Abstract
Previous observations by our laboratory indicate that the presence of anti–IL-8 autoantibody:IL-8 immune complexes in lung fluids from patients with acute lung injury/acute respiratory distress syndrome (ALI/ARDS) comprises an important prognostic indicator in the development and ultimate outcome of ALI/ARDS. We also showed that these complexes display proinflammatory activity toward neutrophils through the engagement of FcγRIIa receptors. Because sepsis is one of the most common risk factors for ALI/ARDS, the initial goal of our present study involved investigating the effects of LPS on the expression of FcγRIIa receptors in neutrophils. Our results indicate that LPS triggers an increase in the expression of FcγRIIa on the neutrophil surface, which leads to shortening of the molecular distance between FcγRIIa and Toll-like receptor–4 (TLR4). When such neutrophils are stimulated with anti–IL-8:IL-8 complexes, the TLR4 cascade becomes activated via the engagement of FcγRIIa. The underlying molecular mechanism has been subsequently examined and involves Bruton’s tyrosine kinase (Btk). In conclusion, our study reveals the existence of Btk-dependent molecular cooperation between FcγRIIa and TLR4 signaling cascades in LPS-“primed” human neutrophils. Furthermore, we used fluorescence lifetime imaging to study the interactions between TLR4 and FcγRIIa in human alveolar neutrophils from patients with ALI/ARDS. The results from these experiments confirm the existence of the molecular cooperation between TLR4 and FcγRIIa.
Keywords: neutrophil, FcуRIIa, TLR4, Btk
Clinical Relevance
Our findings indicate that crosstalk occurs between FcγRIIa and Toll-like receptor–4 in alveolar neutrophils from patients with acute lung injury/acute respiratory distress syndrome, and that Bruton’s tyrosine kinase mediates molecular cooperation between these two receptors.
Acute lung injury/acute respiratory distress syndrome (ALI/ARDS) is characterized by diffuse, acute lung injury with a significant increase in both the total number of neutrophils and the proportion of neutrophils within alveolar spaces (1). Although neutrophils constitute only 0.8–3% of nonstructural alveolar cells in normal subjects, they account for 70–80% of the cells in bronchoalveolar (BAL) fluid from patients with ARDS (2, 3). Previous observations by our laboratory indicate that the presence of anti–IL-8 autoantibody:IL-8 immune complexes (IL-8 associated with anti–IL-8 autoantibodies) in lung fluid from patients with ALI/ARDS comprises an important prognostic indicator of the development and ultimate outcome of ALI/ARDS (4–6). In addition, our studies were the first to show that purified anti–IL-8 autoantibody:IL-8 immune complexes display proinflammatory activity toward neutrophils and endothelial cells through the engagement of FcγRIIa (7–9). Further, when we examined lung tissue from patients with lung injury for anti–IL-8 autoantibody:IL-8 immune complexes, we found these complexes in the lungs of patients with ARDS, in association with FcγRIIa (10). Our previous findings also indicate that the expression of FcγRIIa is substantially increased in the lungs of patients with ARDS (9, 10).
Sepsis is a major risk factor for the development of ALI/ARDS, and LPS is a likely cause of progression to ALI/ARDS (1). Toll-like receptor–4 (TLR4), a receptor for LPS, is the prototypical member of the family of Type I transmembrane receptors, with an extracellular leucine-rich repeat domain and an intracellular Toll/IL-1 receptor (TIR) domain. Moreover, neutrophils were reported to express several tyrosine-protein kinases (Tec kinases). The Tec kinases (i.e., Bruton’s tyrosine kinase, Tec, intracellular tyrosine kinase, BMX nonceptor tyrosine kinase, and TXK tyrosine kinase) belong to a family of nonreceptor tyrosine kinases. Tec kinases normally reside in the cytoplasm. After the stimulation of various cell-surface molecules (e.g., TLR4 receptors), the Tec kinases are translocated to the cell membrane, where they initiate downstream signaling cascades (11–15).
The binding of LPS to TLR4 triggers the recruitment of adaptor proteins, namely myeloid differentiation factor–88 (MyD88) and the TIR-domain–containing adaptor protein/MyD88 adaptor–like protein (TIRAP/MAL). According to several reports, Bruton’s tyrosine kinase (Btk) may interact with the TIR domains of MyD88 and TIRAP/MAL, and may subsequently phosphorylate TIRAP/MAL. The phosphorylation of TIRAP/MAL seems necessary for TIRAP/MAL to signal NF-κB activation. However, the role of Btk in orchestrating cellular signaling events has not been sufficiently defined (11, 16–19).
Our preliminary experiments showed that LPS triggers an increase in the expression of FcγRIIa on the neutrophil surface, which leads to crosstalk between TLR4 and FcγRIIa signaling cascades (20). The present study investigated the mechanism underlying this crosstalk. We have established that anti–IL-8:IL-8 complexes activate TLR4 receptors via the engagement of the Btk–MyD88 signaling cascade.
Materials and Methods
For additional details, please see the online supplement.
Human Subjects
All research involving human subjects was approved by the Institutional Human Subject Committee at the University of Texas Health Science Center (Tyler, TX).
Cell Culture Conditions
Blood was drawn from healthy volunteers, and neutrophils were purified according to a protocol routinely used in our laboratory (8). Cells were stimulated with anti–IL-8:IL-8 immune complexes or LPS (Escherichia coli O26:B6, 20 ng/ml; Sigma Chemical Co., St. Louis, MO) for various lengths of time. Anti–IL-8:IL-8 complexes used for neutrophil stimulation consisted of a monoclonal anti–IL-8 antibody and a recombinant human IL-8 (PeproTech, Rocky Hill, NJ) (4, 21, 22).
In some experiments, aliquots of whole blood were incubated with LPS (E. coli O26:B6, 20 ng/ml; Sigma Chemical Co.) for 2 hours at 37°C with gentle shaking. Blood was then lysed to obtain neutrophils.
Pharmacological Inhibitors and Antibodies
Neutrophils were incubated with pharmacological inhibitors of Btk (LFM-A13 and Bruton’s Tyrosine Kinase Inhibitor III; Calbiochem, La Jolla, CA) for 20 minutes in the dark. In some experiments, cells were preincubated with a monoclonal antibody against FcγRIIa (IV.3; F(ab); Medarex Corp., West Lebanon, NJ), or with a monoclonal antibody against TLR4 (HTA125; GeneTex, Irvine, CA). F(ab)2 fragments of the TLR4 antibody were obtained using a F(ab)2 Preparation Kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions.
Immunoprecipitation, Cell-Surface Biotinylation, and Western Blotting
Btk in cell lysates was immunoprecipitated with anti-human Btk antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and detected using specific anti-human Btk, MyD88, and MAL/TIRAP antibodies (all from Santa Cruz Biotechnology). In some experiments, FcγRIIa receptors were immunoprecipitated with an anti-FcγRIIa (N-20; Santa Cruz Biotechnology) antibody, and detected using an anti-FcγRIIa antibody (IV.3; StemCell Technologies, Vancouver, BC, Canada). On other occasions, neutrophils were subjected to surface biotinylation, as previously described (23). The expression of Btk, MyD88, and phospho Btk (pBtk) (pY551; Invitrogen, Carlsbad, CA) was also analyzed directly in whole neutrophil cell lysates.
