Abstract
This chapter provides a short review of various biophysical experiments that have been applied to the inhibitor of kappa B, IκBα, and its binding partner, nuclear factor kappa B, or NF-κB. The picture that emerges from amide hydrogen/deuterium exchange, NMR, and binding kinetics experiments is one in which parts of both proteins are “fuzzy” in the free-state, and some parts remain “fuzzy” in the NF-κB•IκBα complex. The NF-κB family of transcription factors responds to inflammatory cytokines with rapid transcriptional activation, in which NF-κB enters the nucleus and binds DNA. Just as rapidly as transcription is activated, it is subsequently repressed by newly synthesized IkBa that also enters the nucleus and removes NF-κB from the DNA. Because IkBa is an ankyrin repeat protein, it’s “fuzziness” can be controlled by mutagenesis to stabilized the folded state. Experimental comparison with such stabilized mutants helps provide evidence that much of the system control depends on the “fuzziness” of IκBα.
Introduction
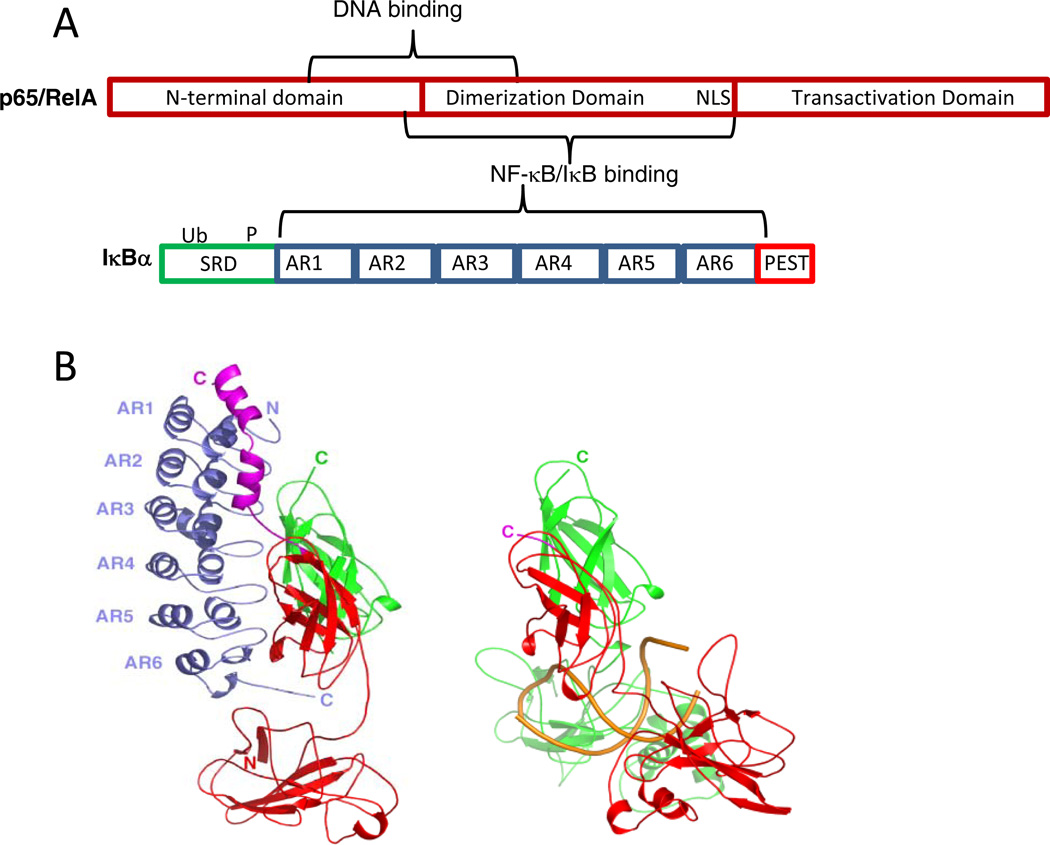
The nuclear factor κB (NF-κB) pathway transduces extra-cellular signals from various receptors to regulate patterns of gene expression 1. Although originally discovered in B-cells because it strongly activates the immunoglobulin kappa-chain gene expression 2, the pathway is ubiquitous, and has been implicated in a variety of cellular functions such as cell growth, proliferation, apoptosis, and stress responses and is missregulated in numerous diseases 3,4. The family of NF-κB proteins includes p65 (RelA), RelB, c-Rel, p50, and p52 subunits, which form homo- and heterodimers 3 (Figure 1A). The most prevalent form in most cell types is a p50/p65 heterodimer, and the crystal structure of this form bound to a canonical kB DNA sequence has been solved 5 (Figure 1B). The inhibitors of NF-κB activity, IκBs, include isoforms IκBα, IκBβ, and IκBε, which block the nuclear localization and transcriptional activity of p65 and c- Rel-containing NF-κB dimers 6 and others that act in different pathways such as IκBδ 7 (Figure 1A). In resting cells, approximately 100,000 NF-κB dimers are nearly all bound to IκBs which keep the NF-κB in the cytoplasm by sequestering the NF-κB nuclear localization signal (NLS) 8,9. The way in which the IκBα binds NF-κB was revealed from crystal structures of the NF-κB•IκBα 10,11.
Figure 1.
(A) Schematic diagram of NF-κB(p65) one of the most abundant NF-κB family members in the cell and of IκBα, the key member of the inhibitor family. (B) LEFT: The crystal structure of IκBα (blue) bound to NF-κB (p50, green; p65, red) 11. RIGHT: The crystal structure of NF-κB (p50, green; p65, red) bound to κB site DNA (gold) 5. (Figure prepared using PyMOL 42).
