Abstract
Silymarin inhibits UVB-induced immunosuppression in mouse skin. To identify the molecular mechanisms underlying this effect, we used an adoptive transfer approach in which dendritic cells (DCs) from the draining lymph nodes of donor mice that had been UVB-exposed and sensitized to 2,4,-dinitrofluorobenzene (DNFB) were transferred into naïve recipient mice. The contact hypersensitivity (CHS) response of the recipient mice to DNFB was then measured. When DCs were obtained from UVB-exposed donor mice that were not treated with silymarin, the CHS response was suppressed confirming the role of DCs in the UVB-induced immunosuppression. Silymarin treatment of UVB-exposed donor mice relieved this suppression of the CHS response in the recipients. Silymarin treatment was associated with rapid repair of UVB-induced cyclobutane pyrimidine dimers (CPDs) in DCs and silymarin treatment did not prevent UV-induced immunosuppression in XPA-deficient mice which are unable to repair UV-induced DNA damage. The CHS response in mice receiving DCs from silymarin-treated UV-exposed donor mice also was associated with enhanced secretion of Th1-type cytokines and stimulation of T cells. Adoptive transfer of T cells revealed that transfer of either CD8+ or CD4+ cells from silymarin-treated, UVB-exposed donors resulted in enhancement of the CHS response. Cell culture study showed enhanced secretion of IL-2 and IFNγ by CD8+ T cells, and reduced secretion of Th2 cytokines by CD4+ cells, obtained from silymarin-treated UVB-exposed mice. These data suggest that DNA repair-dependent functional activation of DCs, a reduction in CD4+ regulatory T-cell activity, and stimulation of CD8+ effector T cells contribute to silymarin-mediated inhibition of UVB-induced immunosuppression.
Keywords: DNA repair, contact hypersensitivity, cyclobutane pyrimidine dimer, nucleotide excision repair, silymarin, ultraviolet radiation
1. Introduction
Excessive exposure of skin to solar ultraviolet (UV) radiation is known to suppress the immune system and UV-induced immunosuppression has been implicated in the promotion of the risk of skin cancer [1-3]. Chronically immunosuppressed patients who live in regions of intense sun exposure have an exceptionally high rate of skin cancer [4]. The incidence of skin cancers, especially squamous cell carcinomas (SCCs), also is increased among organ transplant recipients who require prolonged immunosuppressive therapy [5-8], which is consistent with the hypothesis that immune surveillance is an important mechanism in the prevention of the generation and maintenance of neoplastic cells [9]. UV radiation-induced damage of epidermal Langerhans cells, a subpopulation of dendritic cells (DCs) in the skin, is considered to be an important mechanism in UV-induced immunosuppression [10, 11]. Indeed, epidermal Langerhans cells are considered to be the principal targets of UV radiation as it inhibits their antigen-presenting activity, their capacity to stimulate type 1 T helper (Th) cells, and renders them tolerogenic [12, 13]. UV-induced DNA damage, predominantly in the form of generation of cyclobutane pyrimidine dimers (CPDs), is a molecular trigger of UV-mediated immunosuppression and the initiation of photocarcinogenesis [14, 15]. UV-induced DNA damage in antigen-presenting cells appears to play a key role in suppression of immune reactions in the skin. For example, UV-irradiated DCs can adoptively transfer immune tolerance when they are injected intravenously into mice that are not irradiated with UV. This further indicates that UV-irradiated DCs have a reduced ability to stimulate helper and effector T cells and implies that DNA damage may contribute to the development of UV-induced tolerogenic DCs [16, 17]. There is evidence indicating that DNA repair mechanisms are related directly to the function of DCs in terms of their stimulation of T cells and the induction of immune reactions [16, 17]. Repair of CPDs in Langerhans cells has been correlated with increased function in terms of induction of contact hypersensitivity (CHS) and reversal of the tolerogenic response as evidenced by an enhancement of interferon (IFNγ) production by T cells [16]. This indicates that Langerhans cells that have incurred UV-induced DNA damage are the key effectors of UV-induced immunosuppression.
Silymarin is isolated from the plant milk thistle (Silybum marianum L. Gaertn.). It is composed of primarily silibinin (≈90%) with small amounts of other silibinin stereoisomers, such as isosilybin and dihydrosilybin, etc. [18]. Silibinin is the major active constituent of silymarin and the anti-carcinogenic properties of silymarin and silibinin are almost identical. The results of studies using animal models have demonstrated that silymarin is an effective skin cancer chemopreventive agent that exhibits no toxicity in vivo [19]. It possesses strong antioxidant and anti-inflammatory properties [19, 20] and has the ability to protect epidermal keratinocytes from UV radiation-induced apoptotic cell death through a mechanism involving repair of the damaged DNA [21]. Topical treatment of mouse skin with silymarin, either before or after UVB exposure, prevents UVB-induced immunosuppression through a currently undefined mechanism that is associated with inhibition of interleukin (IL)-10 expression and stimulation of IL-12 production in skin and draining lymph nodes [22]. Thus, the focus of the current study was to define the chemopreventive mechanisms and molecular targets in the protection afforded by silymarin against UV-induced immunosuppression with an emphasis on the association of repair of UVB-induced DNA damage and immunomodulation.
Here, we report the results of analysis of the effects of silymarin in UVB-exposed wild-type and xeroderma pigmentosum complementation group A (XPA)-knockout mice, which are deficient in repair of UV-induced DNA damage because of the absence of a nucleotide excision repair (NER) mechanism. The data indicate that prevention of UVB-induced immunosuppression by silymarin is mediated through enhanced repair of damaged DNA in UVB-irradiated DCs, which leads to restoration of their functional activity. We further investigated whether silymarin affects the development of CD8+ effector T cells or CD4+ regulatory T cells, which have been shown to play critical roles in the CHS response in UV-exposed mice. Our data suggest that silymarin inhibits UVB-induced immunosuppression by stimulating CD8+ effector T cells and diminishing the activity of CD4+ regulatory T cells.
2. Materials and methods
2.1. Silymarin, chemicals, and antibodies
Silymarin was procured from Sigma Chemical Co. (St. Louis, MO) and stored at -20°C. For topical application, silymarin was dissolved in acetone and applied uniformly at a concentration of 1.0 mg/cm2 skin area. The dose of silymarin was selected based on its chemopreventive effects against UV-induced immunosuppression in mice [22].
Lipopolysaccharide (LPS) and carboxyfluorescein succinimidyl ester (CFSE) were purchased from Sigma Chemical Co. (St. Louis, MO). Anti-mouse CD3e and GM-CSF were purchased from BD Bioscience (San Diego, CA). ELISA kits for analysis of mouse IFNγ, IL-12, IL-4 and IL-10 were purchased from eBioscience (San Diego, CA), while antibody specific for CPDs was obtained from Kamiya Biomedical (Seattle, WA). Anti-mouse Langerin/CD207 antibody was purchased from Dendritics (Dardilly France). Microbeads conjugated to monoclonal anti-mouse CD8/CD4 or anti-mouse CD11c antibodies and the MACS system used for the purification of immune cells were obtained from Miltenyi Biotec (Auburn, CA).