Laser Confocal Microscopy and Fluorescence Lifetime Imaging
Neutrophils mounted on cytospins were incubated with primary antibodies used for laser confocal microscopy, namely, anti-human FcγRIIa (N-20), anti-human TLR4 (HTA125; GeneTex), anti-human pBtk (pY551; Invitrogen), anti-human Btk, and anti-human MyD88 (Santa Cruz Biotechnology), followed by fluorescence dye–conjugated secondary antibodies.
The monoclonal antibodies used for fluorescence lifetime imaging (FLIM)/fluorescence resonance energy transfer (FRET) included anti-human FcγRIIa (clone AT10, specific for neutrophil FcγRIIa) (24) (based also on our unpublished observations), FITC (CALTAG Laboratories, Burlingame, CA), anti-human TLR4 (clone HTA125, Alexa Fluor 647; eBioscience, San Diego, CA), anti-human human leukocyte antigen-ABC locus (clone W6/32, FITC; eBioscience), and anti-human TLR4 (clone 76B357.1, FITC; Imgenex, San Diego, CA), all specific for extracellular domains of target receptors. FRET was performed on fixed neutrophils, and analyzed as previously described (25).
Human Embryonic Kidney Cells and Transfection Experiments
Human embryonic kidney (HEK)-Blue TLR4 cells were transfected with a human cDNA clone of Btk or a control human cDNA clone of a kinase-deficient mutant (K430M) of Btk (OriGene Technologies, Inc., Rockville, MD) according to the manufacturer’s recommendations, and as previously described (26).
Statistical Analysis
Differences between groups were evaluated by a simple one-way ANOVA or t test when appropriate. P < 0.05 was considered significant. All statistics were performed using SIGMASTAT (SPSS Science, Chicago, IL).
Results
Fluorescence Lifetime Imaging
Because our preliminary results suggested possible crosstalk between FcγRIIa and TLR4 in LPS-treated human blood neutrophils (20), we used FLIM-FRET to study interactions between these two receptors in the membrane region of purified human blood neutrophils. A major histocompatibility complex (MHC) Class I/TLR4 pair was used as a negative control, and a TLR4/TLR4 pair (each labeled with an antibody against a different epitope of TLR4) served as a positive control (Figure 1A). Color changes from brown to green for the FcγRIIa/TLR4 pair and the TLR4/TLR4 pair in LPS-treated neutrophils are indicative of substantial changes in the fluorescence lifetime of these cells. Next, we performed FLIM analyses of BAL fluid neutrophils from patients with ALI/ARDS. Average lifetimes for donors (BAL fluid neutrophils stained with anti-FcγRIIa FITC) and donors/acceptors (BAL fluid neutrophils stained with anti-FcγRIIa FITC and anti-TLR4 Alexa Fluor 647) were 1.00 ns and 0.72 ns, respectively. Further, BAL neutrophils stained with anti–MHC Class I FITC only (donor) exhibited an average lifetime of 0.87 ns, whereas those stained with anti–MHC Class I FITC and anti-TLR4 Alexa Fluor 647 monoclonal antibodies (donors/acceptors) exhibited an average lifetime of 0.85 ns (Figure 1B).
Figure 1.
Measurements of fluorescence lifetime imaging (FLIM)/fluorescence resonance energy transfer (FRET). (A) Analysis of association between FcγRIIa and Toll-like receptor–4 (TLR4) in purified human blood neutrophils cultured in medium only (Med) or stimulated with LPS (20 ng/ml) for 2 hours, and between MHC Class I or TLR4 and TLR4 in LPS-treated cells. Representative cells are shown. Changes in color (from brown to green) represent changes in fluorescence lifetime (τ). D, donor; D/A, donor/acceptor pair. (B) Histograms depict changes in the lifetimes of bronchoalveolar lavage (BAL) fluid neutrophils from patients with acute respiratory distress syndrome (ARDS) for the FcγRIIa/TLR4 and major histocompatibility complex (MHC) Class I/TLR4 pairs. Representative data from one of three or four experiments are shown. (C) Calculated FRET efficiencies. PMNs, neutrophils. Representative data from 3–4 experiments are shown.
Based on average changes in lifetime, the energy transfer efficiency (E) was calculated using the equation E = (1 − (τDA/τD)) × 100%, where τ = fluorescence lifetime, D = donor, and DA = donor/acceptor pair. We found that FRET occurred between FcγRIIa and TLR4 (E = 18.2% ± 2.7%) and between two different epitopes of TLR4 (TLR4/TLR4) (E = 13.9% ± 1.1%) in LPS-treated blood neutrophils. FRET did not occur between FcγRIIa and TLR4 in neutrophils cultured in medium only (E = 2.1% ± 1.7%). Similarly, we detected no FRET between MHC Class I receptors and TLR4 (E = 3.0% ± 0.9%), although MHC Class I expression increased in LPS-stimulated neutrophils (27) (also based on our unpublished data) (Figure 1C). Next, we evaluated BAL fluid neutrophils from patients with ALI/ARDS. The calculated energy transfer efficiency measured 26.5% ± 11.1% for FcγRIIa/TLR4, and 2.5% ± 2.1% for MHC Class I/TLR4 in BAL fluid neutrophils from patients with ALI/ARDS (Figure 1C).
Expression of FcγRIIa and TLR4 in Human Neutrophils
Next, we evaluated the expression levels of FcγRIIa and TLR4 in stimulated neutrophils. We chose a 2-hour time point, based on published information (28, 29) and our kinetic studies. We noted a substantial increase in the amount of FcγRIIa in immunoprecipitated samples, and of TLR4 in cell lysates after LPS treatment (Figure 2A). Further, we used laser confocal microscopy and antibodies that recognize extracellular portions of FcγRIIa and TLR4 to assess the surface expression of these receptors. More FcγRIIa was detected on the surface of purified human neutrophils that were cultured with LPS for 2 hours (Figure 2B). Similarly, we found that the expression of TLR4 on the neutrophil surface was up-regulated after culturing purified cells with LPS for 2 hours (Figure 2C).
Figure 2.
Analysis of expression of FcγRIIa and TLR4 in human blood neutrophils. (A) Western blot analysis (WB) was performed on neutrophil lysates immunoprecipitated (IP) with anti-FcγRIIa or on whole-neutrophil lysates, using specific antibodies against FcγRIIa or TLR4. IgG control (FcγRIIa) and actin (TLR4) are also shown. Neutrophils were incubated with medium or LPS before analysis, and the same numbers of cells were evaluated in each case. (B) Images depict FcγRIIa (green) on the surface of purified human blood neutrophils cultured in medium only (Med) or stimulated with LPS (20 ng/ml) for 2 hours. Sec Ab, secondary antibody control. (C) Images depict TLR4 on the surface of purified human blood neutrophils cultured in medium only (Med) or stimulated with LPS (20 ng/ml) for 2 hours. (D) Images depict FcγRIIa (green) on the surface of neutrophils obtained from human blood incubated with medium only (Med) or with medium supplemented with LPS (20 ng/ml) for 2 hours. Nuclei are stained blue. (E) Western blot analysis of neutrophil lysates immunoprecipitated with anti-FcγRIIa, using streptavidin–horseradish peroxidase (HRP). Neutrophils were incubated with medium or LPS and then with biotin before analysis, and the same numbers of cells were evaluated in each case. The vertical bar charts in Figures 2B–2D depict the fold increase in cell-surface concentrations of FcγRIIa and TLR4 after LPS treatment. Between 600 and 700 cells were analyzed in these experiments. The histograms in Figures 2B and 2C show change in the numbers of FcγRIIa and TLR4 on the surface of representative neutrophils. The graphs in Figures 2A and 2E depict the fold increase in the amount of FcγRIIa and TLR4, compared with medium. Representative data from three experiments are shown. A.U., arbitrary units.