When a cell receives an extracellular signal such as a viral insult or cytokine, extracellular receptors activate the assembly of the IκB kinase (IKK), which in turn phosphorylates the N-terminal signal response domain of NF-κB-bound IκBα, leading to subsequent ubiquitination and degradation of the IκBα by the proteasome 12. NF-κB dimers then translocate to the nucleus, bind DNA, and regulate transcription of numerous NF-κB target genes 13. NF-κB activated genes show widely varying transcription levels, activation kinetics, and post-induction repression, but how this single system results in so many different transcription effects is not well understood 14,15. The gene coding for IκBα is one of the strongly NF-κB-activated genes 16–18. When NF-κB transcription is activated, the resulting newly synthesized IκBα translocates to the nucleus, binds to NF-κB, and the complex is exported from the nucleus 1,19.
Free IκBα is rapidly degraded by a proteasome-dependent but ubiquitinin-dependent mechanism, with a half-life is less than 10 min. On the other hand, NF-κB-bound IκBα is incredibly stable, with an intracellular half-life of many hours consistent with the binding constant of the complex, of 40 pM 9,20. The IκBα in complex with NF-κB is only degraded if it is first phosphorylated, then ubiquitinated, and finally degraded by the proteasome in a ubiquitin-dependent fashion resulting in free, active NF-κB. A wealth of experimental data now suggests that the various functions of IκBα depend the partially folded character or “fuzziness” of parts of NF-κB and IκBα. These functions include the rapid degradation of the free protein, its tight binding to NF-κB, and its ability to dissociate NF-κB from the DNA. This review will briefly summarize the evidence for “fuzziness” and show the functional consequences of “fuzziness” in the NF-κB signaling system.
EXPERIMENTAL EVIDENCE OF IκBα “FUZZINESS”
NMR evidence of IκBα "fuzziness"
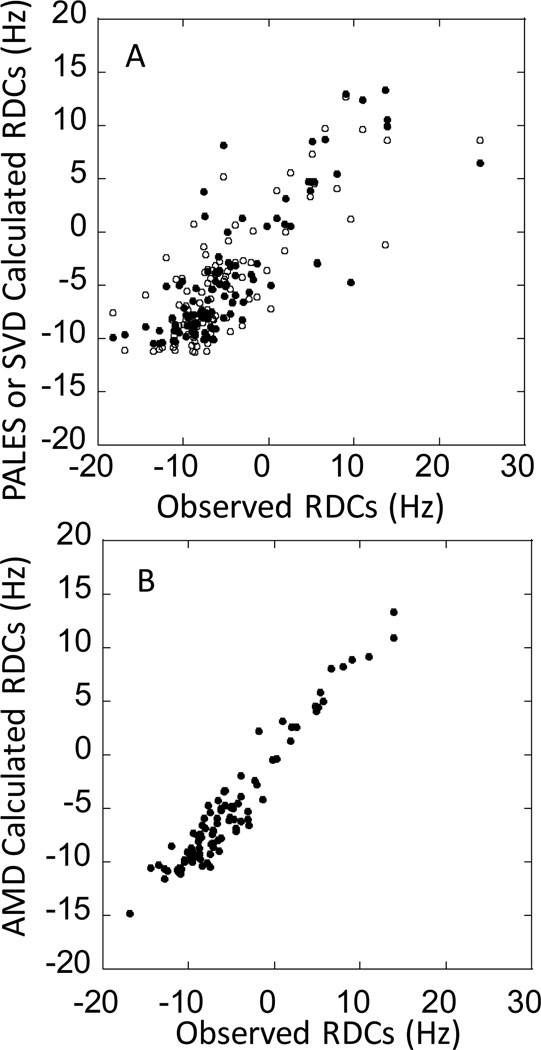
NMR studies of the entire 6-AR ARD of IκBα, residues 67–287, revealed that most of the cross peaks for AR5 and AR6 were missing, which likely indicated conformational exchange processes (CF Cervantes, unpublished data). In contrast, all of the resonances could be assigned for the 4-AR fragment containing residues 67–206 21. When the chemical shift values for these residues were compared to those of the NF-κB-bound IκBα(67–287), they were nearly identical indicating that the structure of this part of IκBα is nearly identical in the free and bound states 22. In addition, backbone dynamics experiments indicated that this part of the IκBα ARD is rigidly structured 21,22. Residual dipolar coupling (RDC) measurements were also performed on this fragment. RDCs predicted from the crystal structure of this part of IκBα bound to NF-κB did not agree well with the measured values and we surmised that this might be due to they report on motions from microseconds to hundreds of milliseconds (Figure 2A) 21. Indeed, RDCs computed for an ensemble of structures of the IκBα(67–206) generated from accelerated molecular dynamics simulations agreed much better with the experimental data (Figure 2B). Thus, RDC measurements combined with AMD simulations might more realistically represent the solution ensemble in all its “fuzziness”.
Figure 2.
(A) Observed vs. theoretical residual dipolar couplings measured by the program PALES for IκBα(67–206) (closed symbols) and SVD (open symbols) using the crystal structure of the IκBα•NF-κB complex (PDB accession code 1IKN, 10). (B) Observed vs. AMD-calculated residual dipolar couplings for IκBα(67–206). The RDCs were measured as previously described 21.