2.2. Animals
Female C3H/HeN mice of 4-6 weeks of age were purchased from Charles River Laboratories. The XPA-KO mice on a C3H/HeN background were bred in the University of Alabama at Birmingham Animal Resource Facility, as described previously [21, 23]. All mice were maintained under standard conditions of a 12-h dark/12-h light cycle, relative humidity of 50 ± 10% and a temperature of 24 ± 2°C. The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
2.3. UVB irradiation of mice
The shaved backs of the mice were UVB irradiated using a band of 4 FS20 UVB lamps (Daavlin; UVA/UVB Research Irradiation Unit, Bryan, OH) equipped with an electronic controller to regulate UV dosage, as described previously [21, 23]. The UV lamps emit UVB (280-320 nm) and UVA (320-375 nm), with UVC emission being insignificant. The majority of the resulting wavelengths of UV radiation are in the UVB range (290-320 nm), with a peak emission at 314 nm. As XPA-KO mice lack NER mechanisms they are susceptible to UVB radiation and were therefore exposed to 20mJ/cm2 of UVB, whereas their wild-type counterparts (C3H/HeN) were exposed to the routine intensity of UVB (150 mJ/cm2).
2.4. Contact hypersensitivity assay
The UVB-induced suppression of CHS in mice was determined as described previously [22]. Briefly, the shaved backs of the mice were UVB irradiated for 4 consecutive days. Topical application of silymarin was started at least 3 days before UVB irradiation and the mice were treated with silymarin at least 30 min before each UVB exposure. Twenty-four hours after the last UVB exposure, the mice were sensitized by painting 25 μL of 0.5% 2, 4-dinitrofluorobenzene (DNFB) on the shaved UVB-irradiated skin site. The CHS response was elicited 5 days later by challenging the ear of each mouse with 20 μL of DNFB (0.2% in acetone). Ear thickness was measured 24 h after the challenge using an engineers’ micrometer (Mitutoyo, Tokyo, Japan) and compared with the thickness just before challenge. Non-UVB irradiated mice that were sensitized with the same dose of DNFB served as a positive control, whereas the non-UVB-irradiated mice that received only ear challenge with DNFB served as a negative control.
2.5. Adoptive transfer of CD11c+ cells
For adoptive transfer of CD11c+ cells, the donor mice were exposed to UVB radiation (wild-type, 150 mJ/cm2; XPA-KO, 20 mJ/cm2) for four consecutive days with or without treatment with silymarin. Twenty-four h after the last UVB exposure, mice were sensitized by painting DNFB (25 μL of 0.5%) on the UVB-irradiated skin site. Twenty-four h after sensitization, the mice were sacrificed, and single-cell suspensions were prepared from the regional lymph nodes. CD11c+ cells were then positively selected using the MACS system following the manufacturer's instructions (Miltenyi Biotec, Inc), which yielded cells that were >90% CD11c+ DCs. These DCs (5× 105 cells/mouse) were injected subcutaneously into untreated naïve C3H/HeN mice. Five days later, DNFB was applied to the ear skin of the recipient mice and the ear swelling response was determined as described above.
2.6. Preparation of bone marrow-derived dendritic cells (BM-DCs)
BM-DCs were prepared from bone marrow as described previously [24, 25]. Briefly, normal mice were sacrificed and the femurs collected, cleaned and sterilized by dipping in 70% ethanol for 5 min. The bone marrow cells were collected in RPMI 1640 media under a sterile hood. After lysis and removal of red blood cells, the B cells and T cells were depleted using Dynal beads. The remaining cells were washed, suspended in dendritic cell medium [RPMI supplemented with 10% FBS, GM-CSF (10 ng/mL) and IL-4 (10 ng/mL)], and cultured in this media for 5 days. LPS (5 μg/mL) was then added to the culture media to induce maturation of dendritic cells and the cells were harvested the following day. These BM-DCs were ≈95% CD11c+ cells.
2.7. Dot-blot assay for DNA damage
UVB-induced DNA damage in BM-DC and its repair by silymarin was determined using dot-blot analysis, as described previously [21, 23]. Briefly, cells were treated with or without silymarin (0, 5, 10 and 20 μg/mL) for 30 min before irradiation to UVB (5 mJ/cm2). Cells were harvested immediately or 24 h later. Genomic DNA from the cells was isolated following standard procedures. Genomic DNA (100 ng) was transferred to a positively charged nitrocellulose membrane by vacuum dot-blotting and fixed by baking the membrane for 2 h at 80°C. After blocking the non-specific binding sites in blocking buffer, the membrane was then incubated with the antibody specific to CPDs for 1 h at room temperature. After washing, the membrane was incubated with HRP-conjugated secondary antibody. The CPDs were detected by chemiluminescence using an ECL detection system.
2.8. Immunohistochemical detection of langerin-positive dendritic cells and langerin- plus CPD-positive (double positive) cells in vivo mouse skin
Dorsal skin area of the mouse was exposed to UV with or without treatment with silymarin. Twenty-four h after exposure, mice were euthanized, and dorsal skin samples were collected and frozen in OCT medium. For immunohistochemical detection of langerin-, CPDs- or double-positive cells, 5μM thick frozen sections were kept in 70mM NaOH solution in 70% ethanol for 2 min to denature nuclear DNA followed by neutralization in 100 mM Tris-HCl buffer in 70% ethanol. The sections were washed and permeabilized with 0.2% Triton-X100 in PBS for 20 min. After blocking the non-specific binding with 5% bovine serum albumin, sections were incubated with antibodies specific for CPDs and biotinylated langerin/CD207. Sections were counterstained with Alexa fluor488 (green color for CPDs) and streptavidin-Alexa fluor594 (red color for langerin). Sections were mounted with Vectashield mounting medium for fluorescence and stained with DAPI. Positive cells were detected under a fluorescence equipped Olympus microscope BX51 fitted with a Qcolor DP71 digital camera, and photographs were taken.
2.9. Dendritic cell isolation from the lymph nodes of mice, in vitro stimulation and analysis of cytokines
Single-cell suspensions of the draining lymph nodes were prepared as described previously [24, 26] and CD11c+ DCs positively selected using a MACS system according to the manufacturer's instructions (Miltenyi Biotec, Inc.). The purified DCs were then incubated with LPS (5μg/mL) for 48 h, the cell culture supernatants collected by centrifugation, and the levels of cytokines, IL-12, IFNγ and IL-10 measured using cytokine-specific ELISA kits.
2.10. In vitro stimulation of CD4+ T cells by DCs and analysis of T-cell proliferation
Mice were UVB irradiated for three consecutive days with or without treatment with silymarin, as described above. Twenty-four h after the last UVB exposure, the mice were sacrificed, the lymph nodes harvested and CD11c+ cells purified using the MACS system. Responder T cells were CD4+ T cells isolated from naïve C3H/HeN mice that were not UVB exposed or silymarin treated. Briefly, single cell suspensions were prepared from spleens of naïve mice and were incubated with ACK buffer (Lonza) on ice for 5 min to lyse red blood cells. The remaining cells were washed and incubated with microbeads conjugated to anti-CD4 antibodies and CD4+ T cells were separated using the MACS system according to the manufacturer's instructions (Miltenyi Biotec, Inc.). To examine T cell division, the purified CD4+ T-cells were labeled with CFSE, a fluorescent dye that is routinely used to monitor T-cell division [27-29]. The CD11c+ cells were cultured with the CFSE labeled CD4+ T cells for 4 days in the complete RPMI 1640 medium in the presence of soluble anti-CD3e (5.0 μg/mL). Cultures without the addition of anti-CD3 antibody served as background controls. Dividing T cells showed decreased levels of fluorescence (low fluorescence intensity) compared to non-dividing cells (high fluorescence intensity), which was analyzed by flow cytometry. Fluorescence positive cells (CFSE labeled CD4+ T cells) were gated and percentages of cells with high or low fluorescence intensity were evaluated. T cells from the negative controls (without anti-CD3 antibody) showed a minimal background level of dividing cells and set as controls for non-dividing cells. High percentages of dividing cells represent high levels of CD4+ T cell proliferation.
