Abstract
Mucous cell metaplasia is a hallmark of airway diseases, such as asthma and chronic obstructive pulmonary disease. The majority of human airway epithelium is pseudostratified, but the cell of origin of mucous cells has not been definitively established in this type of airway epithelium. There is evidence that ciliated, club cell (Clara), and basal cells can all give rise to mucus-producing cells in different contexts. Because pseudostratified airway epithelium contains distinct progenitor cells from simple columnar airway epithelium, the lineage relationships of progenitor cells to mucous cells may be different in these two epithelial types. We therefore performed lineage tracing of the ciliated cells of the murine basal cell–containing airway epithelium in conjunction with the ovalbumin (OVA)-induced murine model of allergic lung disease. We genetically labeled ciliated cells with enhanced Yellow Fluorescent Protein (eYFP) before the allergen challenge, and followed the fate of these cells to determine whether they gave rise to newly formed mucous cells. Although ciliated cells increased in number after the OVA challenge, the newly formed mucous cells were not labeled with the eYFP lineage tag. Even small numbers of labeled mucous cells could not be detected, implying that ciliated cells make virtually no contribution to the new goblet cell pool. This demonstrates that, after OVA challenge, new mucous cells do not originate from ciliated cells in a pseudostratified basal cell–containing airway epithelium.
Keywords: mucous cell metaplasia, pseudostratified airway epithelium, ovalbumin, ciliated cell, goblet or mucous cell
Clinical Relevance
Mucous metaplasia is a pathologic process in airway diseases, such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis. The cell of origin of newly formed mucous cells remains controversial. Knowing the progenitors of airway mucous cells will help to understand the cellular and molecular biology behind the pathologic process, and will also help to prevent the mucous cell metaplasia that eventually may produce the death of the patient.
Mucous metaplasia is one of hallmarks of respiratory diseases, such as asthma (1) and chronic obstructive pulmonary disease (COPD) (2). It is characterized by an increased number of mucous cells (goblet cells) in the airway epithelium and, consequently, an excess of airway mucus that can contribute to airway obstruction, mucus plugging, chronic cough, decreased pulmonary function, increased risk of infection, and death (3–7). Mouse models of the acute allergic response to inhaled allergens, such as ovalbumin (OVA), are frequently used to study features of clinical asthma, including the appearance of goblet cells (8–10). Mechanistically, IL-13 is known to promote goblet cell metaplasia in murine models of allergic lung disease (11–14), and increased levels of IL-13 are associated with asthma and COPD (3,15,16). Recently, SAM pointed domain containing ets transcription factor (SPDEF) and Forkhead box protein A3 (FOXA3) have been identified as transcription factors exclusively expressed in airway goblet cells, and the expression of both is increased after pulmonary allergen and IL-13 exposure (17, 18).
Prior animal studies of allergic lung disease largely focused on the murine small airway epithelium and, in aggregate, suggested that club cells (Clara) are the cells of origin of goblet cells after an allergen challenge. A decrease in the number of club cells accompanied by an increase in the number of mucous cells in the small airway columnar epithelium of OVA-challenged mice suggested that, in this region of the airway, club cells convert to mucous cells (19). Furthermore, using the OVA mouse model of airway disease, Evans and colleagues (20) observed mucin expression in a subset of club cells, again suggesting that club cells serve as progenitors of goblet cells. These observations were also consistent with another study showing that mucous cells produced in the airways of OVA-treated mice include many ultrastructural characteristics of club cells documented by electron microscopy (21). Finally, Chen and colleagues (18) used a direct lineage tracing approach to follow the fate of club cells after an OVA challenge and demonstrated that goblet cells do indeed arise from club cells. However, this study could not exclude the possibility that there was also a contribution of some small airway ciliated cells to the goblet cell pool. The study also did not address the cell of origin of mucous cells in pseudostratified basal cell–containing airway epithelium.
Of note, several studies have shown that ciliated cells can give rise to goblet cells in other contexts. In a mouse model of Sendai virus infection, epidermal growth factor receptor (EGFR) activation in ciliated cells inhibited apoptosis and led to the expression of goblet cell markers in these ciliated cells (22). The presence of cells possessing characteristic markers of both ciliated and mucous cells in the mouse and human airway epithelium, in vivo and in vitro, led the authors to postulate that ciliated cells transdifferentiate into mucous cells after IL-13 treatment (22, 23). Very recently, Turner and colleagues (24) also suggested that ciliated cells could convert to goblet cells using human airway epithelial cells in culture. These cells were transduced with lentiviral vectors containing Cre recombinase under the control of the Forkhead box protein J1 (FOXJ1) promoter and a cytomegalovirus (CMV)-driven floxed-EGFP construct to label ciliated cells. After IL-13 treatment, newly formed goblets cells were EGFP+, suggesting that the goblet cells were derived from FOXJ1-expressing ciliated cells. Thus, there may be differences in the cells of origin of mucous cells, depending upon the nature of the injury that induced the mucous metaplasia or the type of airway epithelium studied.
The majority of the human small airway epithelium affected in asthma and COPD is a pseudostratified basal cell–containing airway epithelium (25). In the mouse, this type of airway epithelium is found only in the trachea and mainstem bronchus. Despite these are the regions that most closely approximate the cellular architecture of the small airways of human lungs (25, 28), there is a paucity of literature characterizing the effects of OVA challenge on this region of the murine airway. We therefore performed lineage tracing of the ciliated cells of the murine pseudostratified airway epithelium in conjunction with the OVA-induced murine model of allergic lung disease. We genetically labeled ciliated cells with enhanced Yellow Fluorescent Protein (eYFP) before the allergen challenge and followed the fate of these cells specifically in the mouse trachea and bronchi to determine whether they gave rise to newly formed mucous cells. We concluded that ciliated cells do not give rise to goblet cells in OVA-induced mucous metaplasia in the region of the mouse airway that most closely approximates the cellular composition of the human small airways affected in COPD and asthma.
Material and Methods
Mouse Models
FOXJ1-Cre mice were previously described (29). Rosa26R-eYFP (Gt(Rosa)26Sortm1(eYFP)Cos)/J) mice were acquired from The Jackson Laboratory (Bar Harbor, ME). The OVA sensitization protocol was previously described (8). Animal protocols were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care in accordance with National Institutes of Health guidelines.