Amide hydrogen/deuterium exchange of the IκBα ARD
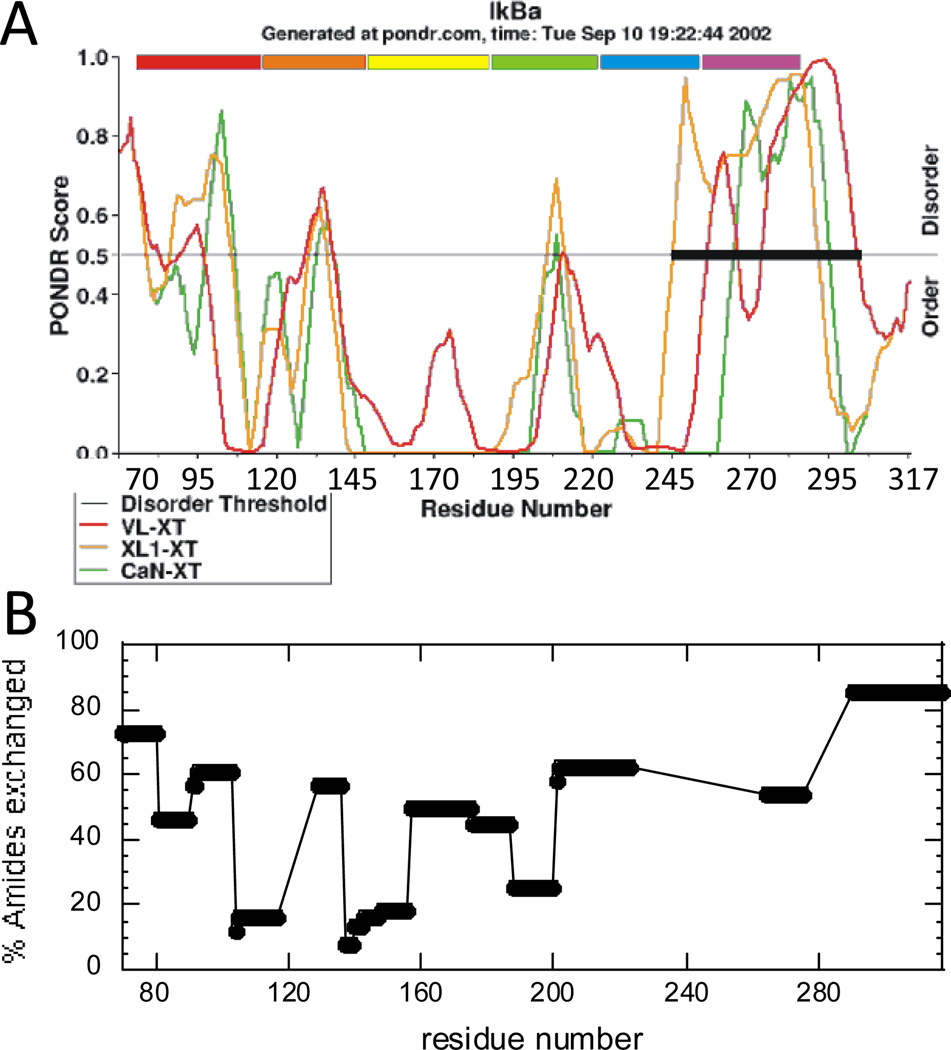
Full-length IκBα is composed of three regions; an N-terminal signal response region of ~70 amino acids, where phosphorylation and ubiquitination occur, an ankyrin repeat domain (ARD) of ~220 amino acids, and a C-terminal PEST sequence that extends from residues 275–317 10,11. The NF-κB binding activity is localized to the ARD and PEST regions, for which high resolution crystal structures were obtained only when in complex with NF-κB. Sequence analyses predict intrinsic disorder in both the N-terminal domain and the PEST region of IκBα as well as in a good portion of the ARD (Figure 1A) 23. IκBα has resisted all attempts to crystallize it in the unbound state, and its biophysical behavior is consistent with a native state that does not adopt a unique compact fold 24. It is interesting to note the qualitative agreement between the the predicted disorder (Figure 3A) and the native state amide exchange (plotted as percent exchanged at 2 min) (Figure 3B).
Figure 3.
(A) PONDR 23 analysis of the intrinsic disorder in the ankyrin repeat domain of IκBα. (B) Summary of native state amide H/D exchange results on free IκBα. IκBα(67–317) was allowed to exchange for 2 min, and after quenching, the protein was digested with pepsin and the amount of exchange in each peptide was analyzed by MALDI mass spectrometry 24.
Both IκBα and NF-κB fold on binding
The NF-κB NLS folds on binding to IκBα
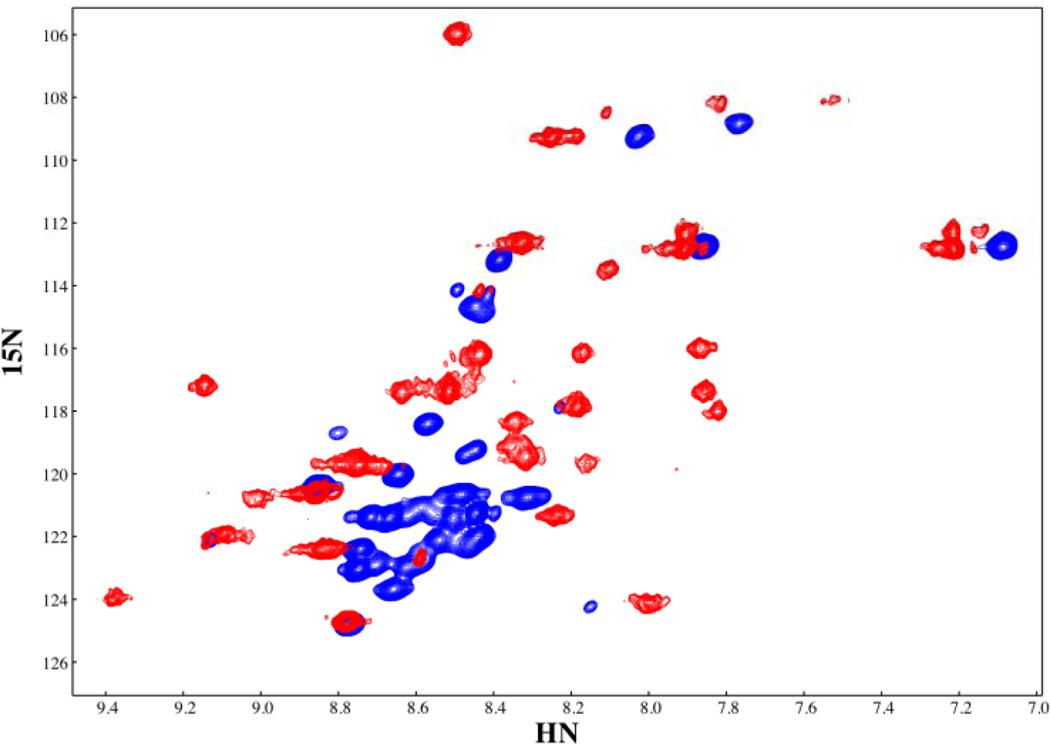
NF-κB family members such as the canonical member of the family, RelA, contain three main domains, the N-terminal domain, the dimerization domain, and the transactivation domain. IκBα binds primarily to the dimerization domain whereas DNA binds in between the N-terminal and dimerization domains (Figure 1A). The crystal structure of the NF-κB•IκBα complex shows that IκBα binds to NF-κB in a head-to-tail fashion and the small sequence of NF-κB containing the nuclear localization signal (NLS) forms two short helices that lay over the top of AR1 of IκBα (Figure 1B) 10,11. The NLS connects the dimerization domain to the transactivation domain in the full length NF-κB(p65) protein. In between the two helices is the KRKR sequence, which constitutes the minimal NLS 11. Theoretical studies of the binding of the NLS polypeptide (residues 291–325) of NF-κB(p65) to IκBα also suggested that this segment of NF-κB folds on binding to IκBα 25. NMR heteronuclear single quantum coherence spectra of the NLS (residues 289–321 of NF-κB (p65) clearly show that in the free-state the chemical shifts of the NLS backbone NHs are not well-dispersed and are mostly at random coil chemical shift values. In contrast, when bound to IκBα, the chemical shifts are well-dispersed and at values expected for helical structure (Figure 4) (C.F. Cervantes, submitted). It has been experimentally observed