Some controversy surrounds the surface expression of FcγRIIa, which is believed to be promptly internalized in stimulated cells (30). Consequently, we analyzed the expression of this receptor, using whole blood to maintain more physiological conditions. The treatment of whole human blood with LPS for 2 hours also induces an increase in the surface expression of FcγRIIa in neutrophils (Figure 2D). Finally, we performed the surface biotinylation of neutrophils, followed by the immunoprecipitation of FcγRIIa from cell lysates. As shown in Figure 2E, the surface expression of FcγRIIa is again up-regulated.
The mechanism of up-regulation for FcγRIIa remains unknown. However, LPS reportedly does not affect the mRNA concentration of FcγRIIa (31). Further, we originally analyzed only the surface expression of FcγRIIa (Figure 2). When entire cells are evaluated, evidence is found of positive staining for FcγRIIa in the cytoplasm of neutrophils treated with medium alone. In contrast, in cells stimulated with LPS, the staining is localized to the cell membrane (Figure E1A in the online supplement). Therefore, we believe that upon stimulation, FcγRIIa migrates from an intracellular pool to the cell surface (Figure E1). In fact, a cytoplasmic localization of FcγRIIa was reported for human monocytes (32). In addition, the expression of other receptors in neutrophils, such as CD300a (inhibitory receptor–containing immunoreceptor tyrosine–based inhibitory motif), is regulated in this way (33).
Moreover, the antibody (clone IV.3) used to detect FcγRIIa in Figure 2A recognizes only the extracellular portion of the receptor (residues 132–137) (34). The total amount of FcγRIIa remains unchanged under the same conditions when the blot is developed using an antibody that can recognize both extracellular and intracellular portions of the receptor (Figure E1B).
Mechanism of FcγRIIa/TLR4 Interactions
After we established that a significant decrease occurs in the molecular distance between FcγRIIa and TLR4 in neutrophils that are “primed” with LPS and activated by anti–IL-8:IL-8 immune complexes, we set out to define an underlying mechanism. The molecular closeness between FcγRIIa and TLR4 could lead to the activation of an adaptor molecule associated with both signaling cascades. One such molecule is Btk (18, 19, 30, 35). Therefore, we evaluated concentrations of Btk in neutrophils treated with either anti–IL-8:IL-8 immune complexes or LPS, and in cells pretreated with LPS and stimulated with the complexes.
As shown in Figure 3A, concentrations of Btk are relatively low in unstimulated cells. However, they are significantly increased in neutrophils treated with anti–IL-8:IL-8 immune complexes, and in cells that were “primed” with LPS and activated by the complexes (Figure 3A, upper blot). Notably, the anti-Btk antibody used in these experiments, as well as in immunofluorescence staining (Figure 3C, bottom), recognizes a kinase domain of Btk, and thereby detects Btk in its activated state (36). On the other hand, when an antibody against total Btk is used, no difference in Btk concentrations is evident (Figure 3A, middle blot).
Figure 3.
Effects of anti–IL-8:IL-8 immune complexes and LPS on expression and activation of Bruton’s tyrosine kinase (Btk) in purified human blood neutrophils. (A) Western blot analysis of whole-cell lysates of neutrophils incubated with medium (Med) or anti–IL-8:IL-8 complexes (IC) or LPS for 5 minutes, or LPS for 2 hours, or “primed” with LPS for 2 hours and stimulated with IC for 5 minutes, using specific antibodies against a kinase-domain Btk (upper blot) or total Btk (middle blot) and actin (bottom blot). The graph depicts the fold increase in the amount of Btk compared with medium. (B) Western blot analysis of whole-cell lysates of neutrophils incubated with medium (Med) or anti–IL-8:IL-8 complexes or LPS for 5 minutes, or LPS for 2 hours, or “primed” with LPS for 2 hours and stimulated with IC for 5 minutes, using specific antibodies against phosphorylated Btk (pBtk) and actin. The graph depicts the fold increase in the amount of pBtk compared with medium. (C) Btk or pBtk (green) is visualized in purified human blood neutrophils, using laser confocal microscopy. P < 0.001, for LPS (5 minutes) and LPS/IC versus medium, and for IC versus LPS/IC for pBtk. P = 0.003 for Btk. Representative cells (in white squares above) are enlarged to show membrane localization of pBtk (arrows). The vertical bar chart depicts levels of Btk or pBtk after treatment with various compounds (as already described). More than 5,000 cells were evaluated. Representative data from three experiments are shown.
We also analyzed the activation of Btk using anti-pBtk antibody. We noted an increase in Btk phosphorylation (activation) in neutrophils treated with either the complexes alone for 5 minutes, or with both LPS and the complexes (Figure 3B). This time we also observed a modest activation of Btk in cells stimulated with LPS for 5 minutes (Figure 3B).
Moreover, the ability of anti–IL-8:IL-8 immune complexes and LPS to induce an increase in Btk activation was confirmed using confocal laser microscopy, which is far more sensitive than immunoblotting. Both the complexes and LPS triggered an increase in concentrations of activated Btk after 5 minutes (P < 0.001; Figure 3C). In agreement with the results presented in Figures 3A and 3B, we did not observe appreciable levels of activated Btk or pBtk in neutrophils stimulated with LPS for 2 hours, whereas a substantial increase in Btk activation occurred in the cells first “primed” with LPS and then activated by anti–IL-8:IL-8 complexes (P < 0.001; Figure 3C). Finally, we stress that ours is the first study to show the activation of Btk through the engagement of FcγRIIa receptors in human neutrophils.
The pretreatment of neutrophils with LPS for 2 hours, followed by stimulation with anti–IL-8:IL-8 immune complexes, results in a greater activation of Btk than does treatment of cells with the complexes alone (P < 0.05; Figures 3A–3C). This could be explained by the increase in numbers of FcγRIIa receptors, or alternatively, Btk could trigger the activation of the TLR4 cascade. To test the second possibility, we analyzed the expression of another adaptor molecule associated with the TLR4 cascade, MyD88, in neutrophils treated with LPS, the complexes, or both. No appreciable change was evident in MyD88 levels when samples were analyzed by Western blotting (Figure 4A). In confocal images, however, MyD88 was detected inside the cytoplasm and close to the cell membrane in neutrophils incubated with either LPS or sequentially with LPS and the complexes (Figure 4B). The translocation of MyD88 from the cytoplasm to the membrane denotes its activation (19). Figure 4B shows that the expression and activation of MyD88 increases (P < 0.001) in neutrophils pretreated with LPS and stimulated with anti–IL-8:IL-8 immune complexes. The amount and activation of MyD88 are also increased in cells stimulated with LPS for 5 minutes (P < 0.001), but not in cells incubated for 2 hours. In addition, as expected, stimulation with anti–IL-8:IL-8 immune complexes exerted no effect on MyD88 expression or activation, because this molecule is not normally associated with the FcγRIIa cascade.