2.11. Purification of T-cell subpopulations (CD8+ and CD4+) and adoptive transfer
For the adoptive transfer of T-cell subpopulations, CD8+ and CD4+ T cells were isolated from single-cell suspensions of the spleens and lymph nodes of DNFB-sensitized donor mice using microbeads conjugated to monoclonal anti-mouse CD8 or anti-CD4 monoclonal antibody and the MACS system according to the manufacturer's instructions (Miltenyi Biotech, Inc.). Briefly, the donor mice (C3H/HeN) with or without pre-treatment with silymarin were exposed to UVB radiation for four consecutive days. Twenty-four h after the last UVB exposure, mice were sensitized with DNFB on the UVB-irradiated skin site. Five days after sensitization, the mice were sacrificed and the spleens and regional lymph nodes were harvested for the isolation of CD8+ and CD4+ T cells.
In one set of experiments, purified CD8+ T cells (8× 106) were injected i.v. into naïve recipients. In these experiments, mice were challenged with DNFB immediately after injection of cells. In a second set of experiments, purified CD4+ T cells (8× 106) were injected i.v. into recipient mice, which were sensitized 24 h later by epicutaneous application of DNFB on the shaved abdominal skin. Five days later, they were challenged by application of DNFB on the ear skin. Ear thickness was measured before and 24 h after challenge. Groups of naïve mice, which were not sensitized but were ear challenged, served as negative controls, while mice which were both sensitized and challenged served as a positive control.
2.12. In vitro stimulation of T-cell subpopulations by BM-DCs and analysis of Th1 and Th2 cytokines
C3H/HeN mice were UVB irradiated with and without treatment with silymarin for three consecutive days, and sensitized with DNFB 24 h after the last UV exposure, as detailed above. The mice were sacrificed 5 days later, the spleens and draining lymph nodes were collected, single-cell suspensions were prepared and the CD8+ and CD4+ T-cell subpopulations were purified as described above. BM-DCs were prepared from naïve mice and were used for in vitro stimulation of primed T cells, as described earlier [24, 25]. The purified CD8+ and CD4+ T cells (2× 106/ml) were stimulated or co-cultured separately with the BM-DCs (2× 105/mL) and the culture supernatants were collected 48 h later by centrifugation. The supernatants were analyzed for Th1 and Th2 cytokines using cytokine-specific ELISA kits.
2.13. Statistical analysis
The difference between experimental groups in terms of the CHS response and the levels of cytokines were analyzed using the Student's t test. A p value <0.05 was considered significant.
3. Results
3.1. Silymarin inhibits UVB-induced suppression of the CHS response by enhancing the functionality of dendritic cells in UVB-exposed mice
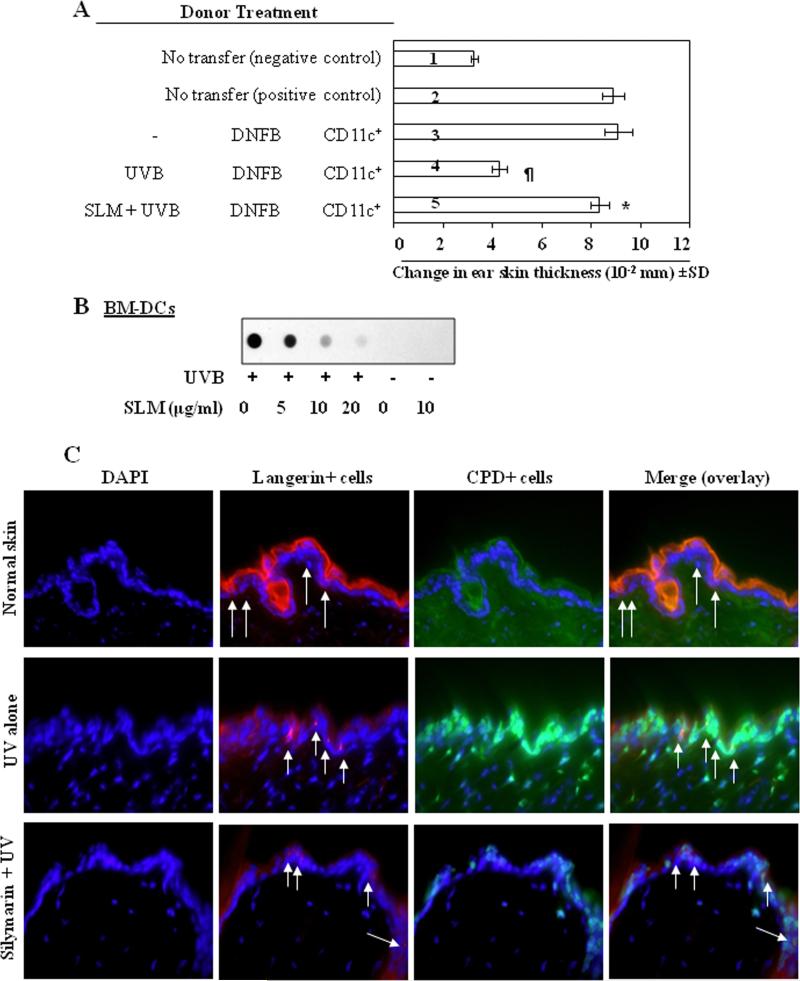
To determine whether inhibition of UVB-induced suppression of CHS by silymarin in mice is mediated through photoprotection of DCs, we used an adoptive transfer approach. As described in detail in the Materials and Methods section, the donor (C3H/HeN) mice were exposed to UVB with or without topical treatment with silymarin, and then sensitized to DNFB. Twenty-four h after sensitization, the mice were sacrificed and DCs (CD11c+ cells) were positively selected from the lymph nodes. The DCs were then injected subcutaneously into naïve mice and the CHS response measured. As shown in Figure 1A, those naïve recipient mice that had received CD11c+ cells from silymarin-treated, UVB-exposed donor mice showed a significantly greater CHS response (5th bar) than the naïve mice that received cells from the UVB-exposed mice that were not treated with silymarin (4th bar). This suggested that the prevention of UVB-induced immunosuppression by silymarin is mediated through a mechanism associated with preservation of the functional activity of the DCs.
Figure 1.
