716–1718. doi: 10.1126/science.1060896. pii:292/5522/1716. [DOI] [PubMed] [Google Scholar]
- Zuzarte M, Rinne S, Schlichthorl G, Schubert A, Daut J, Preisig-Muller R. A di-acidic sequence motif enhances the surface expression of the potassium channel TASK-3. Traffic. 2007;8(8):1093–1100. doi: 10.1111/j.1600-0854.2007.00593.x. pii:TRA593. [DOI] [PubMed] [Google Scholar]