Figure 4.
Effects of anti–IL-8:IL-8 immune complexes and LPS on expression and activation of myeloid differentiation factor–88 (MyD88) in purified human blood neutrophils. (A) Western blot analysis of whole-cell lysates of neutrophils incubated with medium (Med) or anti–IL-8:IL-8 complexes (IC) or LPS for 5 minutes, or LPS for 2 hours, or “primed” with LPS for 2 hours and stimulated with IC for 5 minutes, using specific antibodies against MyD88 and actin. The graph depicts the fold increase in the amount of MyD88 compared with medium. (B) MyD88 (green) is visualized in purified human blood neutrophils, using laser confocal microscopy. P < 0.001, for LPS (5 minutes) and LPS/IC versus medium. Representative cells (in white squares above) are enlarged to show membrane localization of MyD88 (arrows). The vertical bar chart depicts levels of MyD88 after treatment with various compounds (as already described). Approximately 3,800 cells were analyzed. Representative data from three experiments are shown.
Interactions between Btk and MyD88 in Human Neutrophils
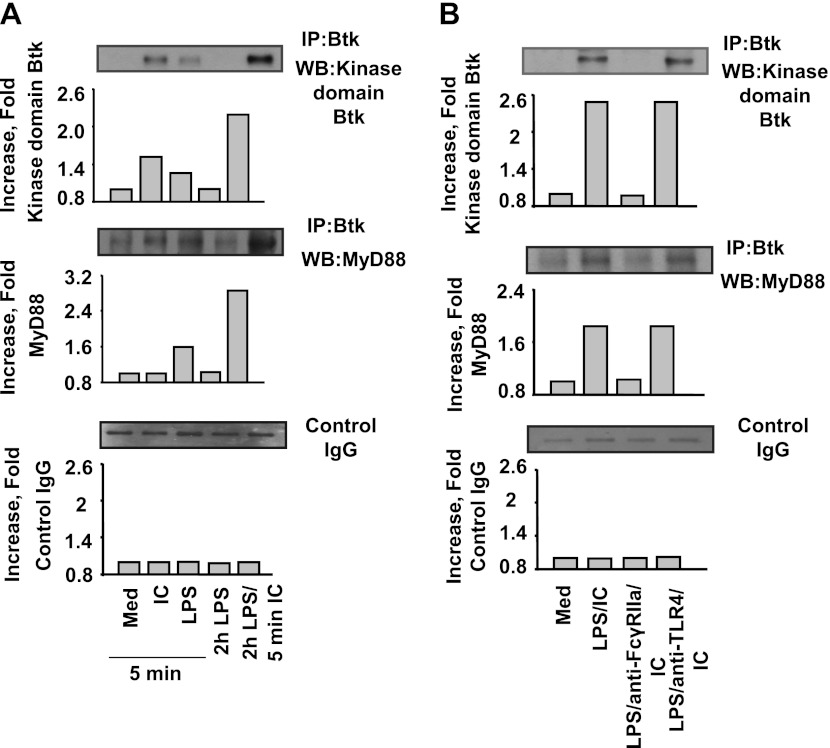
Some evidence from the literature indicates that Btk may directly interact with MyD88, for example, in the monocyte-like cell line, human acute monocytic leukemia cell line (THP-1) (11). Therefore, the purpose of the next series of experiments was to establish whether Btk has the ability to interact with MyD88 in neutrophils. We performed immunoprecipitation experiments using antibodies against Btk. The results presented in Figure 5 demonstrate an interaction occurs between Btk and MyD88 in human neutrophils. First, we found an increase in the amount of Btk in neutrophils stimulated with either anti–IL-8:IL-8 immune complexes or LPS for 5 minutes, but not in cells stimulated with LPS alone for 2 hours. We also noted a substantial increase of Btk in cells first “primed” with LPS and then activated by the complexes (Figure 5A). These observations agree with the data presented in Figures 3B and 3C. We note that the anti-Btk antibody used in these experiments (Western blotting) recognizes a kinase domain of Btk, thereby detecting Btk in its active state (36).
Figure 5.
Association of Btk with MyD88. (A, upper blot) Immunoprecipitation (IP) of neutrophil cell lysates with anti-Btk antibody, and Western blot analysis (WB) of obtained samples for Btk. Neutrophils were incubated with medium (Med) or anti–IL-8:IL-8 complexes (IC) or LPS for 5 minutes, or LPS for 2 hours, or “primed” with LPS for 2 hours and stimulated with IC for 5 minutes The graph depicts the fold increase in the amount of active Btk compared with medium. Middle blot: Immunoprecipitation (IP) of neutrophil cell lysates with anti-Btk antibody, and Western blot analysis (WB) of obtained samples for MyD88. Neutrophils were incubated with medium (Med) or anti–IL-8:IL-8 complexes (IC) or LPS for 5 minutes, or LPS for 2 hours, or “primed” with LPS for 2 hours and stimulated with IC for 5 minutes. The graph depicts the fold increase in the amount of MyD88 compared with medium. Representative data from three experiments are shown. Bottom blot: IgG control. (B) Effect of blocking FcγRIIa or TLR4 receptors on the concentrations of Btk (upper blot) and MyD88 in complex with Btk (middle blot). Purified human blood neutrophils were “primed” with LPS for 2 hours and stimulated with anti–IL-8:IL-8 immune complexes (IC) for 5 minutes in the presence or absence of anti-FcγRIIa or anti-TLR4–blocking antibodies. Cell lysates were immunoprecipitated with anti-Btk antibody, and levels of Btk and MyD88 were analyzed. The graphs depict the fold increase in the amount of active Btk or MyD88 compared with medium. Bottom blot: IgG control. Representative data from three experiments are shown.
Moreover, we detected MyD88 in some of the immunoprecipitates. MyD88 was present in samples obtained by stimulating neutrophils with LPS for 5 minutes (Figure 5A). The pretreatment of cells with LPS for 2 hours, followed by stimulation with anti–IL-8:IL-8 immune complexes, resulted in a substantial increase in the amount of MyD88 associated with Btk (Figure 5A). This increase was even greater than observed for LPS alone (Figure 5A). As before (Figure 4B), no MyD88 was detectable in samples incubated exclusively with anti–IL-8:IL-8 immune complexes for 5 minutes, or with LPS alone for 2 hours.
Role of FcγRIIa and TLR4 Receptors
Our findings indicate that the increase in amounts of MyD88 in samples obtained from neutrophils stimulated sequentially with LPS and anti–IL-8:IL-8 immune complexes (2 hours and 5 minutes, respectively) may be brought about by an activation of the TLR4 cascade. The complexes may trigger the activation of TLR4 receptors, insofar as we did not detect MyD88 in samples from cells stimulated with LPS alone for 2 hours (Figures 4B and 5A). To address this possibility, we preincubated neutrophils with an anti-FcγRIIa antibody before performing stimulations with anti–IL-8:IL-8 immune complexes and immunoprecipitations. Anti-FcγRIIa antibody pretreatment suppressed the increase in amounts of MyD88 associated with Btk in cells “primed” with LPS and stimulated with anti–IL-8:IL-8 immune complexes (Figure 5B). In contrast, an antibody against TLR4 exerted no effect (Figure 5B). Similar results were obtained for Btk (Figure 5B), in agreement with our observations that FcγRIIa mediates the activation of Btk in neutrophils “primed” with LPS and stimulated with anti–IL-8:IL-8 immune complexes. We note that the anti-Btk antibody used in these experiments (Western blotting) recognizes a kinase domain of Btk, thereby detecting Btk in its active state (36).