Effect of silymarin on UVB-induced suppression of the CHS response and DNA damage in C3H/HeN mice. (A), Topical treatment of mice with silymarin improves the ability of DCs to induce the CHS response. Donor mice (C3H/HeN) treated with or without silymarin were UVB-irradiated and sensitized with DNFB 24 h after the last UVB exposure. Mice were sacrificed 24 h after sensitization, single-cell suspensions of the lymph nodes were prepared, and CD11c+ cells were positively selected using MACS system, as detailed in the Materials and methods. Naïve recipient mice were injected subcutaneously with the CD11c+ cells (5× 105) obtained from donor mice. Recipient mice were ear challenged with DNFB 5 days after injection of cells, and the ear thickness was measured before and 24 h after challenge. The change in ear thickness is reported as the mean of millimeters (× 10-2) ± SD, n=5 per group. *Significantly greater CHS response versus recipient of CD11c+ from UVB plus DNFB treated mice, P<0.001; ¶Significantly lower CHS response versus the positive control (DNFB-sensitized) group, P<0.001. (B), Analysis of CPDs by dot-blot assay. Silymarin repairs UVB-induced DNA damage in vitro in BM-DCs obtained from C3H/HeN mice. BM-DCs were exposed to UVB radiation (5 mJ/cm2) with or without pretreatment with silymarin, harvested either immediately or 24 hours later. Genomic DNA from various treatment groups was isolated and subjected to dot-blot analysis using an antibody specific to CPDs. SLM= silymarin. (C), Treatment of mice with silymarin enhanced the repair of UV-induced DNA damage in epidermal DCs (langerin-positive cells). Langerin-positive cells are shown by red fluorescence and CPD-positive cells are shown by green fluorescence. Arrows indicate langerin-positive or double-positive cells (langerin + CPDs). Representative photomicrographs are shown, n=3/group. Magnification, ×400.
3.2. Silymarin enhances the repair of UV-induced DNA damage in BM-DCs
As it has been shown that UV-induced CPDs are an important molecular trigger for UV-induced immunosuppression [14, 15], we next determined whether silymarin treatment enhanced the repair of UVB-induced CPDs in DCs. For this purpose, BM-DCs, with or without pretreatment with silymarin (0, 5, 10 and 20 μg/mL for 30 min), were exposed to UVB radiation (5mJ/cm2). The cells were harvested immediately or 24 h after UVB exposure. Immediately after UVB exposure, there was no significant difference in the levels of CPDs as analyzed by dot-blot analysis, whether the cells were treated with silymarin or not treated with silymarin (data not shown). However, when the cells were analyzed 24 h after UVB irradiation, a marked reduction in the intensity of the dot-blot of the UVB-induced CPDs as compared with nonsilymarin-treated UVB-exposed BM-DCs was observed (Figure 1B), suggesting that silymarin acts to repair UVB-induced CPDs in BM-DCs.
3.3. Repair of UVB-induced DNA damage in DC by silymarin is required for enhancement of the functional ability of DCs
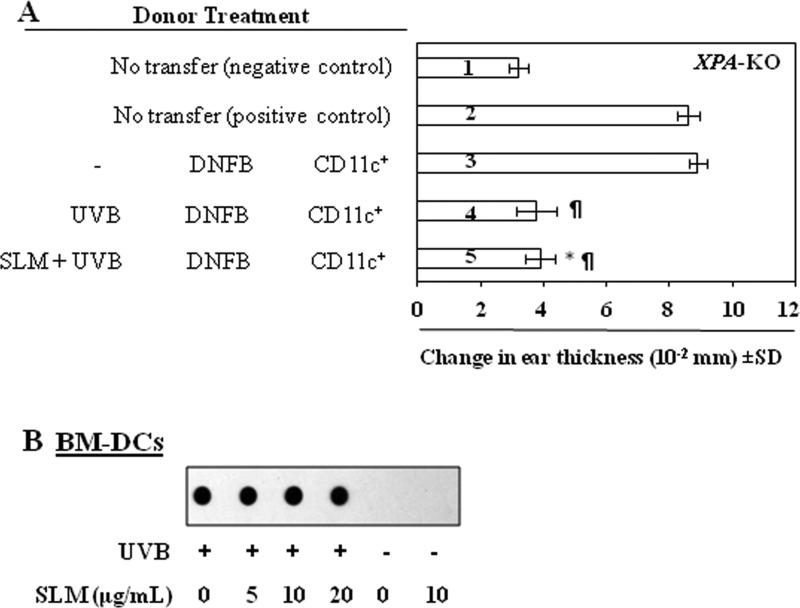
To verify whether silymarin acts to inhibit UVB-induced immunosuppression through rapid repair of damaged DNA that promotes the restoration of the functional ability of DCs, we used XPA-KO mice. As these mice are devoid of NER mechanisms they are unable to repair CPDs. The adoptive transfer protocol was as described above except that CD11c+ cells isolated from XPA-KO mice were injected subcutaneously into naïve wild-type (C3H/HeN) mice. As shown in Figure 2A, the mice that received CD11c+ cells from silymarin-treated, UVB-exposed XPA-KO donor mice did not show a greater CHS response (5th bar) than mice that received cells from the UVB-exposed XPA-KO mice that were not treated with silymarin (4th bar). This suggested that silymarin treatment was not able to enhance the functional ability of CD11c+ cells obtained from UVB-exposed XPA-KO mice. We further tested whether silymarin was able to repair UVB-induced CPD formation in BM-DCs obtained from the XPA-KO mice. Twenty-four h after UVB-irradiation there was no significant difference in the levels of CPDs, as determined by dot-blot analysis (Figure 2B), in BM-DCs from XPA-KO mice whether they were treated with silymarin or not treated with silymarin.
Figure 2.
Effect of silymarin on UVB-induced suppression of CHS response and DNA damage in XPA-deficient mice. (A), Topical treatment of XPA-deficient mice with silymarin does not improve the ability of DCs to induce CHS in naïve mice. Donor mice (XPA-deficient) were treated with or without silymarin and CD11c+ cells from lymph nodes were positively selected using MACS system as described in Materials and methods. Recipient mice (C3H/HeN) were injected subcutaneously with 5× 105 CD11c+ cells obtained from donor mice (XPA-deficient). Recipient mice were ear challenged with DNFB 5 days after injection of cells, and ear skin thickness was measured before and 24 h after challenge. The change in ear thickness is reported as the mean of millimeters (× 10-2) ± SD, n=5 per group. *No significantly greater CHS response in silymarin treated mice versus recipient of CD11c+ from UVB plus DNFB treated mice. ¶Significantly lower CHS response versus the positive control (DNFB-sensitized) group, P<0.001. (B), Analysis of CPDs by dot-blot assay. Silymarin does not stimulate repair of UVB-induced DNA damage in BM-DCs obtained from XPA-deficient mice. Results are shown from a single experiment that is representative of two independent assays.
3.4. Silymarin enhances repair of UVB-induced DNA damage in epidermal DCs in wild-type mice but does not repair in DCs from XPA-KO mice
The DNA repair ability of silymarin was further verified in DCs in vivo mouse model. As shown in Figure 1C, compared to non-UV-exposed mouse skin, UV exposure to the skin of wild-type mice induced a large number of CPD-positive cells (green color) while decreased the number of langerin-positive DCs (red color) in the epidermis. In contrast, the numbers of CPD-positive cells were less while the number of langerin-positive cells were higher in the skin of silymarin-treated group of mice compared to non-silymarin treated UV exposed mouse skin. The data on double-positive staining panel indicate that the majority of langerin-positive cells did not show the presence of DNA damage in silymarin-treated group of mice compared to those which were langerin and CPD double positive in the skin of non-silymarin-treated mice but exposed to UV. These results suggest that silymarin enhanced the repair of UV-induced DNA damage in langerin-positive subset of dendritic cells. This effect of silymarin was not observed in the DCs of XPA-KO mouse skin under identical experimental conditions (data not shown).