that this segment binds with a 1µM KD to IκBα, and with a large ΔCP,obs for IκBα binding to this NLS segment (−1.30 ± 0.03 kcal mol−1 K−1) that could not be accounted for by burial of polar and non polar surface area calculations derived from the crystal structures 26–28. Thus, the thermodynamic signatures of the binding interaction cannot be accounted for by merely docking the individucal static structures, and larger structural rearrangements must be implicated, as is often observed for protein-DNA interactions 29. Chemical shift values in the random coil region are a good indicator of lack of persistent structure in the free NLS, which must, therefore, be more “fuzzy” in the free-state than in the bound state where persistent helical structure is observed. The “head” of IκBα (ARs 1–3) appears to be folded based on H/D exchange experiments, and the “tail” of NF-κB (the NLS polypeptide) folds upon binding to it.
Figure 3.
The HSQC spectra of free and IκBα-bound p65(289–321). The secondary chemical shifts of bound p65(289–321) show the characteristic positive values characteristic of helical regions, conforming to the helical areas seen in these same residues in the crystal structure of the IκBα•NF-κB complex (PDB accession code 1NFI, 11). The chemical shifts for free p65(289–321) are indicative of an unfolded peptide.
IκBα folds on binding to NF-κB
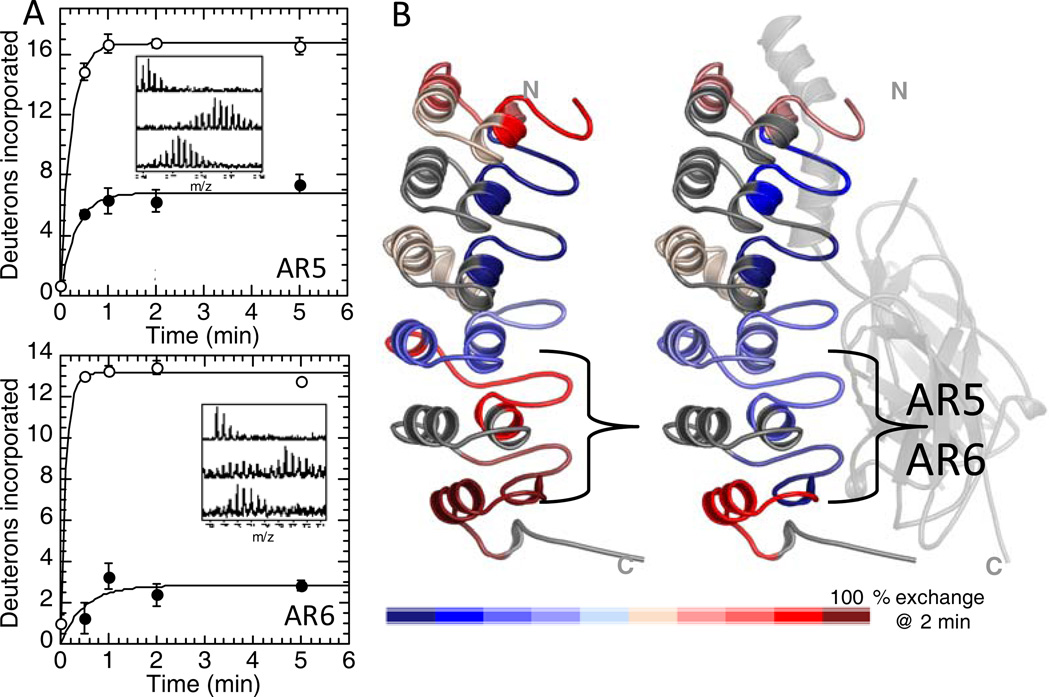
Native state amide H/D exchange experiments revealed that the fifth and sixth ARs exchange all of their amides within 2 minutes whereas the β-hairpins of AR2 and AR3 were remarkably resistant to exchange. (Figure 5) 30. The decrease in the number of exchanging amides could not be accounted for just by interface protection suggesting that IκBα undergoes a folding transition upon binding. Amide exchange is an interesting probe of “fuzziness”. Although the rate of amide exchange depends on many factors that often cannot be teased apart, it can reliably report on relative differences. Thus, it is possible to compare the β-turns of each AR relative to one another as we did for the free protein, and it is also possible to compare the β-turn of a particular AR in the free vs. NF-κB-bound state. In this latter case, one must consider the decrease in amide exchange due to decreased solvent accessibility at the protein-protein interface. In the case of IκBα, we compared the solvent accessible surface areas calculated from the crystal structure of the NF-κB•IκBα complex to the results from amide exchange. These comparisons revealed that whereas the difference in exchange between bound and free was some 10 amides, the solvent accessible surface area was expected to change only by 2–3 amides (Figure 5) 31. The large difference in amide exchange upon binding should therefore be attributed to differences in protein dynamics or “fuzziness” of the free protein that is lost upon binding.
