Abstract
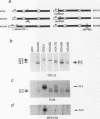
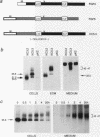
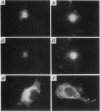
The fibroblast growth factors (FGFs) fall into two distinct groups with respect to their mode of release from cells. Whereas FGF1 and FGF2 lack conventional signal peptides, the remaining members have typical features of secreted proteins. However, the behavior of mouse FGF3 is anomalous, since, despite entering the secretory pathway and undergoing primary glycosylation, its release from transfected COS-1 cells is very inefficient compared with that of FGF4 and FGF5. To investigate the unusual properties of FGF3, we analyzed the processing, secretion, and intracellular localization of a series of site-directed mutants as well as chimeras produced by fusing parts of FGF3, FGF4, and FGF5. Wild-type FGF3 was shown to accumulate in an immature form in the Golgi complex, from where it is slowly released into the extracellular matrix. Removing or relocating the Asn-linked glycosylation site further impaired its release, and exchanging the signal peptide or carboxy terminus had little effect. In contrast, a chimeric protein with an amino terminus from FGF5 was efficiently secreted and biologically active in cell transformation assays. The data suggest that a structural feature of FGF3 involving the amino-terminal region and glycosylation site has a significant bearing on its passage through the Golgi complex and may regulate the secretion of the ligand.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Baird A., Klagsbrun M. The fibroblast growth factor family. Cancer Cells. 1991 Jun;3(6):239–243. [PubMed] [Google Scholar]
- Bashkin P., Doctrow S., Klagsbrun M., Svahn C. M., Folkman J., Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989 Feb 21;28(4):1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- Bates B., Hardin J., Zhan X., Drickamer K., Goldfarb M. Biosynthesis of human fibroblast growth factor-5. Mol Cell Biol. 1991 Apr;11(4):1840–1845. doi: 10.1128/mcb.11.4.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blam S. B., Mitchell R., Tischer E., Rubin J. S., Silva M., Silver S., Fiddes J. C., Abraham J. A., Aaronson S. A. Addition of growth hormone secretion signal to basic fibroblast growth factor results in cell transformation and secretion of aberrant forms of the protein. Oncogene. 1988 Aug;3(2):129–136. [PubMed] [Google Scholar]
- Bradley R. S., Brown A. M. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990 May;9(5):1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Papkoff J., Fung Y. K., Shackleford G. M., Varmus H. E. Identification of protein products encoded by the proto-oncogene int-1. Mol Cell Biol. 1987 Nov;7(11):3971–3977. doi: 10.1128/mcb.7.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Basilico C. Isolation of a rearranged human transforming gene following transfection of Kaposi sarcoma DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5660–5664. doi: 10.1073/pnas.84.16.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Deed R., Dixon M., Peters G. The structure and function of the int-2 oncogene. Prog Growth Factor Res. 1989;1(3):123–132. doi: 10.1016/0955-2235(89)90006-9. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Dixon M., Deed R., Acland P., Moore R., Whyte A., Peters G., Dickson C. Detection and characterization of the fibroblast growth factor-related oncoprotein INT-2. Mol Cell Biol. 1989 Nov;9(11):4896–4902. doi: 10.1128/mcb.9.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Kaufman R. J. Analysis of synthesis, processing, and secretion of proteins expressed in mammalian cells. Methods Enzymol. 1990;185:577–596. doi: 10.1016/0076-6879(90)85046-q. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmentation of asparagine-linked oligosaccharide processing in the Golgi apparatus. J Cell Biol. 1983 Jul;97(1):270–275. doi: 10.1083/jcb.97.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Yokota S., Takatsuki A., Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988 Dec 5;263(34):18545–18552. [PubMed] [Google Scholar]
- Fuller-Pace F., Peters G., Dickson C. Cell transformation by kFGF requires secretion but not glycosylation. J Cell Biol. 1991 Oct;115(2):547–555. doi: 10.1083/jcb.115.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Deed R., MacAllan D., Walther W., Dickson C., Peters G. Cell transformation by Int-2--a member of the fibroblast growth factor family. Oncogene. 1991 Jan;6(1):65–71. [PubMed] [Google Scholar]
- Goldfarb M. The fibroblast growth factor family. Cell Growth Differ. 1990 Sep;1(9):439–445. [PubMed] [Google Scholar]
- Guan J. L., Machamer C. E., Rose J. K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985 Sep;42(2):489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Haub O., Drucker B., Goldfarb M. Expression of the murine fibroblast growth factor 5 gene in the adult central nervous system. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8022–8026. doi: 10.1073/pnas.87.20.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert J. M., Basilico C., Goldfarb M., Haub O., Martin G. R. Isolation of cDNAs encoding four mouse FGF family members and characterization of their expression patterns during embryogenesis. Dev Biol. 1990 Apr;138(2):454–463. doi: 10.1016/0012-1606(90)90211-z. [DOI] [PubMed] [Google Scholar]
- Jackson A., Friedman S., Zhan X., Engleka K. A., Forough R., Maciag T. Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10691–10695. doi: 10.1073/pnas.89.22.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer P., Peters G., Dickson C. The Int-2/Fgf-3 oncogene product is secreted and associates with extracellular matrix: implications for cell transformation. Mol Cell Biol. 1991 Dec;11(12):5929–5936. doi: 10.1128/mcb.11.12.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajewski J., Mason J. O., Varmus H. E. Interaction of Wnt-1 proteins with the binding protein BiP. Mol Cell Biol. 1992 Feb;12(2):784–790. doi: 10.1128/mcb.12.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Baird A. A dual receptor system is required for basic fibroblast growth factor activity. Cell. 1991 Oct 18;67(2):229–231. doi: 10.1016/0092-8674(91)90173-v. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Influence of new glycosylation sites on expression of the vesicular stomatitis virus G protein at the plasma membrane. J Biol Chem. 1988 Apr 25;263(12):5948–5954. [PubMed] [Google Scholar]