3.5. Silymarin increases the levels of cytokines in CD11c+ cells obtained from UVB-exposed wild-type mice but not UVB-exposed XPA-deficient mice
Next we determined whether the repair of the DNA damage and restoration of the functionality of DCs is associated with the ability of silymarin to enhance the production of Th1 type cytokines by UVB-irradiated DCs. Both XPA-KO mice and their wild-type counterparts, with or without silymarin treatment, were UVB irradiated for three consecutive days. Twenty-four h after the last UVB exposure, CD11c+ cells were isolated from the lymph nodes, cultured in the presence of LPS for 48 h and the levels of cytokines in the supernatants determined by ELISA. As shown in Table 1, the DCs obtained from UVB-exposed wild-type mice produced significantly lower amounts of IFNγ and IL-12 (55% and 79% respectively, P<0.001) than DCs obtained from wild-type mice not exposed to UVB. The DCs obtained from wild-type mice that had been treated by topical application of silymarin prior to UVB-irradiation produced significantly higher levels of IFNγ and IL-12 (70% and 283% respectively, P<0.001) than DCs obtained from wild-type mice that had not been treated with silymarin. Moreover, the levels of IL-10, an immunosuppressive cytokine, were significantly lower (P<0.05) in the DCs obtained from the silymarin-treated UVB-exposed wild-type mice. In contrast, the production of IFNγ, IL-12, or IL-10 by DCs obtained from silymarin-treated UVB-exposed XPA-KO mice was no different than DCs obtained from silymarin-untreated UVB-exposed XPA-KO mice.
Table 1.
Silymarin enhances the production of IFNγ and IL-12 by DCs obtained from UVB-irradiated wild-type mice but not DCs obtained from XPA-KO mice.
| Cytokines | Wild-type |
XPA-KO |
||||
|---|---|---|---|---|---|---|
| Control | UVB | SLM +UVB | Control | UVB | SLM+ UVB | |
| IFNγ | 3989 | 1765¶ | 3010* | 2508 | 1764¶ | 1883 |
| IL-12 | 57 | 12¶ | 46* | 21 | 5¶ | 7 |
| IL-10 | 288 | 394** | 300† | 203 | 339** | 371 |
Wild-type and XPA-KO mice that were treated or not-treated with silymarin were exposed to UVB irradiation three times on consecutive days. Mice were sacrificed 24 h after the last UVB exposure and DCs (CD11c+ cells) were positively selected from the lymph nodes. DCs were stimulated with LPS (5μg/mL) for 48 h. After incubation, cells were harvested and supernatants collected. The concentrations of cytokines in the cell supernatants were estimated by ELISA. The data are presented as the mean in terms of pg/2 million cells, n=5.
Significant decrease versus non-UVB control, P<.001
significant increase versus UVB alone, P<0.001.
Significant decrease versus UVB alone, P<0.05.
Significant increase versus non-UVB control P<0.01.
SLM= silymarin.
3.6. Silymarin promotes the ability of DCs from UVB-irradiated wild-type, but not XPA deficient, mice to stimulate T cell proliferation in vitro
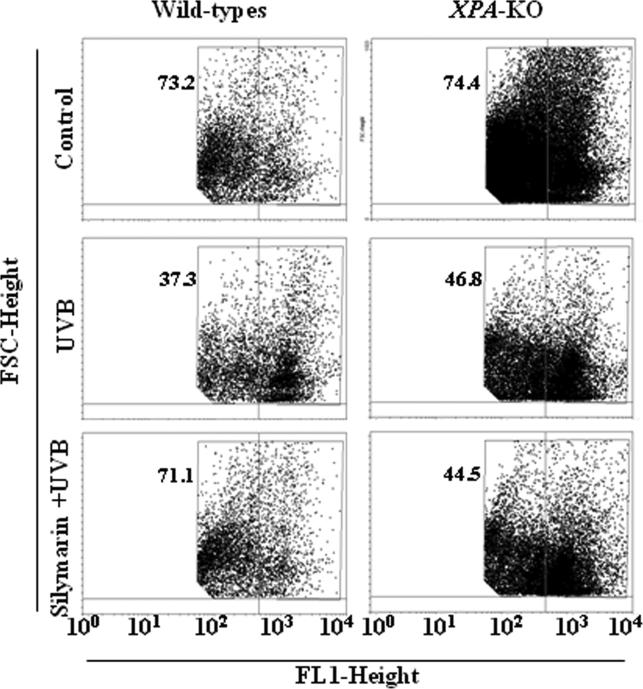
We then sought to determine whether the silymarin-mediated chemopreventive effects on UVB-induced immunosuppression are associated with DC-induced stimulation of T-cell development. CD4+ T cells were isolated from the spleens of naïve wild-type mice, labeled with CFSE, and co-cultured with CD11c+ cells isolated from the lymph nodes of variously treated wild-type and XPA-KO mice. After 4 days, the cells were harvested and the proliferation index of the CD4+ T cells was determined by FACS analysis. The proliferative index of the CD4+ T cells co-cultured with DCs prepared from UVB-irradiated wild-type mice was significantly lower than that of CD4+ T cells co-cultured with DCs from wild-type mice that were not irradiated with UVB. The proliferative index of the CD4+ T cells co-cultured with DCs from silymarin-treated UVB-irradiated wild-type mice was significantly higher (P<0.001) than that of CD4+ T cells co-cultured with non-silymarin-treated UVB-exposed wild-type mice (Figure 3, left panels). In contrast, there was no significant difference in the proliferation index of CD4+ T cells co-cultured with DCs obtained from silymarin-treated and silymarin-untreated UVB-exposed XPA-KO mice (Figure 3, right panels). The data on proliferating cells in different treatment groups is presented in terms of percentage of proliferating CD4+ T cells, and there was a 2-4% standard deviation in each treatment group of 2 separate experiments.
Figure 3.
Topical treatment of mice with silymarin improves the functionality of DCs from UVB-irradiated wild-type mice and enhances the proliferation of CD4+ T cells, but not in XPAKO mice. CD4+ T cells isolated from the spleens of naïve mice (wild-type) were labeled with CFSE and co-cultured with CD11c+ cells (DCs) isolated from lymph nodes of the different treatment groups of wild-type and XPA-KO mice in the presence of anti-CD3e (5.0 μg/ml). After 4 days of co-culture, cells were harvested and analyzed for their proliferation index using FACS. Representative histograms from one experiment are shown from a total of two independent experiments. Numerical values in different treatment groups indicate percentage of proliferating CD4+ T cells.