Figure 5.
(A) Native state amide H/D exchange data for the region of IκBα corresponding to the β-turn of AR5 (TOP) and AR6 (bottom) in the free-state (open circles) and in the NF-κB-bound state (closed circles). (B) Structural summary of the amide H/D exchange data (red is highly exchanging and blue is slowly exchanging) for IκBα in the free state (LEFT) and in the NF-κB-bound state (RIGHT) showing that exchange is similar for most of the IκBα molecule in each state, except for the β-turns of AR5 and AR6 that are exchanging much less in the bound state.
REMAINING FUZZINESS IN THE NF-κB•IκBα COMPLEX
At the other end of the IκBα ARD, deletion of the PEST sequence (residues 276–287) reduces the NF-κB binding by some 5 kcal/mol 32. Taken together, the binding affinity losses due to deletion at the ends of the interface are more than enough to account for the entire binding energy of complex formation. Interestingly, the PEST region does not become completely ordered upon binding to NF-κB according to high resolution NMR spectroscopy data 33. The native state of the NF-κB•IκBα complex thus retains regions with highly dynamic character.
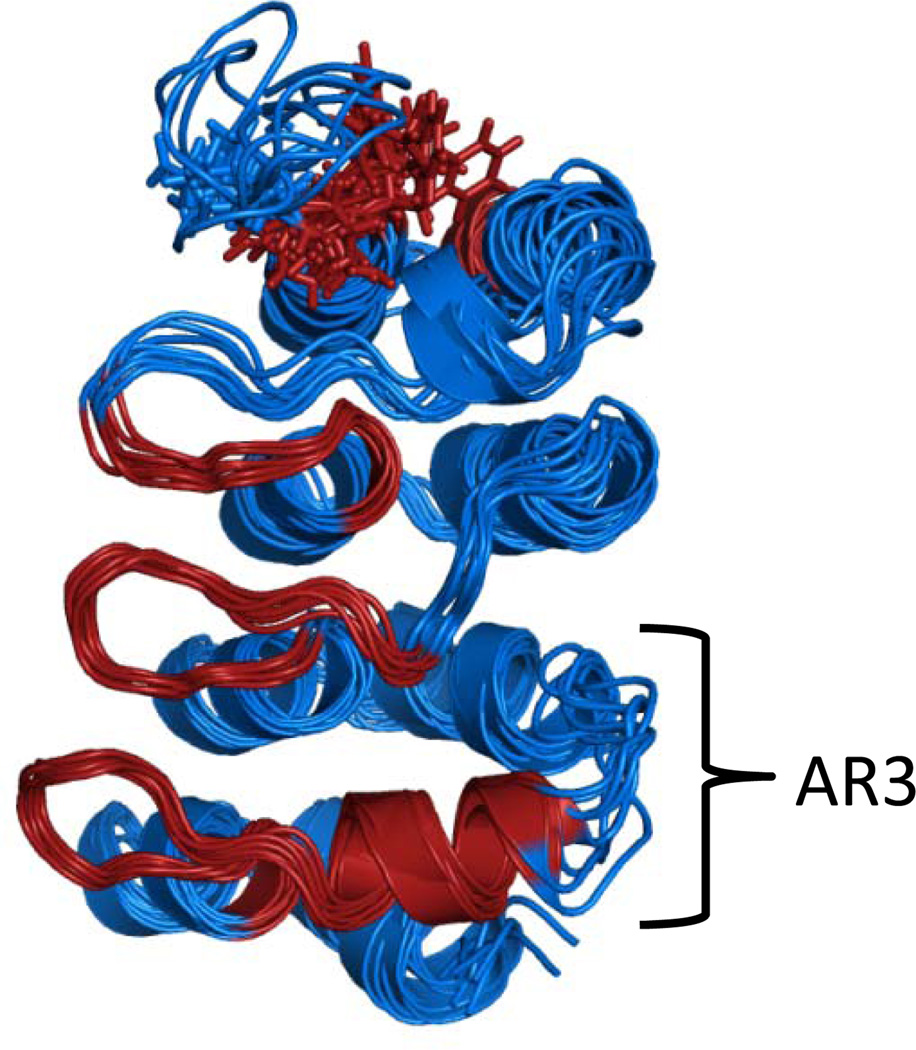
NMR experiments on the NF-κB•IκBα complex revealed another interesting feature. Although amide exchange was very low in AR2 and AR3, NMR relaxation experiments on IκBα(67–206) indicated that the backbone of AR3 was more dynamic than was observed for the other ARs 21. By finding the best AMD acceleration that matched the experimental RDCs, we were able to obtain a “picture” of the ensemble of structures that represents the solution state of the first four ARs of the IκBα ARD (Figure 6). It is clear that the variable loop connecting AR3 to AR4 is more “fuzzy” and the NMR data also indicated conformational exchange dynamics on the msec to msec time scale in the β-loop and helix of AR3 (colored red in Figure 6) 21. NMR experiments further revealed that the dynamics observed in AR3 of free IκBα become even more excentuated upon binding to NF-κB. In the complex, many of the cross peaks for AR3 are not observed indicating strong conformational exchange to a range of chemical shift values making the peaks so broad as to become unobservable under the conditions of the experiment 22. These results strongly support the idea that while some parts of IκBα (AR5 and AR6) become less “fuzzy” in the complex, other parts become more “fuzzy”.