Immunofluorescence, histology, quantitative RT (qRT)-PCR, Western blot, and FACS protocols are specified in detail in the supplemental Material and Methods.
Statistical Analysis
Data were compared among groups using the Student’s t test. A P value of less than 0.05 was considered significant.
Results
Detailed Characterization of Mucous Cell Fate Induction in Pseudostratified Airway Epithelium after OVA Challenge
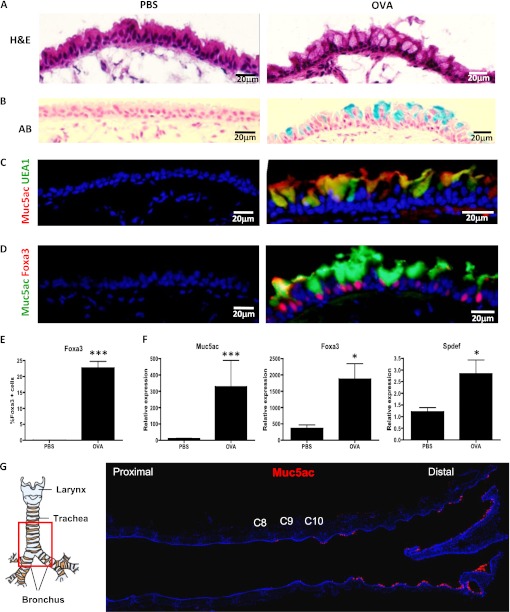
We used OVA challenge to induce mucous cell metaplasia in the mouse airways. We assessed mucous cell differentiation after the allergen challenge using immunohistochemistry for classic markers of mucous cells (mucins) as well as for newly identified transcription factors associated specifically with goblet cell fate (SPDEF and FOXA3) (30, 31). We then performed a numerical analysis of the cell fate distribution of airway epithelial cell types after OVA challenge using a standardized OVA challenge protocol in mice with a specific genetic background and at a specific region of the airway tree to ensure the reproducibility of our assays. C57BL6/J males (6 wk old) received two intraperitoneal injections of OVA on Days 0 and 10. At 10 days after the second injection, the mice were challenged with 1% OVA in PBS or saline alone for 20 minutes using a nebulizer. This procedure was repeated on three consecutive days and the mice were killed 48 hours after the third OVA or PBS challenge. We stained airway sections with hematoxylin and eosin and observed an increase in goblet cells in the distal trachea and major bronchi of mice subjected to nebulized OVA as compared with control mice that received nebulized saline (PBS) (Figure 1A).
Figure 1.
Mucous cells in the pseudostratified airway epithelium of ovalbumin (OVA)-challenged mice. Immunostaining of frozen sections of control mice (PBS) (left) and OVA-treated mice (right). The analysis was restricted to the distal part of the trachea and bronchi. (A) Hematoxylin and eosin (H&E) staining of mouse proximal airways 48 hours after OVA challenge demonstrates the presence of goblet cells. (B) Alcian Blue (AB) stains the newly formed goblet cells in OVA-treated mice. Blue staining identifies mucous-producing cells (C) Immunofluorescence for Muc5ac (red) and UEA1 (green) (D) Immunofluorescence for Muc5ac (green) and Foxa3 (red) on airway sections of PBS- and OVA-treated mice. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). (E) Percent of Foxa3+ cells in control and OVA-treated airways. (F) mRNA expression of mucous genes, Muc5ac, Foxa3, Spdef, assessed by quantitative RT (qRT)-PCR in airway epithelial cells from OVA-treated mice compared with control (PBS). mRNA was extracted from epithelial cells of the distal trachea and main bronchus of each mouse (n = 4/condition). The y axis represents relative quantification normalized to glyceraldehyde 3-phosphate dehydrogenase (Gapdh). Data shown are means (±SEM). *P < 0.05; ***P < 0.001. (G) The specific region of the murine proximal airways analyzed in this study. The red square delimits the region of the distal trachea and main bronchus that was studied. Transverse sections of the proximal airways stained for Muc5ac (red). Cartilage rings C8, C9, and C10 are highlighted. Figures are representative of three independent experiments (n = 4 mice/condition in each experiment). Scale bars, 20 μm.
In contrast to control airways, we detected Alcian Blue+ cells in the airways of OVA-treated animals, confirming that mucous-producing cells were induced in response to allergen (Figure 1B). We also confirmed the formation of bona fide mucous cells by immunofluorescence for Muc5ac, UEA1, and Foxa3 (Figures 1C and 1D). Almost all of the Muc5ac+ cells were positive for the lectin, UEA1 (Figure 1C), and all of the Muc5ac+ cells stained for Foxa3 (Figure 1D). The number of Foxa3+ cells in the OVA-treated airways was 377 out of a total of 1,676 epithelial cells (22.7 ± 9.4%) (Figure 1E). In control airways, we were unable to detect any cells that were positive for these markers. To ensure that a mucous cell differentiation program had been activated after OVA challenge, we analyzed the expression of mucous genes. We isolated RNA from airway epithelial cells obtained after papain dissociation of the distal trachea and mainstem bronchus of OVA- or PBS-treated mice and performed quantitative real-time PCR. As expected, the expression of the mucous genes, Muc5ac, Spdef, and Foxa3, was increased in the airway epithelial cells from OVA mice in comparison to those from control mice (Figure 1F). These data indicate that OVA exposure leads to the development of fully differentiated mucous cells in the mouse pseudostratified airway epithelium. Of note, in all of the airways analyzed we detected the development of mucous cells beginning at cartilage numbers 8 or 9, and the numbers of mucous cells increased distally along the trachea and the mainstem bronchi (Figure 1G). For this reason, we focused our analysis of mucous cell metaplasia in this region (Figure 1G).