3.7. Silymarin prevents UVB-induced suppression of CHS through the activation of CD8+ effector T cells
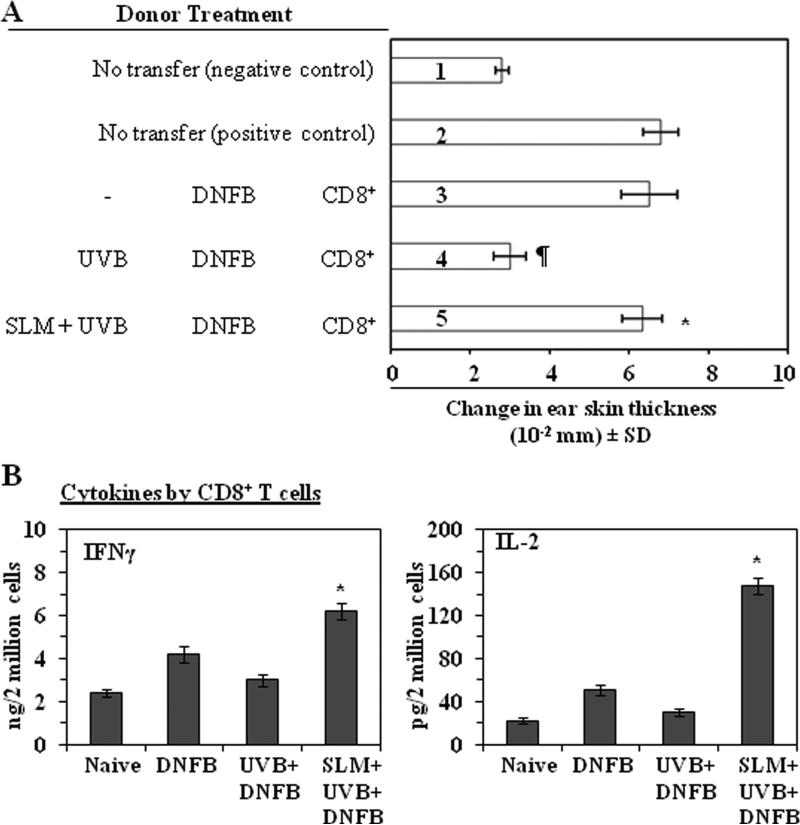
To identify the T-cell subpopulations responsible for the silymarin-induced prevention of immunosuppression we used the adoptive transfer model described in detail in the Materials and methods section in which CD8+ cells positively selected from the spleens and regional lymph nodes of C3H/HeN donor mice that had been sensitized to DNFB were injected into naïve mice, which were then challenged immediately by application of DNFB on the ear skin. The ear swelling response was measured 24 h later. As shown in Figure 4A, naïve mice that received CD8+ T cells from silymarin-treated, UVB-exposed donor mice showed a significant greater CHS response (113%, 5th bar) than naïve mice that received CD8+ cells from UVB-exposed mice that were not treated with silymarin (4th bar). These data suggested that the prevention of UVB-induced immunosuppression by silymarin is transferable to naïve mice by CD8+ effector T cells, and suggested that the CD8+ T-cell subpopulation plays a role in the silymarin-enhanced CHS response in UVB-exposed mice.
Figure 4.
Silymarin prevents transferable UVB-induced suppression of CHS through modulation of activity or function of CD8+ T cells. (A), Donor mice (C3H/HeN) that were topically treated with and without silymarin were UVB-irradiated, DNFB sensitized, and sacrificed 5 days later. CD8+ T cells were positively selected from the single-cell suspensions prepared from spleens and lymph nodes cells, as detailed in Materials and methods. The CD8+ T cells (8 × 106) were injected i.v. into naïve mice. The recipient mice were ear challenged immediately and ear swelling response was measured before and 24 h after challenge. (B), Treatment of mice with silymarin enhances the production of IFN! and IL-2 by CD8+ T cells. The treatment groups were as described in Figure. CD8+ T cells were isolated from silymarin treated or untreated UVB-irradiated mice, then CD8+ T cells were co-cultured with DNBS-labeled BM-DC for 48 h. The concentration of cytokines in the cell culture supernatants were estimated by cytokine-specific ELISA kits and are presented as mean ± SD in terms of pg or ng per 2 million cells, n=5/group. *Significant increase versus UVB+DNFB group, P<0.001.
To verify that silymarin treatment activates CD8+ T cells, we determined the Th1 and Th2 cytokine profiles of CD8+ effector T cells that had been isolated from the lymph nodes and spleens of wild-type mice and then stimulated in vitro for 48 h with DNBS-labeled BM-DC obtained from naïve mice. As shown in Figure 4B, CD8+ T cells from UVB-exposed mice that had been treated with silymarin produced significantly higher levels of IFNγ (>2-fold, P<0.001) and IL-2 (5-fold, P<0.001) than CD8+ T cells from UVB-exposed mice not treated silymarin. Th2 cytokines were barely detectable (data not shown). The significantly higher production of Th1 cytokines by the CD8+ T-cells in mice that were treated with silymarin further suggested that activation of CD8+ T cells may play a role in the greater CHS response observed in the silymarin-treated, UVB-exposed mice.
3.8. Silymarin treatment of UVB-exposed mice inhibits the development CD4 suppressor T cells
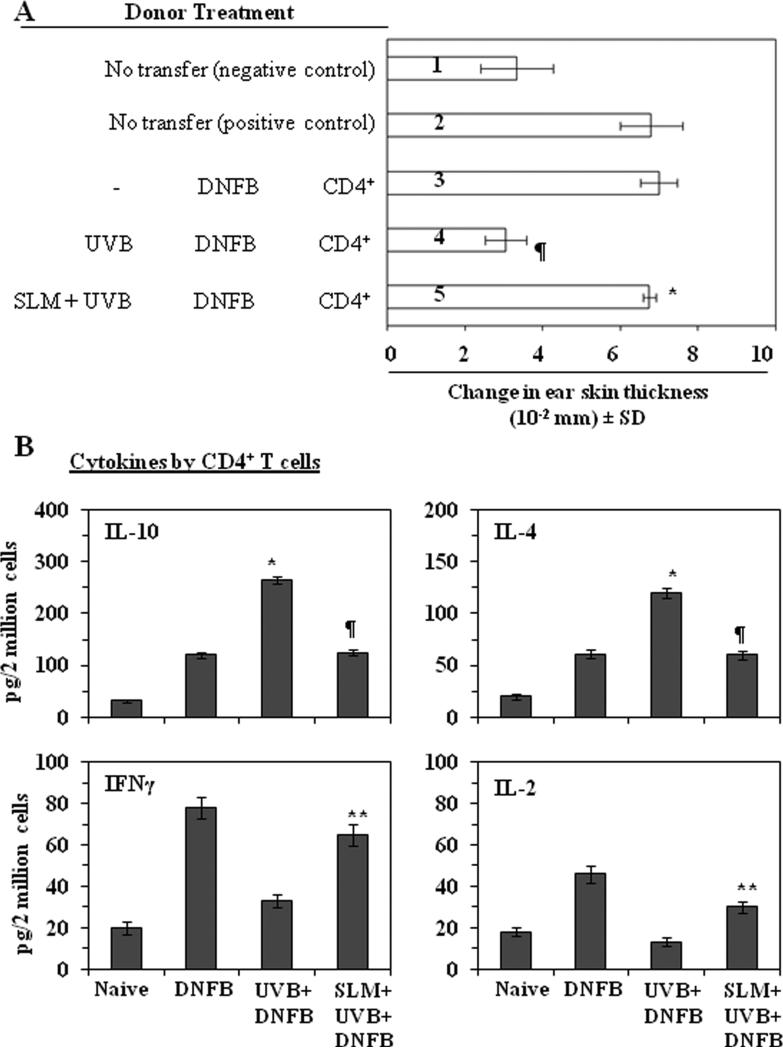
CHS response can be mediated by CD8+ or CD4+ T cells. Some investigators suggest that CD8+ T cells are primary effector cells in CHS response, whereas CD4+ T cells are able to develop to Th2 cells which produced IL-4 and IL-10 [25, 30-34]. UV induced regulatory cells are CD4+ T cells and UV induced immunosuppression can be transferred by CD4+ T cells from UV-exposed mice [35]. Moreover, the functions of UV induced CD4+ regulatory cells are dependent on IL-10 [36]. To investigate the effect of silymarin on the development of UV induced CD4+ regulatory T cells, we used the adoptive transfer protocol described in the Materials and methods section in which positively selected CD4+ T cells from the spleens and regional lymph nodes of C3H/HeN donor mice that had been sensitized to DNFB 5 days earlier were injected into naïve recipient mice. The recipient mice were sensitized to DNFB 24 h later and then challenged by application of DNFB on the ear skin 5 days later. As shown in Figure 5A, the recipient mice that received CD4+ T cells from UVB-irradiated and DNFB-sensitized donor mice had a significantly lower CHS response (>90%, P<0.01) (4th bar) than mice that were transferred with CD4+ T cells from the donor mice which were sensitized but not exposed to UVB (3rd bar). This result implicates that CD4+ T cells from the UVB treated donors inhibits the induction of CHS responses in the recipient mice. The recipient mice that had received CD4+ T cells from the silymarin-treated, UVB-exposed donor mice had a greater CHS response (123%, 5th bar) than the recipient mice that received CD4+ T cells from the UVB-exposed donor mice that were not treated with silymarin (4th bar). The result indicates that the treatment with silymarin abrogates the development of UVB induced CD4+ regulatory T cells. It suggests that a mechanism for prevention of UVB-induced suppression of CHS response by silymarin is the inhibition of UVB induced CD4+ regulatory T cells in addition to the activation of CD8+ effector T cells.