Transfection of HEK-Blue TLR4 Cells
To determine whether the observed activation of the TLR4 cascade by anti–IL-8:IL-8 immune complexes is mediated by Btk, we used HEK-Blue TLR4 cells that naturally express negligible amounts of Btk, and could not be stimulated with anti–IL-8:IL-8 immune complexes (not shown). The transfection of these cells with cDNA for Btk resulted in an increase in the basal expression of Btk (not shown), and a substantial increase in the amount of Btk in cells treated with the complexes (Figures 6A and 6B). The data presented in Figures 6A–6C were obtained either anti-total Btk antibody (Figure 6A) or the antibody that recognizes a kinase domain of Btk, and therefore detects Btk in its active state (Figures 6A–6C) (36).
Figure 6.

Btk in human embryonic kidney (HEK)-Blue TLR4 cells transfected with Btk-specific cDNA or control cDNA. (A) Western blot analysis of whole-cell lysates of HEK-Blue TLR4 transfected with Btk-specific cDNA, using specific antibodies against a kinase-domain Btk (upper blot) or total Btk (middle blot) and actin (bottom blot). The graph depicts the fold increase in the amount of Btk compared with medium. HEK-Blue TLR4 was either cultured in medium only, or stimulated for 5 minutes with the complexes (IC), or treated for 2 hours with granulocyte/macrophage colony-stimulating factor (GM-CSF), or “primed” with GM-CSF for 2 hours and then stimulated with anti–IL-8:IL-8 immune complexes (IC) for 5 minutes. (B) Analysis of Btk levels in cells transfected with Btk-specific cDNA, using confocal microscopy. The vertical bar chart depicts levels of active Btk after treatment with various compounds (as already described). P < 0.001, for IC (5 minutes) and LPS/IC versus medium, and LPS/IC versus IC. (C) Analysis of Btk levels in cells transfected with control cDNA, using confocal microscopy. Cells were cultured in medium only, or stimulated for 5 minutes with the complexes (IC), or treated for 2 hours with GM-CSF, or “primed” with GM-CSF for 2 hours and then stimulated with IC for 5 minutes The vertical bar chart depicts levels of Btk after treatment with various compounds (as already described). Representative data from three experiments are shown. (D) Analysis of MyD88 levels in nontransfected cells, using confocal microscopy. HEK-Blue TLR4 was either cultured in medium only, or stimulated for 5 minutes with the complexes (IC), or treated for 2 hours with GM-CSF, or “primed” with GM-CSF for 2 hours and then stimulated with IC for 5 minutes. (E) Analysis of MyD88 levels in cells transfected with Btk-specific cDNA, using confocal microscopy. Cells were cultured in medium only, or stimulated for 5 minutes with the complexes (IC), or treated for 2 hours with GM-CSF, or “primed” with GM-CSF for 2 hours and then stimulated with IC for 5 minutes. The vertical bar chart depicts levels of MyD88 after treatment with various compounds (as already described). P < 0.001, for IC (5 minutes) and LPS/IC versus medium, and LPS/IC versus IC. (F) Analysis of MyD88 levels in cells transfected with control cDNA, using confocal microscopy. Cells were cultured in medium only, or stimulated for 5 minutes with the complexes (IC), or treated for 2 hours with GM-CSF, or “primed” with GM-CSF for 2 hours and then stimulated with IC for 5 minutes Representative data from three experiments are shown.
Because HEK-Blue TLR4 cells already have more TLR4 receptors, we used granulocyte/macrophage colony-stimulating factor (GM-CSF) to up-regulate FcγRIIa receptors selectively, to mimic what happens in neutrophils “primed” with LPS. We stress that GM-CSF has the ability to increase numbers of FcγRIIa receptors in HEK-Blue TLR4 cells (data not shown). GM-CSF by itself exerted no effect on Btk activation, but more Btk was evident in cells treated with GM-CSF and stimulated with anti–IL-8:IL-8 immune complexes (P < 0.001; Figures 6A and 6B). The stimulation of cells transfected with control cDNA in the same manner as already described did not induce an increase in the amount of active Btk (Figure 6C).
Next, we analyzed the expression and activation of MyD88 (Figures 6D–6F). MyD88 is expressed in nontransfected cells (Figure 6D), but in the absence of appreciable concentrations of Btk, we did not observe an activation of MyD88 in cells “primed” with GM-CSF and incubated with anti–IL-8:IL-8 immune complexes. After transfection with cDNA specific for Btk, no change was evident in the levels of MyD88 in cells stimulated with the complexes (Figure 6E), in agreement with the results in Figures 4B and 5A. On the other hand, we detected MyD88 in cells treated in sequence with GM-CSF and the complexes (P < 0.001; Figure 6E), confirming the role of Btk in mediating the activation of MyD88 via the engagement of FcγRIIa receptors. The transfection of HEK-Blue cells with the control cDNA is depicted in Figure 6F. The stimulation of these cells with anti–IL-8:IL-8 immune complexes, GM-CSF, or both did not affect concentrations of MyD88 (Figure 6F).
Blocking of Btk with Specific Inhibitors
To confirm further the importance of Btk in mediating the effects of anti–IL-8:IL-8 immune complexes on the activity of TLR4, we used specific inhibitors (LMF-A13 and BTK INH III; Calbiochem) to block Btk (Figure 7A). Figure 7A shows that no Btk and MyD88 were detectable in neutrophils treated with LMF-A13 or BTK INHIII. Further, Btk was recently shown to associate with MAL/TIRAP in human neutrophils (37). We demonstrate that Btk can indeed interact with MAL/TIRAP, and that this interaction can be blocked using these inhibitors (Figure 7A).
Figure 7.
(A) Effects of blocking of Btk on levels of Btk, MyD88, and MyD88 adaptor–like protein/ Toll/IL-1 receptor–domain–containing adaptor protein (MAL/TIRAP) in complex with Btk. Purified human blood neutrophils were “primed” with LPS for 2 hours and stimulated with anti–IL-8:IL-8 immune complexes (IC) for 5 minutes in the presence or absence of Btk inhibitors (LFM-A13 or INH III). Cell lysates were immunoprecipitated with anti-Btk antibody, and levels of Btk, MyD88, and MAL/TIRAP were analyzed. The graphs depict the fold increase in the amount of active Btk, MyD88, or MAL/TIRAP compared with medium. Representative data from three experiments are shown. (B) Activation of NF-κB. Purified human blood neutrophils were cultured in medium only, or in the presence of LPS for 5 minutes, or in the presence of LPS for 2 hours, or “primed” with LPS for 2 hours and then stimulated with anti–IL-8:IL-8 immune complexes (IC) for 5 minutes. The expression of phosphorylated p65 (pp65) was analyzed by laser confocal microscopy. pp65, green; nuclei, red (pseudocolor); nuclear translocation/colocalization, yellow. Cells were also cultured in medium only, or in the presence of LPS for 2 hours, or “primed” with LPS for 2 hours and then stimulated with anti–IL-8:IL-8 immune complexes (IC) for 5 minutes. The expression of pIκB was analyzed in cell lysates, using specific antibodies against phospho IkappaB kinase beta (pIκB) and actin. The graph depicts the fold increase in the amount of pIκB compared with medium. (C) Release of the active form of matrix metalloproteinase-9 (Ac–MMP-9) was studied using Western blot analysis of supernatants from cultured cells and specific antibodies (anti–Ac–MMP-9 and anti-actin). The graph depicts the fold increase in the amount of Ac–MMP-9 compared with medium. Representative data from three experiments are shown.