Ciliated Cell Hyperplasia Occurs in the Murine Airway Epithelium after OVA Challenge
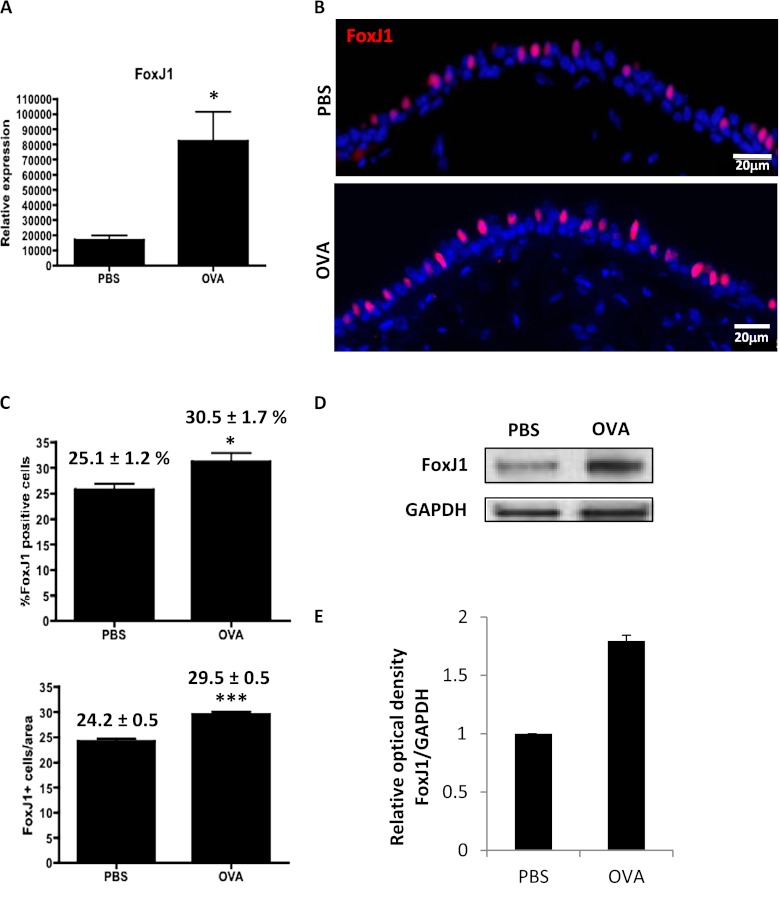
We first performed a quantitative examination of the behavior of ciliated cells in response to the OVA challenge. FoxJ1, a ciliated cell transcription factor, has been shown to be regulated by IL-13 (23), a cardinal proinflammatory cytokine that plays an important role in goblet cell hyperplasia and metaplasia in human asthma. Given a known increase in IL-13 after OVA exposure (13, 14), we examined whether the expression of FoxJ1 was altered as a consequence of OVA exposure. In fact, the expression of FoxJ1, as detected by qRT-PCR, was significantly up-regulated in the airway epithelium of OVA-challenged mice (Figure 2A). We proceeded to examine whether the increase in FoxJ1 reflected an expansion in the numbers of ciliated cells. Immunostaining for FoxJ1 was performed to detect ciliated cells (Figure 2B). Quantification of FoxJ1+ cells in these airways revealed a significant increase in the number of ciliated cells in the distal trachea of the OVA-treated mice, where mucous cells develop (Figure 2C; see also Table E1 in the online supplement). Control mice possessed 24.2 (±0.5) FoxJ1+ cells per 250 μm basement membrane, representing 25.1 (±1.2)% of the total cells (1,201 out of 4,679 airway epithelial cells), whereas the OVA-treated mice showed 29.5 (±0.5) FoxJ1+ cells per 250 μm basement membrane, representing 30.5 (±1.7)% of total cells (1,659 out of 5,330 airway epithelial cells). Consistent with these results, we observed an increase in the protein levels of FoxJ1 in the airway epithelial cells of the OVA-treated mice measured by Western Blot (Figure 2D). Densitometric measurement of the bands and normalization to glyceraldehyde 3-phosphate dehydrogenase revealed an optical density of 1.8 (±0.1) in the OVA-challenged airways compared with controls (Figure 2E). These results demonstrate that an early response of the airways to OVA challenges includes not only an increase in the number of mucous cells, but also of ciliated cells.
Figure 2.
Ciliated cell hyperplasia occurs in the OVA-induced mucous cell metaplasia model. (A) mRNA expression of FoxJ1 assessed by qRT-PCR in airway epithelial cells from OVA-treated mice compared with control (PBS). The y axis represents relative quantification normalized to Gapdh. (B) Immunofluorescence for FoxJ1 (red) on airway sections of PBS- and OVA-treated mice. Sections were counterstained with DAPI (blue) (C) Percent and absolute number of FoxJ1+ cells in control (PBS) and OVA-treated airways. A total of 4,679 epithelial cells were counted in controls, and 5,330 were counted in OVA-challenged airways. The numbers represent the percentages of ciliated cells (FoxJ1+) and the absolute number of FoxJ1+ cells/250 μm basement membrane counted in the distal trachea and main bronchus (average of posterior, middle, and anterior regions) of four animals in three different experiments. (D) Expression of FoxJ1 at the protein level is detected by Western Blot. GAPDH is shown as loading control. (E) Quantification was performed by measuring the optical density of the FoxJ1 band relative to that of GAPDH. Bars represent normalized relative optical density that was arbitrarily set to 1 for the control (PBS). The mean value of the optical density ratio for the OVA-treated airways is expressed as a fold of the control value. Figures are representative of three independent experiments (n = 4 mice/condition each). Scale bars, 20 μm. mRNA and protein were extracted from epithelial cells isolated from distal trachea and main bronchus in five mice per condition. Total protein extracts were isolated and pooled from airway epithelial cells of five mice per condition. Data shown are means (±SEM). *P < 0.05; ***P < 0.001.
Neither Ciliated Cells nor Mucous Cells Proliferate after OVA Challenge
We reasoned that the increased number of ciliated cells might result from their own proliferation or the proliferation of a different cell type and subsequent differentiation of this other cell type to a ciliated cell. Even though ciliated cells are believed to be postmitotic (32), OVA exposure may impact the behavior of ciliated cells by stimulating them to re-enter the cell cycle and proliferate, a phenomenon seen with other cell types that were previously thought to be postmitotic.