Figure 5.
Silymarin prevents transferable UVB-induced suppression of CHS through modulation of activity or function of CD4+ suppressor T cells. (A), The donor mice were treated as described in Figure 4, Panel A. CD4+ T cells were positively selected from the spleens and lymph nodes using the MACS system. CD4+ T cells (8×106) were injected i.v. into naïve recipient mice. The recipient mice were sensitized with DNFB and ear was challenged 5 days after sensitization. The change in ear thickness is reported as the mean of millimeters (mm × 10-2) ±SD, n=5 per group. Those naïve mice that received CD4+ T cells from UVB-exposed donor mice that were treated with silymarin showed a greater CHS response than UVB-exposed mice that were not silymarin treated. *Significantly greater CHS response versus recipient of T cells from UVB plus DNFB treated mice (4th bar), P<0.001; ¶Significantly lower CHS response versus the positive control group (2nd and 3rd bar), P<0.001. (B), Treatment of mice with silymarin decreases the production of IL-4 and IL-10 but increases the secretion of IFNγ and IL-2 by CD4+ T cells. CD4+ T cells were isolated from the different treatment groups, as described in Materials and methods. The CD4+ T cells were then co-cultured with DNBS-labeled BM-DC for 48 h. The concentrations of cytokines in the cell culture supernatants were estimated by ELISA and are presented as mean ± SD in terms of pg/2 million cells, n=5/group. *Significant increase versus positive control, P<0.001. ¶Significant decrease versus UVB+ DNFB group, P<0.001. **Significant increase versus UVB+ DNFB group, P<0.01.
To further determine the effects of silymarin on UVB induced CD4+ regulatory T cells, we compared the cytokine profiles of CD4+ T cells from mice in the different treatment groups. As described in detail in the Materials and methods section, purified CD4+ T cells were prepared and stimulated in vitro for 48 h with DNBS-labeled BM-DCs. The production of Th2 type immune suppressive cytokines IL-4 and IL-10 by the CD4+ T cells from the silymarin-treated, UVB-exposed mice was significantly lower (IL-4, 51%; IL-10, 60%, P<0.001) than the production of these cytokines by CD4+ T cells from UVB-exposed mice not treated with silymarin (Figure 5B). In contrast, although both IFNγ and IL-2 were detectable in the supernatants of CD4+ T cells, the levels of these cytokines were low particularly when compared with the levels of the cytokines in the supernatants of the CD8+ T cells. CD4+ Th2 cells which produce IL-4 and IL-10 are regulatory cells which inhibit CHS responses [25]. The low level production of Th2 cytokines IL-4 and IL-10 by the CD4+ T cells obtained from silymarin-treated mice suggests that silymarin inhibits the development of CD4+ Th2 cells in UVB treated mice.
4. Discussion
Nonmelanoma skin cancer, including basal and squamous cell carcinoma, represent the most common malignant neoplasms in humans. They have a tremendous impact on public health and healthcare expenditures. Exposure of the skin to UV radiation inhibits the antigen-presenting activity of DCs and their capacity to stimulate Th1 cytokines by T cells [12, 13]. Moreover, UV-treated Langerhans cells can induce immune tolerance if they are adoptively transferred into naïve mice that are not UVB-irradiated. In the present study, using adoptive transfer of CD11c+ cells, we demonstrate clearly that topical treatment with silymarin protects mouse skin from the photodamaging effects of UV radiation on DCs. We found that silymarin inhibits UVB-induced suppression of the CHS response by enhancing the functionality of DCs in UVB-exposed mice. Our study also provides evidence that silymarin enhances repair of UVB-induced DNA damage in BM-DCs obtained from wild-type mice, but in BM-DC from XPA-KO mice. Further, treatment of silymarin was found to enhance the repair of UV-induced DNA damage in the form of CPDs in epidermal DCs (langerin-positive cells) in wild-type mice, but this effect of silymarin was not observed in the epidermal DCs of UV-exposed skin of XPA-KO mice. These findings suggest that repair of UVB-induced DNA damage in DCs by silymarin is mediated through an NER mechanism and that this is required for the enhancement of the functionality of DCs. A similar DNA repair-dependent inhibition of UVB-induced immunosuppression was observed when mice were treated with green tea polyphenols [37]. This may be a characteristic of plant polyphenols and flavonols.
Our in vivo animal experiments in which we used an adoptive transfer approach to further characterize the cell populations that mediate the immunoprotective effects of silymarin revealed that silymarin prevents UVB-induced immunosuppression through stimulation and/or enhanced development of CD8+ effector T cells and that topical application of silymarin enhances the ability of the CD8+ T cells to secrete the Th1-type cytokines, IFNγ and IL-2. In addition, silymarin inhibits the ability of UVB induced CD4+ T cells to produce Th2 immune suppressive cytokines, IL-10 and IL-4.
As cytokines play a crucial role in modulating the immune system [38], we were interested in comparing the cytokine profile of CD8+ and CD4+ T cells obtained from mice that were exposed to UVB radiation and to delineate the relationship of these cytokine profiles with the inhibitory effect of silymarin on the UVB-induced immunosuppression. We found that the production of Th1-type cytokines (IFNγ, IL-2) was greatly enhanced in CD8+ T cells from silymarin-treated UVB-irradiated mice whereas the levels of Th2 immune suppressive cytokines (IL-4 and IL-10) produced by CD4+ T cells were significantly decreased. The silymarin-associated alteration in the cytokine profile of both CD4+ and CD8+ T cells may have a role in the enhancement of immune reactions in UVB irradiated mice. IFNγ-producing T cells are important effector cells in the CHS response as well as being involved in reducing the development of UVB-induced skin tumors [39]. However, the protective effect of silymarin against UVB-induced immunosuppression may also be mediated, at least in part, through the inactivation or inhibition of the development of CD4+ Th2 T cells that are induced by UVB irradiation, as there was a significant reduction in the production of Th2 cytokines IL-4 and IL-10 by BM-DC-stimulated CD4+ T cells obtained from silymarin-treated UVB irradiated mice. IL-10 is required for the suppressive function of UVB induced CD4+ regulatory T cells [36] and IL-4 and IL-10 have been implicated in immunosuppression and the development of regulatory T-cells in UV-skin carcinogenesis [38]. As the silymarin-associated enhanced production of Th1 cytokines by DCs (CD11c+ cells) from UVB-exposed C3H/HeN (wild-type) mice was not evident in DCs from UVB-exposed XPA-deficient mice, the findings suggest that repair of UVB-induced DNA damage by silymarin contributes to its ability to protect the immune system in UVB-irradiated wild-type mice.
