Abstract
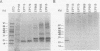
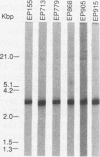
Plant-pathogenic fungi produce cutinase, an enzyme required to degrade plant cuticles and facilitate penetration into the host. The absence of cutinase or a decrease in its production has been associated with a decrease in pathogenicity of the fungus. A set of isogenic strains of Cryphonectria parasitica, the chestnut blight fungus, was tested for the presence and amounts of cutinase activity. The virulent strain of C. parasitica produced and secreted significantly higher amounts of cutinase than the hypovirulent strains. Use of both nucleic acid and polyclonal antibody probes for cutinase from Fusarium solani f. sp. pisi showed that cutinase in C. parasitica is 25 kDa in size and is coded by a 1.1-kb mRNA. Both mRNA and protein were inducible by cutin hydrolysate, while hypovirulence agents suppressed the level of mRNA and the enzyme. Since all the strains had the cutinase gene, the suppression of expression was due to the hypovirulence agents. The data presented are the first report indicating that hypovirulence agents in C. parasitica regulate a gene associated with pathogenicity in other plant-pathogenic fungi.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostakis S. L. Biological control of chestnut blight. Science. 1982 Jan 29;215(4532):466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Nuss D. L. A viral gene confers hypovirulence-associated traits to the chestnut blight fungus. EMBO J. 1992 Feb;11(2):473–477. doi: 10.1002/j.1460-2075.1992.tb05077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Shapira R., Nuss D. L. Cotranslational autoproteolysis involved in gene expression from a double-stranded RNA genetic element associated with hypovirulence of the chestnut blight fungus. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1167–1171. doi: 10.1073/pnas.88.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Hiremath L. S., Webb N. R., Rhoads R. E. Immunological detection of the messenger RNA cap-binding protein. J Biol Chem. 1985 Jul 5;260(13):7843–7849. [PubMed] [Google Scholar]
- Hiremath S., L'Hostis B., Ghabrial S. A., Rhoads R. E. Terminal structure of hypovirulence-associated dsRNAs in the chestnut blight fungus Endothia parasitica. Nucleic Acids Res. 1986 Dec 22;14(24):9877–9896. doi: 10.1093/nar/14.24.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieberman P., Laitman J. T., Reidenberg J. S., Landahl K., Gannon P. J. Folk psychology and talking hyoids. Nature. 1989 Nov 30;342(6249):486–487. doi: 10.1038/342486a0. [DOI] [PubMed] [Google Scholar]
- Lin T. S., Kolattukudy P. E. Induction of a biopolyester hydrolase (cutinase) by low levels of cutin monomers in Fusarium solani f.sp. pisi. J Bacteriol. 1978 Feb;133(2):942–951. doi: 10.1128/jb.133.2.942-951.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti I. B., Kolattukudy P. E. Prevention of fungal infection of plants by specific inhibition of cutinase. Science. 1979 Aug 3;205(4405):507–508. doi: 10.1126/science.205.4405.507. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Powell W. A., Jr, Van Alfen N. K. Two nonhomologus viruses of Cryphonectria (Endothia) parasitica reduce accumulation of specific virulence-associated polypeptides. J Bacteriol. 1987 Nov;169(11):5324–5326. doi: 10.1128/jb.169.11.5324-5326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Van Alfen N. K. Differential accumulation of poly(A)+ RNA between virulent and double-stranded RNA-induced hypovirulent strains of Cryphonectria (Endothia) parasitica. Mol Cell Biol. 1987 Oct;7(10):3688–3693. doi: 10.1128/mcb.7.10.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae B. P., Hillman B. I., Tartaglia J., Nuss D. L. Characterization of double-stranded RNA genetic elements associated with biological control of chestnut blight: organization of terminal domains and identification of gene products. EMBO J. 1989 Mar;8(3):657–663. doi: 10.1002/j.1460-2075.1989.tb03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigling D., Van Alfen N. K. Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J Bacteriol. 1991 Dec;173(24):8000–8003. doi: 10.1128/jb.173.24.8000-8003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R., Choi G. H., Nuss D. L. Virus-like genetic organization and expression strategy for a double-stranded RNA genetic element associated with biological control of chestnut blight. EMBO J. 1991 Apr;10(4):731–739. doi: 10.1002/j.1460-2075.1991.tb08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykh M., Soliday C., Kolattukudy P. E. Proof for the Production of Cutinase by Fusarium solani f. pisi during Penetration into Its Host, Pisum sativum. Plant Physiol. 1977 Jul;60(1):170–172. doi: 10.1104/pp.60.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Flurkey W. H., Okita T. W., Kolattukudy P. E. Cloning and structure determination of cDNA for cutinase, an enzyme involved in fungal penetration of plants. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3939–3943. doi: 10.1073/pnas.81.13.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia J., Paul C. P., Fulbright D. W., Nuss D. L. Structural properties of double-stranded RNAs associated with biological control of chestnut blight fungus. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9109–9113. doi: 10.1073/pnas.83.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]