We analyzed cell proliferation in OVA-treated airways and found that there was a dramatic increase in the number of the proliferation marker Ki67+ cells in the OVA-challenged epithelium (28.9 ± 10.0%) compared with controls (5.3 ± 2.0%) (Figure 3A). However, this proliferation was almost exclusively in nonciliated cells (Figure 3B). Among a total of 487 epithelial cells, 141 were Ki67+ and only 1 was FoxJ1+ Ki67+. Furthermore, 5-bromo-2′-deoxyuridine (BrdU) incorporation assays also demonstrated that even though OVA challenge induced a significant increase in proliferation, FoxJ1+ ciliated cells were not proliferating (0 out of 29 BrdU+ cells, in a total of 319 epithelial cells; Figure 3C). Immunostaining for p63 (basal cell marker) and Scgb1a1 (club cell marker) revealed that 42.2 (±7.0)% of the proliferating cells (Ki67+) were basal cells (p63+) (Figure 3D), whereas 46.7 (±5.4)% were club cells (Scgb1a1+) (Figure 3E). In addition, after the continuous administration of BrdU to label all replicating cells during and after OVA challenge, none of the newly formed mucous cells had incorporated BrdU, as no BrdU+ Foxa3+ cells were detected (Figure 3F). Thus, mucous cells do not replicate, and they do not arise from a replicating club or replicating basal cell after acute OVA challenge.
Figure 3.
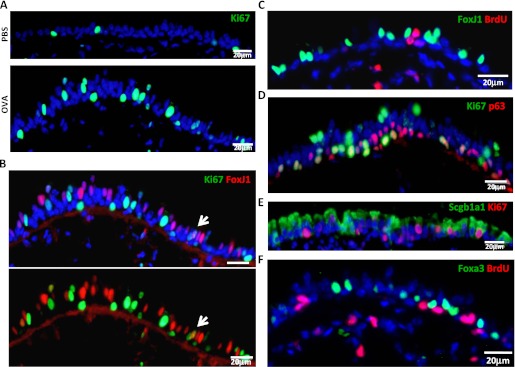
Neither ciliated cells nor mucous cells proliferate after acute OVA challenge, and mucous cells do not arise from replicating progenitor cells. (A) Immunostaining for the Ki67 protein (green) of control and OVA-challenged airway sections. (B) Double immunostaining for FoxJ1 (red) and Ki67 (green) in OVA-challenged airways. The arrow points to the only double-positive cell detected. Ciliated cells do not proliferate after OVA exposure, because the proliferation marker, Ki67, does not colocalize with the ciliated marker, FoxJ1 (C) The 5-bromo-2′-deoxyuridine (BrdU) immunodetection (red) in combination with FoxJ1 (green) in OVA-treated airway section also demonstrates that ciliated cells do not replicate. Immunostaining for Ki67 (red) and p63 (green) (D) or Scgb1a1 (E) demonstrates that both club and basal cells do replicate after OVA challenge. (F) BrdU (red) is not incorporated into mucous cells (Foxa3+) (green) after the continuous administration of BrdU (1 mg/ml) in drinking water during mucous cell development (a total of 5 d). Images are representative of three independent experiments (n = 4 mice/condition in each experiment). Images are taken from the distal trachea and main bronchus. Scale bars, 20 μm.
Newly Formed Mucous Cells Do Not Express Ciliated Cell Markers
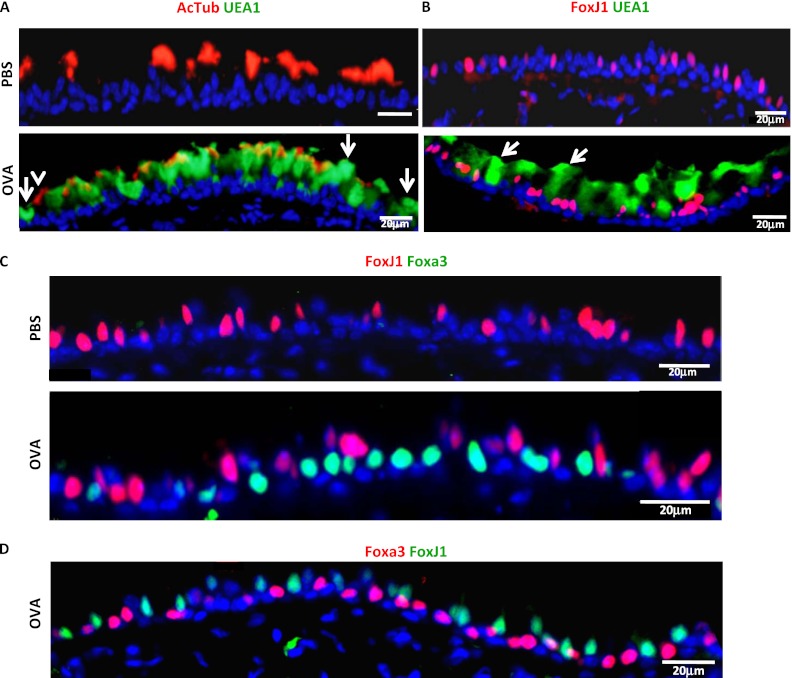
If ciliated cells can act as mucous cell progenitors, one might expect to detect a transitional cell expressing ciliated and mucous markers at the same time. To assess whether newly formed mucous cells show markers of ciliated cell origin, we first analyzed whether the mucous cells coexpress the ciliated cell markers, FoxJ1 or acetylated tubulin. We stained our samples with an antibody against acetylated tubulin (cilia marker) and UEA1 (a lectin that binds to mucous cells) (18) (Figures 1C and 4A). We also used the transcription factor, FoxJ1, to identify ciliated cells (Figure 4B), and we did not detect any FoxJ1+UEA1+ cells (Figure 4B). Furthermore, we performed double immunofluorescence for FoxJ1 and Foxa3. These transcription factors identify the nuclei of ciliated and mucous cells, respectively, allowing for definitive localization of markers to a single cell. We demonstrated that, in the airways of OVA-challenged mice, FoxJ1 and Foxa3 are mutually exclusive (Figure 4C). We found 0 FoxJ1+ cells out of 141 Foxa3+ cells. Therefore, newly formed mucous cells (Foxa3+) do not express the ciliated cell transcription factor, FoxJ1. Control airways showed no positive cells for UEA1 or Foxa3 (Figures 4A–4C). Because this analysis was performed late in the differentiation process (48 h after the third OVA challenge, when functional Muc5ac+ mucous cells have already formed), we assayed for the presence of transitional double-positive cells earlier. The earliest time point at which we detected immature mucous cells (Foxa3+) not yet expressing Muc5ac was 8 hours after the third OVA exposure (data not shown). At that time, we confirmed that none of the immature Foxa3+ mucous cells expressed the ciliated cell transcription factor, FoxJ1 (Figure 4D). These data indicate that the goblet cells, which develop as a consequence of OVA challenge, do not express any ciliated cell markers, suggesting that the cell of origin of a mucous cell is not a ciliated cell in the pseudostratified epithelium of the mouse.