The significance of our study relates to the immunoprotective effect of silymarin against UVB-induced immunosuppression, which is considered to be a risk factor for skin cancer development. Our study suggests that silymarin acts to protect DC from UV radiation-induced DNA damage in the skin. It means that silymarin enhanced repair of UVB-induced DNA damage in DCs. As damaged DNA was repaired in DCs, they were able to present antigen effectively to T cells, and that leads to improved immune system in mice. These results demonstrate that the photoprotective effect of silymarin can be used as an alternative strategy to stimulate the functionality of dendritic cells, which could lead to the stimulation of CD8+ effector T cells which produce IL-2 and IFN-! and inhibition of CD4+ Th2 T cells which produce IL-4 and IL-10. They may be important mechanism for the prevention of skin cancer risk in humans.
Acknowledgements
This work was supported by the funds from National Institutes of Health (Grant # CA140197) to S.K.K. and H.X., the UAB Skin Diseases Research Center (AR050948), and the Veterans Administration Merit Review Award (5I01BX001059 & 1I01BX001410) to C.A.E. and S.K.K. We thank Dr. Fiona Hunter for her assistance in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest There is no potential conflict of interest to declare.
References
- 1.Yoshikawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–6. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 2.Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev Med Interne. 1998;19:247–54. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- 3.Donawho CK, Kripke ML. Evidence that the local effect of ultraviolet radiation on the growth of murine melanomas is immunologically mediated. Cancer Res. 1991;51:4176–81. [PubMed] [Google Scholar]
- 4.Kinlen L, Sheil A, Peta J, Doll R. Collaborative United Kingdom-Australia study of cancer in patients treated with immunosuppressive drugs. Br J Med. 1979;2:1461–6. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ondrus D, Pribylincova V, Breza J, Bujdak P, Miklosi M, Reznicek J, et al. The incidence of tumors in renal transplant recipients with long-term immunosuppressive therapy. Int Urol Nephrol. 1999;31:417–22. doi: 10.1023/a:1007194607496. [DOI] [PubMed] [Google Scholar]
- 6.Cowen EW, Billingsley EM. Awareness of skin cancer by kidney transplant patients. J Am Acad Dermatol. 1999;40:697–701. doi: 10.1016/s0190-9622(99)70149-0. [DOI] [PubMed] [Google Scholar]
- 7.Otley CC, Pittelkow MR. Skin cancer in liver transplant recipients. Liver Transpl. 2000;6:253–62. doi: 10.1053/lv.2000.6352. [DOI] [PubMed] [Google Scholar]
- 8.Fortina AB, Caforio AL, Piaserico S, Alaibac M, Tona F, Feltrin G, et al. Skin cancer in heart transplant recipients: frequency and risk factor analysis. J Heart Lung Transplant. 2000;19:249–55. doi: 10.1016/s1053-2498(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 9.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 10.Cooper KD, Oberhelman L, Hamilton TA, Baadsgaard O, Terhune M, LeVee G, et al. UV Exposure Reduces Immunization Rates and Promotes Tolerance to Epicutaneous Antigens in Humans: Relationship to Dose, CD1a-DR+ Epidermal Macrophage Induction, and Langerhans Cell Depletion. Proc Natl Acad Sci USA. 1992;89:8497–501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53. [PubMed] [Google Scholar]
- 12.Meunier L. Ultraviolet light and dendritic cells. Eur J Dermatol. 1999;9:269–75. [PubMed] [Google Scholar]
- 13.Alcalay J, Kripke ML. Antigen-presenting activity of draining lymph node cells from mice painted with a contact allergen during ultraviolet carcinogenesis. J Immunol. 1991;146:1717–21. [PubMed] [Google Scholar]
- 14.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of molecular targets for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989;170:1117–31. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516–20. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vink AA, Moodycliffe AM, Shreedhar V, Ullrich SE, Roza L, Yarosh DB, et al. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc Natl Acad Sci USA. 1997;94:5255–60. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yarosh DB, et al. Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J Exp Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner H, Hörhammer L, Münster R. [On the chemistry of silymarin (silybin), the active principle of the fruits from Silybum marianum (L.) Gaertn. (Carduus marianus L.)]. Arzneimittelforschung. 1968;18:688–96. [PubMed] [Google Scholar]
- 19.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:556–66. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 20.Katiyar SK. Silymarin and skin cancer prevention: anti-inflammatory, antioxidant and immunomodulatory effects. Int J Oncol. 2005;26:169–76. [PubMed] [Google Scholar]
- 21.Katiyar SK, Mantena SK, Meeran SM. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS ONE. 2011;6:e21410. doi: 10.1371/journal.pone.0021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol Cancer Ther. 2006;5:1660–8. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- 23.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev Res. 2010;3:179–89. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaid M, Singh T, Li A, Katiyar N, Sharma S, Elmets CA, et al. Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells. Cancer Prev Res. 2011;4:238–47. doi: 10.1158/1940-6207.CAPR-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 27.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 28.Parish CR, Glidden MH, Quah BJ, Warren HS. Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im0409s84. Chapter 4:Unit4.9. [DOI] [PubMed] [Google Scholar]
- 29.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–56. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 30.Anderson C, Hehr A, Robbins R, Hasan R, Athar M, Mukhtar H, et al. Metabolic requirements for induction of contact hypersensitivity to immunotoxic polyaromatic hydrocarbons. J Immunol. 1995;155:3530–7. [PubMed] [Google Scholar]
- 31.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–8. [PubMed] [Google Scholar]
- 32.Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, et al. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–10. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 33.Kondo S, Beissert S, Wang B, Fujisawa H, Kooshesh F, Stratigos A, et al. Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene targeted mouse. J Invest Dermatol. 1996;106:993–1000. doi: 10.1111/1523-1747.ep12338505. [DOI] [PubMed] [Google Scholar]
- 34.Hauser C. Cultured epidermal Langerhans cells activate effector T cells for contact sensitivity. J Invest Dermatol. 1990;95:436–40. doi: 10.1111/1523-1747.ep12555587. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y, et al. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 36.Maeda A, Beissert S, Schwarz T, Schwarz A. Phenotypic and functional characterization of ultraviolet radiation-induced regulatory T cells. J Immunol. 2008;180:3065–3071. doi: 10.4049/jimmunol.180.5.3065. [DOI] [PubMed] [Google Scholar]
- 37.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (-)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clinical Cancer Res. 2006;12:2272–80. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 38.Mukhtar H, Elmets CA. Photocarcinogenesis: Mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf N, Nasti TH, Katiyar SK, Jacobs MK, Seibert MD, Ginsburg AC, et al. Antagonistic roles of CD4+ and CD8+ T-cells in 7,12-dimethylbenz(a)anthracene cutaneous carcinogenesis. Cancer Res. 2008;68:3924–30. doi: 10.1158/0008-5472.CAN-07-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]