Figure 6.
Structural ensemble of IκBα(67–206) showing representative structures from the AMD simulation. The molecule is colored red where NMR experiments indicated residues were undergoing conformational exchange (Rex) 21.
FUNCTIONAL CONSEQUENCES OF FUZZINESS IN THE NF-κB•IκBα COMPLEX
Tight binding to multiple partners
NF-κB is a family of homo and heterodimeric molecules made of at least five different proteins. While the most prevalent form in many cell types is NF-κB(p50/p65), under certain conditions, the homodimeric form NF- κB(p65/p65) also becomes prevalent 34. The structure of this form of NF-κB bound to IκBβ has been solved, and it is remarkably similar to the structure of NF-κB(p50/p65) bound to IκBα 35. This is despite the fact that the sequences are not that similar. Kinetic and thermodynamic measurements of binding affinity show that IκBα binds to NF-κB(p50/p65) with nearly the same affinity as to NF-κB(p65/p65) 9.
“Fuzziness” determines the degradation rate of IκBα
Free IκBα, which is marginally stable 24, has a very short intracellular half-life of less than 10 minutes 20,36. This rapid degradation rate depends in part on the presence of the C-terminal PEST sequence 37–39. The degradation of the free protein appears to be independent of ubiquitinylation, since all of the Lys residues in IκBα can be mutated without changing the degradation rate of the free protein 39. In addition, although free IκBα can be phosphorylated and ubiquitinylated, its degradation rate is not different in IKK−/− cells indicating that ubiquitin-independent degradation is the primary route for free IκBα 20.
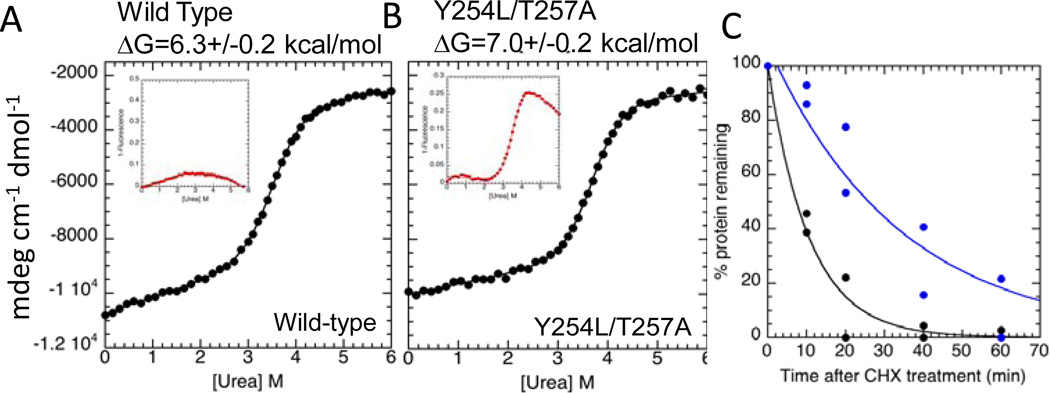
To further probe how “fuzziness” was related to free IκBα degradation rate, we prepared a mutant in which two residues in AR6 were mutated to more commonly-found residues at those positions in other AR sequences. Taking advantage of the single tryptophan residue in IκBα at position 258, we showed that in the wild type protein, W258 did not show a cooperative transition whereas in the mutant it did (Figure 7A, B). This is a strong indication that AR6 is not part of the cooperatively folding ARD in wild type IκBα, but the mutations stabilize AR6 so that now the fluorescence signal from W258 follows the major cooperative transition (Figure 7B). Importantly, the Y254L, T257A mutant IκBα is degraded more slowly than wild-type IκBα both in vitro by the 20S proteasome and in vivo suggesting that in addition to the PEST sequence, the “fuzzy” AR6 of IκBα is important for rapid ubiquitin-independent degradation (Figure 7C) 40. In contrast to the marginally-stable free IκBα, the IκBα•NF-κB complex has a very long intracellular half-life of the complex, which is completely stable in the absence of IκB kinase (IKK) phosphorylation and subsequent ubiquitinylation (>12 hrs). Thus, IκBα “fuzziness”, controlled by binding to NF-κB, switches its degradation mechanisms 39.
Figure 7.
(A) Equilibrium unfolding experiments with wild type IκBα and (B) Y254L,T257A mutant IκBα. The insets show the change in fluorescence of W258, a naturally-occurring Trp258 in AR6. In the wild type protein, this residue does not change fluorescence appreciably with denaturant, however in the stabilized mutant, its fluorescence changes in a manner similar to the CD signal indicating it follows the major cooperative folding transition of the protein. (C) IκB isoforms were measured by quantitative Western blot. The plot shows the levels of IκBα(Y254L,T257A) (blue) and wild-type IκBα (black) after cyclohexamide treatment in NF-κB −/− cells.