Figure 4.
Newly formed mucous cells do not express ciliated cell markers. Double immunofluorescence for acetylated tubulin (red) and UEA1 (green) (A), FoxJ1 (red) and UEA1 (green) (B), and FoxJ1 (red) and Foxa3 (green) (C) was performed on airways of the distal trachea and main bronchus from PBS- or OVA-treated mice 48 hours after OVA challenge. Arrows in (A) indicate cells positive for UEA1 only, whereas the arrowhead indicates a cell positive only for acetylated tubulin. Arrows in (B) points to cells positive for UEA1 only. (D) Immunostaining for FoxJ1 (green) and Foxa3 (red) 8 hours after the last OVA challenge. In all cases, ciliated cell markers do not colocalize with mucous cell markers. AcTub, acetylated tubulin. Scale bars, 20 μm. Images are representative of three independent experiments (n = 4 mice/condition in each experiment).
FOXJ1-Cre–Based eYFP Lineage Labeling Marks the Majority of Ciliated Cells
To determine whether ciliated cells give rise to goblet cells after OVA challenge, we used a genetic lineage tracing strategy to permanently label ciliated cells and all of their potential descendants (33). This strategy is the most direct approach to determine whether ciliated cells are the parents of the newly formed mucous cells in our in vivo model of mucous metaplasia. To lineage trace ciliated cells and determine whether they contribute to goblet cell formation, we used FOXJ1-Cre; Rosa26R eYFP mice (29) in which Cre recombinase is placed under the control of the human FOXJ1 promoter to mediate loxP site recombination and subsequent eYFP expression in ciliated cells. It has been previously shown that this mouse specifically labels ciliated cells of the airways and ciliated cells of other tissues (29).
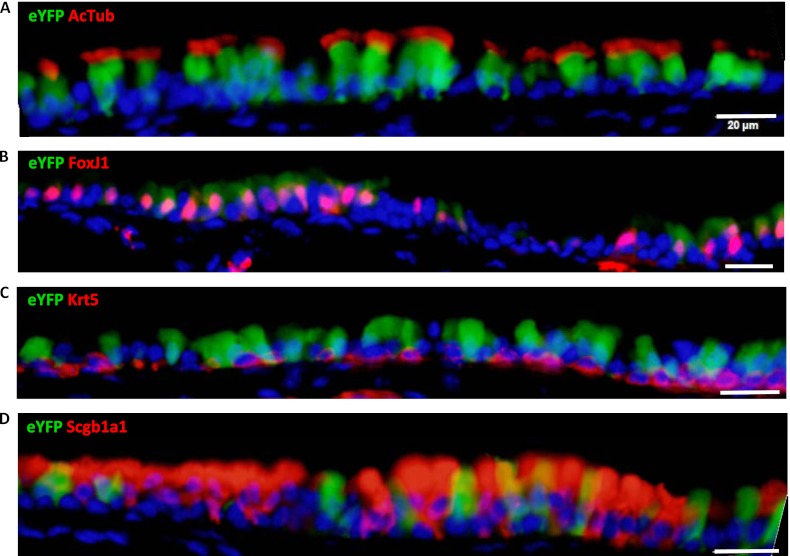
We first examined the specificity of the recombination in our specific mouse strains in the pseudostratified large airway epithelium by assessing eYFP expression. We observed eYFP expression in ciliated cells as marked by coexpression of acetylated tubulin and FoxJ1 (Figures 5A and 5B) and not in basal cells, detected by cytokeratin 5 (Figure 5C). The majority of the FoxJ1+ cells were labeled (80 eYFP+ cells out of 96 FoxJ1+ cells). However, we also detected some unlabeled FoxJ1+ cells (16 out of 96 eYFP+ cells) and a small minority of club cells (Scgb1a1+) that were also labeled (11 eYFP+ out of 109 Scgb1a1+ cells; 11 Scgb1a1+ out of 75 eYFP+ cells) (Figure 5D). This observation is consistent with the possibility that there are club-to-ciliated transitional cells expressing both FoxJ1 and Scgb1a1 in the murine tracheal epithelium.
Figure 5.
FOXJ1-Cre; Rosa26R eYFP mice show most of their airway ciliated cells labeled. Airway sections from FOXJ1-Cre; Rosa26R eYFP mice (n = 4) were stained for enhanced Yellow Fluorescent Protein (eYFP) (green) and markers for ciliated cells (acetylated tubulin [A] and FoxJ1 [B]), basal cells (cytokeratin 5 [Krt5]) (C), and club cells (Scgb1a1) (D) in red. eYFP colocalizes with acetylated tubulin and FoxJ1, indicating that the majority of the ciliated cells are labeled. In rare cases, eYFP is detected in club cells (Scgb1a1+ cells), but not in basal cells. eYFP expression in club cells likely reflects club cells that are in the process of becoming ciliated cells. Scale bars, 20 μm.