This experiment provides additional data in support of our hypothesis that Btk is essential for the initiation of the TLR4 cascade by activated FcγRIIa. In summary, our results suggest that Btk-dependent crosstalk occurs between TLR4 and FcуRIIa pathways in LPS-“primed” neutrophils.
Activation of NF-κB and Release of Active Matrix Metalloproteinase-9
We also analyzed the functional consequences of receptor crosstalk. We present the activation of NF-κB in Figure 7B. This activation regulates the biological functions of neutrophils (38) and the release of the active form of matrix metalloproteinase–9 (Figures 7C and E6). Both are substantially increased after the “priming” of cells with LPS that was followed by stimulation with the complexes.
Discussion
In the present study, we evaluated the effects of LPS on the expression and function of FcγRIIa in neutrophils. Firstly, we conclude that LPS increases the concentrations of both FcγRIIa and TLR4 receptors on the surface of neutrophils. A bovine whey protein extract and TNF-α have been shown to induce the up-regulation of expression of FcγRIIa on the neutrophil surface after overnight incubation (39, 40). However, we were not able to detect appreciable amounts of TNF-α in our experiments (i.e., in supernatant samples collected from neutrophils stimulated with LPS for 2 hours; data not shown). Further, to the best of our knowledge, no studies have shown an increase in the surface levels of TLR4 in LPS-treated human neutrophils. However, one report describing the up-regulation of the expression level of TLR4 in neutrophils infected with Mycobacterium bovis bacille Calmette-Guerin has been published (41).
Moreover, we found that the increase in the level of expression of both receptors on the neutrophil surface leads to a decrease in the molecular distance between FcγRIIa and TLR4. A similar phenomenon occurs in monocytes, where LPS promotes the enhanced expression of various membrane-associated proteins, and decreases the physical proximity between these proteins (42, 43). We used FLIM-FRET in our studies to evaluate the interactions between FcγRIIa and TLR4 in human blood and BAL fluid neutrophils. We stress that although FLIM-FRET is a more complex imaging technique, it has a number of advantages over other more traditional microscopy approaches (44). Because it is only concerned with donor fluorophore data, FLIM-FRET does not have to contend with spectral bleed-through. Other FRET methods generate “apparent” energy transfer efficiencies (E), because non-FRET–participating and FRET-participating donors cannot be separated in the calculation of E. In contrast, FLIM-FRET discriminates between non-FRET–participating and FRET-participating donors by way of different fluorescence lifetimes.
Possible interactions between FcγRIIa and TLR4 in human whole blood and between murine FcγRIII and TLR4 in murine neutrophils were postulated previously (45, 46). The first study (45) described a new anti-TLR4 antibody. The inhibitory capacity of this antibody in human whole blood was strengthened by FcγRIIa, suggesting that FcγRIIa may down-regulate LPS-induced inflammatory responses in blood cells. Our results indicate an opposite effect of FcγRIIa, namely, the activation of TLR4 via the engagement of FcγRIIa by anti–IL-8:IL-8 immune complexes. This phenomenon could not be caused by the endotoxin contamination of the complexes. When we stimulated HEK-Blue TLR4 cells with anti–IL-8:IL-8 immune complexes, no activation of MyD88 was detectable. The stimulated cells actually did not differ from control cells cultured in medium only. We stress that HEK-Blue TLR4 cells are specifically designed to respond to small quantities of endotoxin.
In addition, Rittirsch and colleagues (46) showed the TLR4 dependence of the activation of murine FcγRIII by BSA–anti-BSA IgG immune complexes. However, TLR4 is not required for triggering the stimulatory activities of anti–IL-8:IL-8 immune complexes, because the complexes were fully functional in control HEK cells deficient in TLR4 (data not shown), and the blockade of TLR4 did not affect the activity of these complexes in human blood neutrophils (Figure 5B). Because Rittirsch and colleagues (46) studied peritoneal neutrophils from C3H/HeJ (TLR4 mutant) mice (which, as the authors pointed out, display many defects in signaling pathways), the FcγRIII/TLR4 interrelationship may depend on the presence of a specific signaling protein or proteins. Adversely, such proteins could be essential components of the FcγRIII cascade, but are not present in neutrophils from C3H/HeJ (TLR4 mutant) mice.
Furthermore, Rittirsch and colleagues (46) observed a direct association between FcγRIII and TLR4 receptors in murine peritoneal neutrophils stimulated with BSA–anti-BSA IgG immune complexes. However, we were not able to detect TLR4 in samples generated by the stimulation of human neutrophils with LPS, followed by the immunoprecipitation of FcγRIIa (data not shown). Several explanations may account for the discrepancies between the studies already mentioned. These explanations may involve different cell types (murine versus human neutrophils) (47), the anti-TLR4 antibody type, or the nature of immune complexes that were investigated in these studies.
Our results also suggest that Btk mediates the crosstalk between FcγRIIa and TLR4 in LPS-treated human neutrophils. Btk belongs to the structurally homologous Btk/Tec family of intracellular tyrosine kinases. It is expressed in all hematopoietic lineages of cells, except for plasma cells and T lymphocytes (48). In B cells, the activation of the B-cell receptor triggers the recruitment of Btk to the plasma membrane, followed by its phosphorylation (49). Btk also mediates signaling via the TLR4 receptor and G-protein–coupled receptor in human neutrophils (12, 50). Further, Btk-deficient murine neutrophils are less readily recruited to sites of inflammation in vivo (51, 52). However, the role of Btk in neutrophil activation remains unclear, and the exact mechanism underlying the phosphorylation of Btk has not been established (47). Our study shows that Btk undergoes activation in human neutrophils stimulated with anti–IL-8:IL-8 immune complexes via the FcγRIIa receptor. Similarly, Btk is activated in platelets by the cross-linking of FcγRIIa receptors (35). Moreover, the total concentration of Btk does not change under the same conditions, and remains unchanged in cells pretreated with LPS and stimulated with the complexes. This finding is in contrast to those in studies showing an increase in the Btk expression of B cells (53, 54).
In addition, Btk is capable of associating with MyD88 in the human monocytic cell line THP-1 (11), and our study shows a similar association in human neutrophils. Btk may also associate with MAL/TIRAP and trigger the phosphorylation of this adaptor protein (11, 16). Recently, Btk was demonstrated to interact with MAL/TIRAP in human neutrophils (37). We also observed this interaction (Figure 7A). Furthermore, we observed an increase in the concentrations of MyD88 in neutrophils pretreated with LPS and stimulated with anti–IL-8:IL-8 immune complexes, and in cells stimulated with LPS alone. In agreement with these findings, the up-regulation of the expression of MyD88 was described in neutrophils infected with Mycobacterium bovis bacille Calmette-Guerin (41).