“Fuzziness” is important for rapid signal repression by IκBα
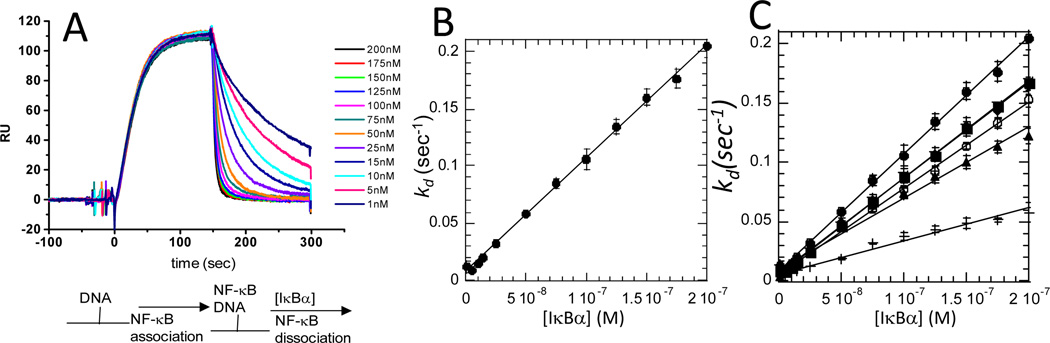
A key feature of the NF-κB negative feedback is the rapidity with which the transcriptional activation is subsequently repressed 1. Rapid post-induction repression is partly explained by the fact that the gene for IκBα is strongly induced by NF-κB, so activation of NF-κB immediately produces newly synthesized IκBα. However, the new IκBα must still escape proteasome degradation, enter the nucleus, and compete for binding to NF-κB with the very large number of κB sites in the DNA. We recently discovered an intriguing kinetic phenomenon in which IκBα is able to markedly increase the rate of dissociation of NF-κB from the DNA 41. The phenomenon was initially discovered by flowing nanomolar concentrations of IκBα over the NF-κB•DNA complex in a co-injection step in an SPR experiment (Figure 8A). IκBα is remarkably efficient at increasing the dissociation rate (kd) of NF-κB from the DNA; the apparent second order rate constant for the IκBα-mediated dissociation is 106 M−1 s−1 (Figure 8B). Several mutant forms of IκBα were also tested for their ability to mediate dissociation of NF-κB from the DNA. The mutations had a variety of effects on NF-κB binding, some bound with the same affinity, and some showed decreased affinity, up to 100-fold. However, all of the thermodynamically stabilized mutants, even the ones that bound with the same affinity, were less able to mediate dissociation of NF-κB from the DNA (Figure 8C) 41. Thus, an important function of the “fuzzy” AR6 in IκBα may be to facilitate dissociation of NF-κB from the DNA to rapidly repress post-induction transcriptional activation.
Figure 8.
(A) Real-time SPR binding experiment in which κB-site DNA was bound to the streptavidin chip at t=0, then NF-κB(p50(19-363)/p651-35) was allowed to associate with the DNA, and finally varying concentrations of IκBα were injected through the second sample loopand the dissociation rate constant (kd) was measured 41. A schematic of the binding events is shown below the graph. (B) Dissociation rate constants for active dissociation are plotted as a function of IκBα variant concentration. (C) Comparison of the active dissociation rate constants of IκBα folding variants: wild type IκBα (●), IκBα(C186P, A220P) (■), IκBα(Q111G) (▲), IκBα(Y254L/Q255H) (►), IκBα(Y254L/T257A) (◄) 41.
CONCLUDING REMARKS AND PERSPECTIVES
The NF-κB signaling regulates many genes, and therefore is highly controlled. Biophysical experiments, including amide H/D exchange and NMR reveal that parts of both proteins are “fuzzy”. From these experiments, we see that “fuzziness” comes in many flavors, and only some parts of each protein are “fuzzy”. In addition, the “fuzziness” of IκBα is reduced in some regions and increased in other regions upon binding to NF-κB. More importantly, we have used biochemical experiments to show that “fuzziness” in IκBα provides kinetic control of dynamic regulatory processes, including its degradation through Ub-dependent and independent pathways and competition with DNA for NF-κB.
Abbreviations
- IκB
inhibitor of kappa B proteins
- NF-κB
nuclear factor kappa B
- AR
ankyrin repeat
- ARD
ankyrin repeat domain.