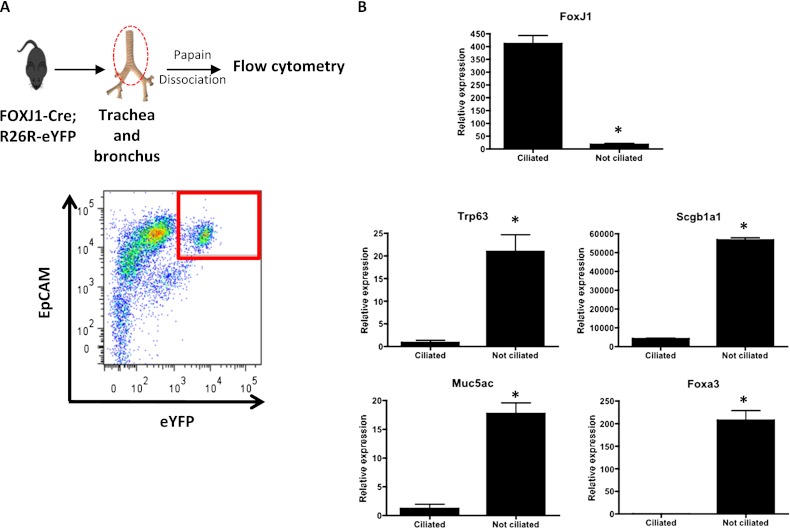
To determine the purity of the labeled population at the transcriptional level, we first isolated epithelial cell adhesion molecule (EpCAM)+ tracheal epithelial cells from FOXJ1-Cre; Rosa26R eYFP mice by flow cytometry, and verified that 15.1% of EpCAM+ cells were eYFP+ (Figure 6A). Furthermore, we verified by immunostaining after the sorting and cytospin that the large majority of the eYFP+ cells expressed ciliated cell markers, and that ∼85% of ciliated cells were labeled after Cre recombination (data not shown), consistent with the immunofluorescence results. To confirm the ciliated cell identity of the labeled population, we sorted out EpCAM+ eYFP+ cells, isolated RNA from these cells, and performed qRT-PCR for cell type–specific genes (Figure 6B). The sorted population expressed FoxJ1, but not Trp63 (basal cells), Scgb1a1 (club cells), Muc5ac, or Foxa3 (goblet cells). These results verify the specificity of the lineage tracing system (FOXJ1-Cre; Rosa26R eYFP mice) in the murine pseudostratified epithelium, and confirm that the labeled population includes most of the airway ciliated cells in this region of the murine airway.
Figure 6.
Labeled cells express FoxJ1 and do not express markers of nonciliated cells. (A) Airway epithelial cells from trachea and major bronchi were isolated from FOXJ1-Cre; Rosa26R eYFP mice (n = 6) and stained with epithelial cell adhesion molecule (EpCAM), a panepithelial marker. EpCAM+ eYFP+ cells were detected and sorted by FACS (delimited by red square in the plot). (B) RNA was isolated from the sorted population, and gene expression was analyzed by qRT-PCR. FoxJ1 is expressed in the labeled sorted population, whereas genes for other cell types (Trp63 [dNp63 isoform], Scgb1a1, Muc5ac, Foxa3) are expressed in the other population of cells, confirming that EpCAM+ eYFP+ cells are ciliated cells. The relative quantification of mRNA expression was normalized to Gapdh. Data shown are means (±SEM). *P < 0.05.
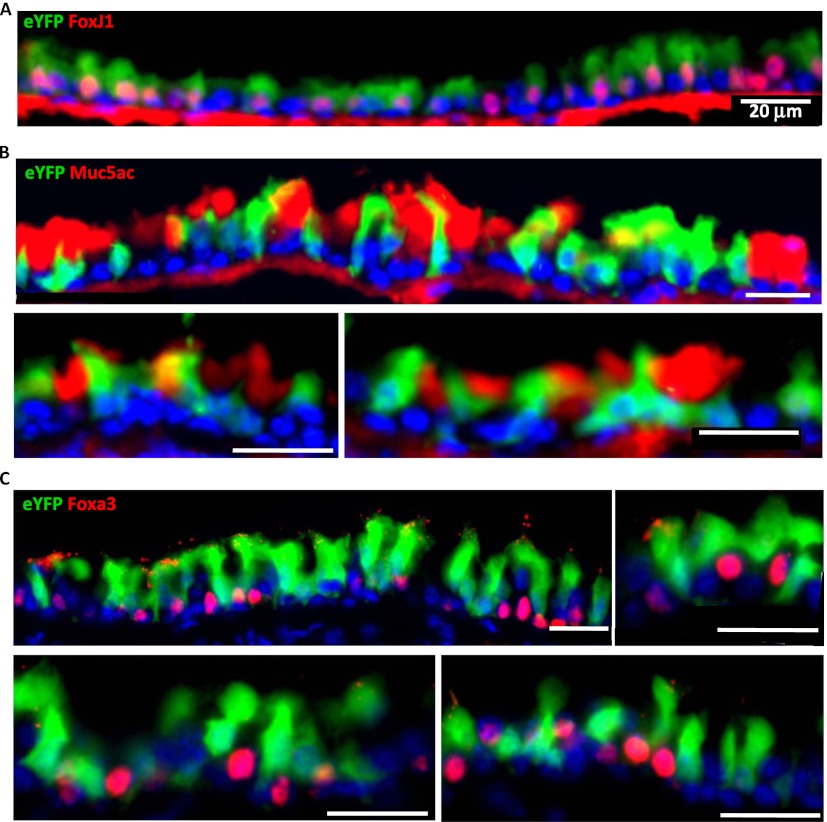
Newly Formed Mucous Cells Are Not Derived from Labeled Ciliated Cells in OVA-Induced Mucous Metaplasia
To definitively establish whether or not ciliated cells are a cell of origin of newly formed mucous cells in our OVA-induced mucous metaplasia model, we challenged 6-week-old FOXJ1-Cre; Rosa26R eYFP male mice with OVA, as previously described here. At 48 hours after the third OVA challenge, airways were collected and processed for analysis. We observed that most of FoxJ1+ cells were eYFP+ (105 eYFP-labeled FoxJ1+ cells out of 117 FoxJ1+ cells; Figure 7A). However, labeled eYFP+ cells did not colocalize with Muc5ac, indicating that mucous cells developed as a consequence of OVA exposure are not derived from FoxJ1+ cells (Figure 7B). Furthermore, Foxa3 (a nuclear mucous cell marker) is not detected in the eYFP-labeled cells (Figure 7C). We found that, out of 85 Foxa3+ cells detected, none were positive for eYFP+, indicating that Foxa3+ mucous cells are not derived from labeled ciliated cells. These results indicate that, in our in vivo mouse model of mucous metaplasia of acute allergic lung disease, the newly formed mucous cells of the basal cell–containing pseudostratified epithelium are not derived from ciliated cells. In fact, there is an absence of evidence that such a phenomenon occurs even as a rare event in this form of injury in this understudied region of the murine respiratory tree.