In conclusion, our observations suggest that the stimulation of LPS-“primed” neutrophils with anti–IL-8:IL-8 immune complexes may initiate the TLR4 cascade through an activation of MyD88. Further, a specific anti-FcγRIIa antibody was found to abrogate the effects of anti–IL-8:IL-8 complexes in LPS-treated neutrophils. We also stress that the MyD88-independent, TLR4-dependent pathway is not activated in human neutrophils (55). The results of our studies also confirm that Btk regulates MyD88 activity after the complex stimulation of LPS-pretreated neutrophils. Together, these findings indicate that anti–IL-8:IL-8 complexes influence the activation of Btk via FcγRIIa in LPS-“primed” cells.
Supplementary Material
Acknowledgments
The authors thank Dr. Thomas R. Martin (Veterans Adminsitration Puget Sound Medical Center, Seattle, WA) for help with the instigation and execution of this study.
Footnotes
This work was supported by National Institutes of Health grant HL073245 (A.K.K.) and American Heart Association grant GRNT12050309.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0039OC on December 13, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349 [DOI] [PubMed] [Google Scholar]
- 2.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. Lung neutrophils in the adult respiratory distress syndrome: clinical and pathological significance. Am Rev Respir Dis 1986;133:218–225 [DOI] [PubMed] [Google Scholar]
- 3.Martin TR, Pistorese BP, Hudson LD, Maunder RJ. Function of lung and blood neutrophils in patients with the adult respiratory distress syndrome: implications for the pathogenesis of lung infections. Am Rev Respir Dis 1991;144:254–262 [DOI] [PubMed] [Google Scholar]
- 4.Kurdowska A, Miller EJ, Noble JM, Baughman RP, Matthay MA, Brelsford WG, Cohen AB. Anti–interleukin-8 autoantibodies in alveolar fluid from patients with the adult respiratory distress syndrome. J Immunol 1996;157:2699–2706 [PubMed] [Google Scholar]
- 5.Kurdowska A, Noble JM, Steinberg KP, Ruzinski J, Hudson LD, Martin TR. Anti–interleukin-8 autoantibody:interleukin-8 complexes in the acute respiratory distress syndrome: relationship between the complexes and clinical disease activity. Am J Respir Crit Care Med 2001;163:463–468 [DOI] [PubMed] [Google Scholar]
- 6.Kurdowska A, Noble JM, Grant IS, Robertson R, Haslett C, Donnelly SC. Anti–interleukin-8 autoantibodies in patients at risk for the acute respiratory distress syndrome. Crit Care Med 2002;30:2335–2337 [DOI] [PubMed] [Google Scholar]
- 7.Krupa A, Kato H, Matthay MA, Kurdowska A. Proinflammatory activity of anti–IL-8 autoantibody:IL-8 complexes in alveolar edema fluid from patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 2004;286:L1105–L1113 [DOI] [PubMed] [Google Scholar]
- 8.Fudala R, Krupa A, Matthay MA, Allen TC, Kurdowska AK. Anti–IL-8 autoantibody:IL-8 immune complexes suppress spontaneous apoptosis of neutrophils. Am J Physiol Lung Cell Mol Physiol 2007;293:L364–L374 [DOI] [PubMed] [Google Scholar]
- 9.Krupa A, Fudala R, Stankowska D, Loyd T, Allen TC, Matthay MA, Gryczynski Z, Gryczynski I, Mettikolla YV, Kurdowska AK. Anti-chemokine autoantibody:chemokine immune complexes activate endothelial cells via IgG receptors. Am J Respir Cell Mol Biol 2009;41:155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen TC, Fudala R, Nash S, Kurdowska A. Anti–interleukin-8 autoantibody:interleukin-8 immune complexes visualized by laser confocal microscopy in injured lung: co-localization with FcγRIIa in lung tissues from patients with acute respiratory distress syndrome. Arch Pathol Lab Med 2007;131:452–456 [DOI] [PubMed] [Google Scholar]
- 11.Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, Walch E, Wirth T, O'Neill LA. Bruton’s tyrosine kinase is a Toll/interleukin-1 receptor domain–binding protein that participates in nuclear factor κB activation by Toll-like receptor 4. J Biol Chem 2003;278:26258–26264 [DOI] [PubMed] [Google Scholar]
- 12.Zemans RL, Arndt PG. Tec kinases regulate actin assembly and cytokine expression in LPS-stimulated human neutrophils via JNK activation. Cell Immunol 2009;258:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaeffer EM, Schwartzberg PL. Tec family kinases in lymphocyte signaling and function. Curr Opin Immunol 2000;12:282–288 [DOI] [PubMed] [Google Scholar]
- 14.Kawakami Y, Kitaura J, Hata D, Yao L, Kawakami T. Functions of Bruton’s tyrosine kinase in mast and B cells. J Leukoc Biol 1999;65:286–290 [DOI] [PubMed] [Google Scholar]
- 15.Yang WC, Collette Y, Nunes JA. Olive DTec kinases: a family with multiple roles in immunity. Immunity 2000;12:373–382 [DOI] [PubMed] [Google Scholar]
- 16.Piao W, Song C, Chen H, Wahl LM, Fitzgerald KA, O’Neill LA, Medvedev AE. Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance. J Biol Chem 2008;283:3109–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O’Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton’s tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem 2006;281:10489–10495 [DOI] [PubMed] [Google Scholar]
- 18.Page TH, Smolinska M, Gillespie J, Urbaniak AM, Foxwell BM. Tyrosine kinases and inflammatory signalling. Curr Mol Med 2009;9:69–85 [DOI] [PubMed] [Google Scholar]
- 19.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511 [DOI] [PubMed] [Google Scholar]
- 20.Fudala R, Stankowska D, Krupa A, Kurdowska AK. Cooperation between IgG receptors (FcgammaRIIa) and TLR4 in human neutrophils. FASEB J 2008;22 (Meeting Abstract Supplement):710 [Google Scholar]
- 21.Sylvester I, Yoshimura T, Sticherling M, Schroder JM, Ceska M, Peichl P, Leonard EJ. Neutrophil attractant protein–1–immunoglobulin G immune complexes and free anti–NAP-1 antibody in normal human serum. J Clin Invest 1992;90:471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurdowska A, Miller EJ, Cohen AB. An anti–interleukin-8 monoclonal antibody that interferes with the binding of interleukin-8 to cellular receptors and the activation of human blood neutrophils. Hybridoma 199514:225–233 [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration: increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem 2001;276:40156–40166 [DOI] [PubMed] [Google Scholar]
- 24.Sanders LA, Feldman RG, Voorhorst-Ogink MM, de Haas M, Rijkers GT, Capel PJ, Zegers BJ, van de Winkel JG. Human immunoglobulin G (IgG) Fc receptor IIA (CD32) polymorphism and IgG2-mediated bacterial phagocytosis by neutrophils. Infect Immun 1995;63:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallrabe H, Periasamy A. Imaging protein molecules using FRET and FLIM microscopy. Curr Opin Biotechnol 2005;16:19–27 [DOI] [PubMed] [Google Scholar]
- 26.Tucker TA, Dean C, Komissarov AA, Koenig K, Mazar AP, Pendurthi U, Allen T, Idell S. The urokinase receptor supports tumorigenesis of human malignant pleural mesothelioma cells. Am J Respir Cell Mol Biol 2010;42:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol 2010;10:1325–1334 [DOI] [PubMed] [Google Scholar]