- Marics I., Adelaide J., Raybaud F., Mattei M. G., Coulier F., Planche J., de Lapeyriere O., Birnbaum D. Characterization of the HST-related FGF.6 gene, a new member of the fibroblast growth factor gene family. Oncogene. 1989 Mar;4(3):335–340. [PubMed] [Google Scholar]
- Merlo G. R., Blondel B. J., Deed R., MacAllan D., Peters G., Dickson C., Liscia D. S., Ciardiello F., Valverius E. M., Salomon D. S. The mouse int-2 gene exhibits basic fibroblast growth factor activity in a basic fibroblast growth factor-responsive cell line. Cell Growth Differ. 1990 Oct;1(10):463–472. [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Bottaro D. P., Rubin J. S., Ron D., Aaronson S. A. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science. 1991 Jan 4;251(4989):72–75. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- Miyagawa K., Sakamoto H., Yoshida T., Yamashita Y., Mitsui Y., Furusawa M., Maeda S., Takaku F., Sugimura T., Terada M. hst-1 transforming protein: expression in silkworm cells and characterization as a novel heparin-binding growth factor. Oncogene. 1988 Oct;3(4):383–389. [PubMed] [Google Scholar]
- Miyazono K., Thyberg J., Heldin C. H. Retention of the transforming growth factor-beta 1 precursor in the Golgi complex in a latent endoglycosidase H-sensitive form. J Biol Chem. 1992 Mar 15;267(8):5668–5675. [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Niswander L., Martin G. R. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992 Mar;114(3):755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Tulsiani D. R., Touster O., Yam A., Novikoff A. B. Immunocytochemical localization of alpha-D-mannosidase II in the Golgi apparatus of rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4364–4368. doi: 10.1073/pnas.80.14.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J. Inducible overexpression and secretion of int-1 protein. Mol Cell Biol. 1989 Aug;9(8):3377–3384. doi: 10.1128/mcb.9.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990 Jun;10(6):2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterno G. D., Gillespie L. L., Dixon M. S., Slack J. M., Heath J. K. Mesoderm-inducing properties of INT-2 and kFGF: two oncogene-encoded growth factors related to FGF. Development. 1989 May;106(1):79–83. doi: 10.1242/dev.106.1.79. [DOI] [PubMed] [Google Scholar]
- Quarto N., Talarico D., Sommer A., Florkiewicz R., Basilico C., Rifkin D. B. Transformation by basic fibroblast growth factor requires high levels of expression: comparison with transformation by hst/K-fgf. Oncogene Res. 1989;5(2):101–110. [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature. 1988 Jan 14;331(6152):173–175. doi: 10.1038/331173a0. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Mori M., Taira M., Yoshida T., Matsukawa S., Shimizu K., Sekiguchi M., Terada M., Sugimura T. Transforming gene from human stomach cancers and a noncancerous portion of stomach mucosa. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3997–4001. doi: 10.1073/pnas.83.11.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Yoshida T., Nakakuki M., Odagiri H., Miyagawa K., Sugimura T., Terada M. Cloned hst gene from normal human leukocyte DNA transforms NIH3T3 cells. Biochem Biophys Res Commun. 1988 Mar 30;151(3):965–972. doi: 10.1016/s0006-291x(88)80460-1. [DOI] [PubMed] [Google Scholar]
- Sasada R., Kurokawa T., Iwane M., Igarashi K. Transformation of mouse BALB/c 3T3 cells with human basic fibroblast growth factor cDNA. Mol Cell Biol. 1988 Feb;8(2):588–594. doi: 10.1128/mcb.8.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico D., Basilico C. The K-fgf/hst oncogene induces transformation through an autocrine mechanism that requires extracellular stimulation of the mitogenic pathway. Mol Cell Biol. 1991 Feb;11(2):1138–1145. doi: 10.1128/mcb.11.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]
- Vlodavsky I., Bar-Shavit R., Ishai-Michaeli R., Bashkin P., Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991 Jul;16(7):268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Lui G. M., Gospodarowicz D. Morphological appearance, growth behavior and migratory activity of human tumor cells maintained on extracellular matrix versus plastic. Cell. 1980 Mar;19(3):607–616. doi: 10.1016/s0092-8674(80)80037-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., McMahon A. P. Expression pattern of the FGF-related proto-oncogene int-2 suggests multiple roles in fetal development. Development. 1989 Jan;105(1):131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Peters G., Dickson C., McMahon A. P. Expression of the FGF-related proto-oncogene int-2 during gastrulation and neurulation in the mouse. EMBO J. 1988 Mar;7(3):691–695. doi: 10.1002/j.1460-2075.1988.tb02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M. Autocrine transformation by chimeric signal peptide-basic fibroblast growth factor: reversal by suramin. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5346–5350. doi: 10.1073/pnas.87.14.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon J., Fried M. Precise gene fusion by PCR. Nucleic Acids Res. 1989 Jun 26;17(12):4895–4895. doi: 10.1093/nar/17.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Culpepper A., Reddy M., Loveless J., Goldfarb M. Human oncogenes detected by a defined medium culture assay. Oncogene. 1987;1(4):369–376. [PubMed] [Google Scholar]