Figure 7.
Newly formed mucous cells after OVA challenge are not derived from ciliated cells. Airways from FOXJ1-Cre; Rosa26R eYFP mice (n = 4) challenged with OVA were collected and analyzed. Immunofluorescence was performed for eYFP (green) and FoxJ1 (A), Muc5ac (B), and Foxa3 (C) (red). Multiple representative panels are shown for each immunostaining. eYFP labels ciliated FoxJ1+ cells, but not mucous cells (Muc5AC+, Foxa3+), indicating that the new goblet cells are not derived from FoxJ1+ cells in murine pseudostratified airway epithelium. Images are representative of the analysis of distal trachea and main bronchus in four animals. Scale bars, 20 μm.
Discussion
The murine OVA model has been extensively used as a model of allergic lung disease and to model features of asthma (8). Most of the previous studies that examined the epithelium have focused on the small airways of the mouse (18–21), which possess a columnar epithelium containing club and ciliated cells, but lack basal progenitor cells (28). Evans and colleagues (20) reported, in great detail, the changes in the number of goblet, club, and ciliated cells exclusively in the non–basal cell–containing mouse airways over a 6-hour to 90-day time course after OVA exposure. A similar study using stereology demonstrated that the cellular changes happened in response to OVA challenge in the distal airway epithelium (19). However, the majority of human airway epithelium affected in asthma and COPD is pseudostratified (25). Because pseudostratified airway epithelium contains distinct progenitor cells from simple columnar airway epithelium, the lineage relationships of progenitor cells to mucous cells may be different in these two epithelial types (26–28). Furthermore, since there is evidence that ciliated, club, and basal cells can all give rise to mucous-producing cells in different contexts, we sought to understand the lineage relationships of progenitor cells to mucous cells in the proximal mouse airway epithelium, because it most closely resembles the cellular architecture of human small airways (25) (Figure 1G). We document and characterize the development of mucous cells in the murine distal trachea and mainstem bronchial pseudostratified epithelium. We characterize the airways by hematoxylin and eosin and Alcian Blue, and we deploy the classical and newly identified transcription factor markers of mucous cells to unambiguously label mucous cells, and to confirm their definitive cell fate (18). In addition, the up-regulation of the mucous cell–specific genes, Muc5ac, Foxa3, and Spdef, confirmed the activation of a mucous differentiation program that results in the development of goblet cells in this region of the airway epithelium of mice challenged with OVA.
Surprisingly, we noted an increase in the number of ciliated cells after OVA challenge. We speculate that the increase in ciliated cells in this basal cell–containing epithelium may allow an early and rapid response of the airway epithelium to an environmental challenge. However, our lineage data demonstrate that this increased pool of ciliated cells does not give rise to mucous cells. Of note, we examined the number of ciliated cells only at 48 hours after the OVA challenge. Thus, we cannot rule out the possibility that, at a later time point, the number of ciliated cells normalizes (20) or even decreases, as may happen in severe recurrent asthma (34).
Furthermore, our Ki67 and BrdU labeling experiments demonstrate that, after the OVA challenge, neither the ciliated cells nor the newly formed mucous cells are replicating. We do however demonstrate that approximately half of the club cells and half of the basal cells are replicating. Because our mucous cells did not label with BrdU even when it was continuously administered throughout the allergen challenge, this suggests that mucous cells are directly arising from club or basal cells that have themselves not replicated at any time during the OVA challenge before they convert to mucous cells. We speculate that the replicating basal and club cell populations are meant to replenish the nonreplicating basal and/or club cells that have been converted to ciliated or mucous cells. It will be of interest to assay whether replicating progenitors are necessary to fuel a continual supply of new mucous cells in chronic models of allergen exposure. If BrdU was administered throughout a chronic allergen challenge, BrdU+ but Ki67− mucous cells would indicate that a progenitor cell incorporated BrdU as a replicating club and/or basal cell, and then subsequently differentiated into a postmitotic mucous cell. Most importantly, lineage tracing of basal and club cells after OVA challenge will clarify which of these cells is the progenitor of the newly formed ciliated and mucous cells in our model system. It is indeed possible that both of these progenitor cell types could act as progenitors for both ciliated and mucous cells.
As caveats to our experiments, we note that, in our ciliated cell lineage tracing experiments, we cannot completely rule out the possibility that the 15% of unlabeled ciliated cells could become mucous cells. We suggest that this is a very unlikely possibility, because approximately 25% of the cells in the airway epithelium become mucous-producing cells without a replication event. CreER drivers are often preferred for lineage tracing experiments in adult mice. In fact, we have repeated the lineage tracing experiments with FOXJ1CreER2T; Rosa26R-eYFP mice (35), where 50.1% of the ciliated cells are labeled with eYFP after a pulse of tamoxifen, and we did not detect any eYFP+ goblet cells (data not shown). This result is also consistent with the literature showing that club cells can serve as the cells of origin of mucous cells in the murine small airway (18).
Our results that demonstrate that ciliated cells do not convert to mucous cells in the murine pseudostratified epithelium should not be generalized to other forms of injury or to other areas of the respiratory tree or across species. Despite their postmitotic state (35–37), ciliated cells are known to change their shape and gene expression during airway repair (38), suggesting that their behavior may depend on the specific type of injury model used in any given study. Evidence for transdifferentiation of ciliated to mucous cells after IL-13 treatment (23, 24), or to squamous cells after naphthalene-induced injury (38), has been reported. Indeed, a recent study used a lineage tracing strategy in vitro, and suggested that FoxJ1-Cre; EGFP cells give rise to goblet cells in an air–liquid interface platform of epithelial cultures (24). This study used human cells, an in vitro system, and a lentiviral infection to introduce FOXJ1-Cre and cytomegalovirus (CMV)-floxed-EGFP constructs into cells. The many differences between this system and our model include the use of modified human cells compared with mouse cells and, most importantly, a reliance on in vitro cultures (23, 24). It is increasingly clear that cells become more plastic in culture systems than they are in vivo, and this may explain the differing results in these studies. Furthermore, because epithelial cells respond to varied mesenchymal and immune cells (39), different in vivo models of mucous metaplasia associated with differing immune responses, such as the Sendai virus model (22) as compared with the OVA model, may cause mucous metaplasia by different mechanisms. As noted previously here, the evidence for human in vitro ciliated cell–to–mucous cell transition could be due to the use of an in vitro assay. However, it cannot be ignored, and merits further exploration in actual human asthma tissues samples, where colabeling for ciliated and mucous cell markers could suggest, but not prove, a ciliated cell origin of human goblet cells in true human asthma.