- 28.O’Mahony DS, Pham U, Iyer R, Hawn TR, Liles WC. Differential constitutive and cytokine-modulated expression of human Toll-like receptors in primary neutrophils, monocytes, and macrophages. Int J Med Sci 2008;5:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomes NE, Brunialti MK, Mendes ME, Freudenberg M, Galanos C, Salomão R. Lipopolysaccharide-induced expression of cell surface receptors and cell activation of neutrophils and monocytes in whole human blood. Braz J Med Biol Res 2010;43:853–858 [DOI] [PubMed] [Google Scholar]
- 30.Jongstra-Bilen J, Puig Cano A, Hasija M, Xiao H, Smith CI, Cybulsky MI. Dual functions of Bruton’s tyrosine kinase and Tec kinase during Fcgamma receptor–induced signaling and phagocytosis. J Immunol 2008;181:288–298 [DOI] [PubMed] [Google Scholar]
- 31.van Mirre E, Breunis WB, Geissler J, Hack CE, de Boer M, Roos D, Kuijpers TW. Neutrophil responsiveness to IgG, as determined by fixed ratios of mRNA levels for activating and inhibitory FcgammaRII (CD32), is stable over time and unaffected by cytokines. Blood 2006;15:108:584–590 [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Masuda E, Blank MC, Kirou KA, Gao X, Park MS, Pricop L. Cytokine-mediated regulation of activating and inhibitory Fc gamma receptors in human monocytes. J Leukoc Biol 2005;77:767–776 [DOI] [PubMed] [Google Scholar]
- 33.Alvarez Y, Tang X, Coligan JE, Borrego F. The CD300a (IRp60) inhibitory receptor is rapidly up-regulated on human neutrophils in response to inflammatory stimuli and modulates CD32a (FcgammaRIIa) mediated signaling. Mol Immunol 2008;45:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, Powell MS, Tan PS, Cendron AC, Wines BD, Scott AM, Hogarth PM. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. J Immunol 2011;187:3208–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukhopadhyay S, Ramars AS, Dash D. Bruton’s tyrosine kinase associates with the actin-based cytoskeleton in activated platelets. J Cell Biochem 2001;81:659–665 [DOI] [PubMed] [Google Scholar]
- 36.Joseph RE, Xie Q, Andreotti AH. Identification of an allosteric signaling network within Tec family kinases. J Mol Biol 2010;403:231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, Takagi M, Mizutani S, Morio T. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat Immunol 2012;13:369–378 [DOI] [PubMed] [Google Scholar]
- 38.Miskolci V, Rollins J, Vu HY, Ghosh CC, Davidson D, Vancurova I. NFkappaB is persistently activated in continuously stimulated human neutrophils. Mol Med 2007;13:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belostocki K, Park MS, Redecha PB, Masuda E, Salmon JE, Pricop L. FcgammaRIIa is a target for modulation by TNF alpha in human neutrophils. Clin Immunol 2005;117:78–86 [DOI] [PubMed] [Google Scholar]
- 40.Rusu D, Drouin R, Pouliot Y, Gauthier S, Poubelle PE. A bovine whey protein extract can enhance innate immunity by priming normal human blood neutrophils. J Nutr 2009;139:386–393 [DOI] [PubMed] [Google Scholar]
- 41.Godaly G, Young DB. Mycobacterium bovis bacille Calmette Guerin infection of human neutrophils induces CXCL8 secretion by MyD88-dependent TLR2 and TLR4 activation. Cell Microbiol 2005;7:591–601 [DOI] [PubMed] [Google Scholar]
- 42.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and Toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol 2000;165:3541–3544 [DOI] [PubMed] [Google Scholar]
- 43.Pfeiffer A, Böttcher A, Orsó E, Kapinsky M, Nagy P, Bodnár A, Spreitzer I, Liebisch G, Drobnik W, Gempel K, et al. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur J Immunol 2001;31:3153–3164 [DOI] [PubMed] [Google Scholar]
- 44.Wallrabe H, Periasamy A, Talati R, Kim C, Barroso M. Confocal FRET and FLIM microscopy to characterize the distribution of transferrin receptors in membranes. Proc SPIE 2006;6089:608905.1–608905.9 [Google Scholar]
- 45.Dunn-Siegrist I, Leger O, Daubeuf B, Poitevin Y, Dépis F, Herren S, Kosco-Vilbois M, Dean Y, Pugin J, Elson G. Pivotal involvement of Fcgamma receptor IIA in the neutralization of lipopolysaccharide signaling via a potent novel anti-TLR4 monoclonal antibody 15C1. J Biol Chem 2007;282:34817–34827 [DOI] [PubMed] [Google Scholar]
- 46.Rittirsch D, Flierl MA, Day DE, Nadeau BA, Zetoune FS, Sarma JV, Werner CM, Wanner GA, Simmen HP, Huber-Lang MS, et al. Cross-talk between TLR4 and Fcgamma receptor III (CD16) pathways. PLoS Pathog 2009;5:e1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez de Diego R, López-Granados E, Pozo M, Rodríguez C, Sabina P, Ferreira A, Fontan G, García-Rodríguez MC, Alemany S. Bruton’s tyrosine kinase is not essential for LPS-induced activation of human monocytes. J Allergy Clin Immunol 2006;117:1462–1469 [DOI] [PubMed] [Google Scholar]
- 48.Mohamed AJ, Yu L, Bäckesjö CM, Vargas L, Faryal R, Aints A, Christensson B, Berglöf A, Vihinen M, Nore BF, et al. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev 2009;228:58–73 [DOI] [PubMed] [Google Scholar]
- 49.Desiderio S. Role of Btk in B cell development and signaling. Curr Opin Immunol 1997;9:534–540 [DOI] [PubMed] [Google Scholar]
- 50.Gilbert C, Levasseur S, Desaulniers P, Dusseault AA, Thibault N, Bourgoin SG, Naccache PH. Chemotactic factor–induced recruitment and activation of Tec family kinases in human neutrophils: II. Effects of LFM-A13, a specific Btk inhibitor. J Immunol 2003;170:5235–5243 [DOI] [PubMed] [Google Scholar]
- 51.Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, Coggeshall KM, McEver RP. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2–mediated slow leukocyte rolling. Blood 2010;116:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mueller H, Stadtmann A, Van Aken H, Hirsch E, Wang D, Ley K, Zarbock A. Tyrosine kinase Btk regulates E-selectin–mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma 2 and PI3K gamma pathways. Blood 2010;115:3118–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nisitani S, Satterthwaite AB, Akashi K, Weissman IL, Witte ON, Wahl MI. Posttranscriptional regulation of Bruton’s tyrosine kinase expression in antigen receptor-stimulated splenic B cells. Proc Natl Acad Sci USA 2000;97:2737–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Z, Mai A, Sun J. Lysine acetylation regulates Bruton’s tyrosine kinase in B cell activation. J Immunol 2010;184:244–254 [DOI] [PubMed] [Google Scholar]
- 55.Tamassia N, Le Moigne V, Calzetti F, Donini M, Gasperini S, Ear T, Cloutier A, Martinez FO, Fabbri M, Locati M, et al. The MyD88-independent pathway is not mobilized in human neutrophils stimulated via TLR4. J Immunol 2007;178:7344–7356 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.