References
- 1.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J. Mol. Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 5.Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature. 1998;391:410–413. doi: 10.1038/34956. [DOI] [PubMed] [Google Scholar]
- 6.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 7.Basak S, et al. A fourth IkappaB protein within the NF-kappaB signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baeuerle PA. IkB-NF-kB structures: at the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- 9.Bergqvist S, et al. Thermodynamics reveal that helix four in the NLS of NF-kappaB p65 anchors IkappaBalpha, forming a very stable complex. J. Mol. Biol. 2006;360:421–434. doi: 10.1016/j.jmb.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IkappaBalpha/NF-kappaB complex reveals mechanisms of NF-kappaB inactivation. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 12.Traenckner EB, Baeuerle PA. Appearance of apparently ubiquitin-conjugated I kappa B-alpha during its phosphorylation-induced degradation in intact cells. J. Cell. Sci. Suppl. 1995;19:79–84. doi: 10.1242/jcs.1995.supplement_19.11. [DOI] [PubMed] [Google Scholar]
- 13.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann A, Leung TH, Baltimore D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO J. 2003;22:5530–5539. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- 16.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott ML, Fujita T, Liou HC, Nolan GP, Baltimore D. The p65 subunit of NF-kappa B regulates I kappa B by two distinct mechanisms. Genes Dev. 1993;7:1266–1276. doi: 10.1101/gad.7.7a.1266. [DOI] [PubMed] [Google Scholar]
- 18.Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 19.Arenzana-Seisdedos F, et al. Nuclear localization of IkBa promotes active transport of NF-kB from the nucleus to the cytoplasm. J. Cell. Sci. 1997;110:369–378. doi: 10.1242/jcs.110.3.369. [DOI] [PubMed] [Google Scholar]
- 20.O'Dea EL, et al. A homeostatic model of IkappaB metabolism to control constitutive NF-kappaB activity. Mol. Syst. Biol. 2007;3:111. doi: 10.1038/msb4100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervantes CF, et al. Functional dynamics of the folded ankyrin repeats of IkappaB alpha revealed by nuclear magnetic resonance. Biochemistry. 2009;48:8023–8031. doi: 10.1021/bi900712r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sue SC, Cervantes C, Komives EA, Dyson HJ. Transfer of Flexibility between Ankyrin Repeats in IkappaBalpha upon Formation of the NF-kappaB Complex. J. Mol. Biol. 2008;380:917–931. doi: 10.1016/j.jmb.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garner E, Romero P, Dunker AK, Brown C, Obradovic Z. Predicting Binding Regions within Disordered Proteins. Genome Informatics. 1999;10:41–50. [PubMed] [Google Scholar]
- 24.Croy CH, Bergqvist S, Huxford T, Ghosh G, Komives EA. Biophysical characterization of the free IkappaBalpha ankyrin repeat domain in solution. Protein Sci. 2004;13:1767–1777. doi: 10.1110/ps.04731004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latzer J, Papoian GA, Prentiss MC, Komives EA, Wolynes PG. Induced fit, folding, and recognition of the NF-kappaB-nuclear localization signals by IkappaBalpha and IkappaBbeta. J Mol Biol. 2007;367:262–274. doi: 10.1016/j.jmb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Ha JH, Spolar RS, Record MT. Role of the Hydrophobic Effect in Stability of Site-Specific Protein-DNA Complexes. J. Mol. Biol. 1989;209:801–816. doi: 10.1016/0022-2836(89)90608-6. [DOI] [PubMed] [Google Scholar]
- 27.Livingstone JR, Spolar RS, Record MT. Contribution to the Thermodynamics of Protein Folding from the Reduction in Water-Accessible Nonpolar Surface-Area. Biochem. 1991;30:4237–4244. doi: 10.1021/bi00231a019. [DOI] [PubMed] [Google Scholar]
- 28.Spolar RS, Livingstone JR, Record MT. Use of Liquid-Hydrocarbon and Amide Transfer Data to Estimate Contributions to Thermodynamic Functions of Protein Folding from the Removal of Nonpolar and Polar Surface from Water. Biochem. 1992;31:3947–3955. doi: 10.1021/bi00131a009. [DOI] [PubMed] [Google Scholar]
- 29.Spolar RS, Record JMT. Coupling of Local Folding to Site-Specific Binding of Proteins to DNA. Science. 1994;263:777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- 30.Truhlar SM, Torpey JW, Komives EA. Regions of IkappaBalpha that are critical for its inhibition of NF-kappaB.DNA interaction fold upon binding to NF-kappaB. Proc. Natl. Acad. Sci. U. S.A. 2006;103:18951–18956. doi: 10.1073/pnas.0605794103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truhlar SM, Croy CH, Torpey JW, Koeppe JR, Komives EA. Solvent accessibility of protein surfaces by amide H/2H exchange MALDI-TOF mass spectrometry. J Am Soc Mass Spectrom. 2006;17:1490–1497. doi: 10.1016/j.jasms.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Bergqvist S, Ghosh G, Komives EA. The IkBa/NF-kB complex has two hotspots, one at either end of the interface. Prot. Sci. 2008;17:2051–2058. doi: 10.1110/ps.037481.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sue SC, Dyson HJ. Interaction of the IkappaBalpha C-terminal PEST sequence with NF-kappaB: insights into the inhibition of NF-kappaB DNA binding by IkappaBalpha. J Mol Biol. 2009;388:824–838. doi: 10.1016/j.jmb.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih VF, et al. Kinetic control of negative feedback regulators of NF-kappaB/RelA determines their pathogen- and cytokine-receptor signaling specificity. Proc Natl Acad Sci USA. 2009;106:9619–9624. doi: 10.1073/pnas.0812367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malek S, Huang DB, Huxford T, Ghosh S, Ghosh G. X-ray crystal structure of an IkappaBbeta x NF-kappaB p65 homodimer complex. J Biol Chem. 2003;278:23094–23100. doi: 10.1074/jbc.M301022200. [DOI] [PubMed] [Google Scholar]
- 36.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 37.Rice NR, Ernst MK. In vivo control of NF-kappa-B activation by I-kappa-Balpha. EMBO J. 1993;12:4685–4695. doi: 10.1002/j.1460-2075.1993.tb06157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pando MP, Verma IM. Signal-dependent and -independent degradation of free and NF-kappa B bound IkappaBalpha. J. Biol. Chem. 2000;275:21278–21286. doi: 10.1074/jbc.M002532200. [DOI] [PubMed] [Google Scholar]
- 39.Mathes E, O'Dea EL, Hoffmann A, Ghosh G. NF-kappaB dictates the degradation pathway of IkappaBalpha. EMBO. J. 2008;27:1357–1367. doi: 10.1038/emboj.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Truhlar SME, Mathes E, Cervantes CF, Ghosh G, Komives EA. Pre-folding IkappaBalpha alters control of NF-kappaB signaling. J. Mol. Biol. 2008;380:67–82. doi: 10.1016/j.jmb.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergqvist S, et al. Kinetic enhancement of NF-kappaB•DNA dissociation by IkappaBalpha. Proc. Nat. Acad. Sci. U.S. A. 2009;106:19328–19333. doi: 10.1073/pnas.0908797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific: San Carlos, CA, USA; 2002. [Google Scholar]