By taking advantage of modern murine genetic models, our results provide complementary and stringent evidence that ciliated cells do not contribute to the development of mucous cells in vivo in the murine pseudostratified airway epithelium after OVA challenge, and are consistent with previous reports showing that club cells give rise to goblet cells after OVA challenge in small airways (18). We note again that we have not clarified whether club cells or basal cells, or both, could serve as mucous cell progenitor populations in the specified region of the murine airway epithelium (40). Further experiments using lineage tracing to label club and basal cells must be done to establish the quantitative contribution of each of these progenitor cell populations to mucous metaplasia. Indeed, it is possible that chronic allergen challenge may require the recruitment of different progenitor cells that are unnecessary in acute challenges, as the supply of functional mucous cells may need to be chronically replenished in the setting of chronic injury.
Supplementary Material
Acknowledgments
The authors thank Michael J. Holtzman for providing the FOXJ1-Cre mice, and Benjamin Medoff for providing advice on the use of the ovalbumin-sensitization protocol. They thank Prescott Woodruff for extended discussion and advice on the appropriate methodology to accurately count cell numbers on tissue sections. They also thank Purushothama Rao Tata, Borja Saez, Jayme Dowdall, and Naveen Nunna for reviewing this manuscript, and all of the members of the Rajagopal Laboratory for their constructive criticisms and comments and for valuable discussion and support.
Footnotes
This work was supported by National Institutes of Health–National Heart, Lung, and Blood Institute Early Career Research New Faculty (P30) award 5P30HL101287-02 and Harvard Stem Cell Institute Junior Investigator Grant (J.R.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0146OC on December 13, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Aikawa T, Shimura S, Sasaki H, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992;101:916–921 [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J. Epidemiological studies in mucus hypersecretion. Novartis Found Symp 2002;248:3–19 [PubMed] [Google Scholar]
- 3.Rogers DF. The airway goblet cell. Int J Biochem Cell Biol 2003;35:1–6 [DOI] [PubMed] [Google Scholar]
- 4.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med 2006;38:116–125 [DOI] [PubMed] [Google Scholar]
- 5.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 2002;109:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev 2000;173:27–38 [DOI] [PubMed] [Google Scholar]
- 7.Randell SH, Boucher RC; University of North Carolina Virtual Lung Group Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 2006;35:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HY, DeKruyff RH, Umetsu AT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 2010;11:577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol 2007;8:369–378 [DOI] [PubMed] [Google Scholar]
- 11.Atherton HC, Jones G, Danahay H. IL-13–induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 2003;285:L730–L739 [DOI] [PubMed] [Google Scholar]
- 12.Alimam MZ, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol 2000;22:253–260 [DOI] [PubMed] [Google Scholar]
- 13.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261 [DOI] [PubMed] [Google Scholar]
- 15.Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol 2006;34:527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278 [DOI] [PubMed] [Google Scholar]
- 17.Park K, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 2007;117:978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 2009;119:2914–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reader JR, Tepper JS, Schelegle ES, Aldrich MC, Putney LF, Pfeiffer JW, Hyde DM. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol 2003;162:2069–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi T, Ishii A, Nakai S, Hasegawa K. Ultrastructure of goblet-cell metaplasia from Clara cell in the allergic asthmatic airway inflammation in a mouse model of asthma in vivo. Virchows Arch 2004;444:66–73 [DOI] [PubMed] [Google Scholar]
- 22.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 2006;116:309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomperts BN, Kim LJ, Flaherty SA, Hackett BP. IL-13 regulates cilia loss and foxj1 expression in human airway epithelium. Am J Respir Cell Mol Biol 2007;37:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner J, Roger J, Fitau J, Combe D, Giddings J, Heeke GV, Jones CE. Goblet cells are derived from a FOXJ1-expressing progenitor in a human airway epithelium. Am J Respir Cell Mol Biol 2011;44:276–284 [DOI] [PubMed] [Google Scholar]
- 25.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driskell RR, Engelhardt JF. Stem cells in the adult lung. : Blau H, Gaerhart J, Hogan B, Melton D, Moore M, Pedersen R, Thomas D, Thomson JA, Verfaillie C, Weissmann I, et al., Handbook of stem cells. Vol. 2 San Diego: Academic Press; 2004. pp. 547–554 [Google Scholar]
- 27.Liu X, Driskell RR, Engelhardt JF. Stem cells in the lung. Methods Enzymol 2006;419:285–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009;106:12771–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Huang G, Shornick LP, Roswit WT, Shipley JM, Brody SL, Holtzman MJ. A transgenic FOXJ1-Cre system for gene inactivation in ciliated epithelial cells. Am J Respir Cell Mol Biol 2007;36:515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akinci E, Banga A, Greder LV, Dutton JR, Slack JM. Reprogramming of pancreatic exocrine cells towards a beta (β) cell character using Pdx1, Ngn3 and MafA. Biochem J 2012;442:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 2006;133:2455–2465 [DOI] [PubMed] [Google Scholar]
- 33.Kretzschmar K, Watt FM. Lineage tracing. Cell 2012;148:33–45 [DOI] [PubMed] [Google Scholar]
- 34.Thomas B, Rutman A, Hirst RA, Haldar P, Wardlaw AJ, Bankart J, Brightling CE, O’Callaghan C. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J Allergy Clin Immunol 2010;126:722–729e2 [DOI] [PubMed] [Google Scholar]
- 35.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol 2008;295:L231–L234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but nor alveolar, epithelium. Cell Stem Cell 2009;4:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA 2007;104:410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 2005;34:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holgate ST, Davies DE, Puddicombe S, Richter A, Lackie P, Lordan J, Howarth P. Mechanisms of airway epithelial damage: epithelial–mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl 2003;44:24s–29s [DOI] [PubMed] [Google Scholar]
- 40.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011;8:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.