Abstract
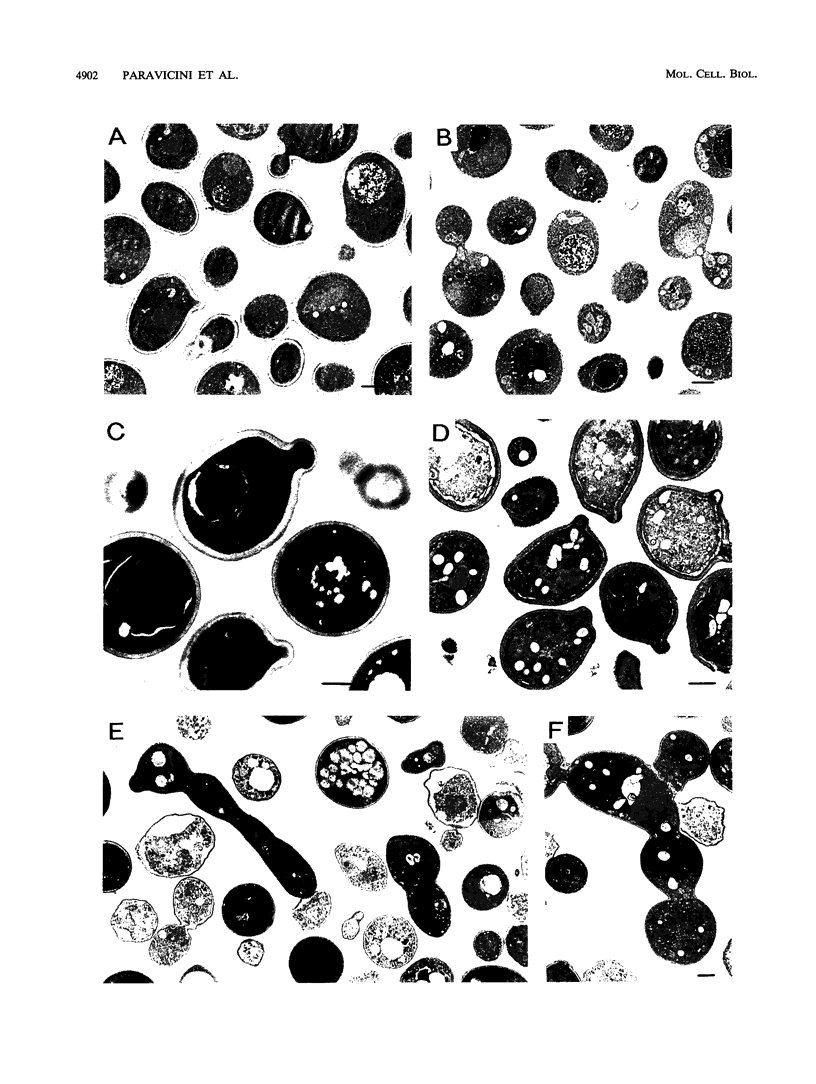
Seven temperature-sensitive cell lysis (cly) mutant strains of Saccharomyces cerevisiae were isolated which lyse at the restrictive temperature on hypotonic but not on osmotically supported medium. The seven mutants fell into four complementation groups, CLY12 to CLY15. The wild-type CLY15 gene was isolated by complementation of the cly15 temperature-sensitive growth defect. Sequence analysis revealed that the complementing DNA fragment encoded a partial PKC1 gene, which has previously been isolated as an S. cerevisiae homolog of mammalian protein kinase C genes (D. E. Levin, F. O. Fields, R. Kunisawa, J. M. Bishop, and J. Thorner, Cell 62:213-224, 1990). Subsequent genetic analysis showed that CLY15 and PKC1 represent identical loci in the yeast genome. A truncated PKC1 gene encoding only the predicted catalytic domain of Pkc1p was able to complement pkc1 mutant strains. Similar to what has been reported recently (D. E. Levin and E. Bartlett-Heubusch, J. Cell Biol. 116:1221-1229, 1992), we observed that cells deleted for the PKC1 gene are viable when grown on osmotically stabilized medium but are osmotically fragile and lyse rapidly after a shift to hypotonic medium. As shown by light and electron microscopic examinations, the delta pkc1 strain exhibits many cells with a strongly elongated bud or chains of incompletely budded cells when grown on solid medium.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone C., Sommer S. S., Hensel A., Bussey H. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J Cell Biol. 1990 May;110(5):1833–1843. doi: 10.1083/jcb.110.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa C. E., Slater M., Cabib E., Au-Young J., Sburlati A., Adair W. L., Jr, Robbins P. W. The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell. 1986 Jul 18;46(2):213–225. doi: 10.1016/0092-8674(86)90738-5. [DOI] [PubMed] [Google Scholar]
- Burke D. J., Church D. Protein synthesis requirements for nuclear division, cytokinesis, and cell separation in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jul;11(7):3691–3698. doi: 10.1128/mcb.11.7.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Preparation of yeast cells for thin-section electron microscopy. Methods Enzymol. 1991;194:602–608. doi: 10.1016/0076-6879(91)94044-d. [DOI] [PubMed] [Google Scholar]
- Cabib E., Bowers B., Sburlati A., Silverman S. J. Fungal cell wall synthesis: the construction of a biological structure. Microbiol Sci. 1988 Dec;5(12):370–375. [PubMed] [Google Scholar]
- Cabib E., Duran A. Simple and sensitive procedure for screening yeast mutants that lyse at nonpermissive temperatures. J Bacteriol. 1975 Dec;124(3):1604–1606. doi: 10.1128/jb.124.3.1604-1606.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Costigan C., Gehrung S., Snyder M. A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol Cell Biol. 1992 Mar;12(3):1162–1178. doi: 10.1128/mcb.12.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Levin D. E. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol Cell Biol. 1992 Jan;12(1):172–182. doi: 10.1128/mcb.12.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol. 1992 Mar;116(5):1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Fields F. O., Kunisawa R., Bishop J. M., Thorner J. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 1990 Jul 27;62(2):213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- Nombela C., Molina M., Cenamor R., Sanchez M. Yeast beta-glucanases: a complex system of secreted enzymes. Microbiol Sci. 1988 Nov;5(11):328–332. [PubMed] [Google Scholar]
- Rivin C. J., Fangman W. L. Cell cycle phase expansion in nitrogen-limited cultures of Saccharomyces cerevisiae. J Cell Biol. 1980 Apr;85(1):96–107. doi: 10.1083/jcb.85.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Silverman S. J., Sburlati A., Slater M. L., Cabib E. Chitin synthase 2 is essential for septum formation and cell division in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4735–4739. doi: 10.1073/pnas.85.13.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stateva L. I., Oliver S. G., Trueman L. J., Venkov P. V. Cloning and characterization of a gene which determines osmotic stability in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Aug;11(8):4235–4243. doi: 10.1128/mcb.11.8.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres L., Martín H., García-Saez M. I., Arroyo J., Molina M., Sánchez M., Nombela C. A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol Microbiol. 1991 Nov;5(11):2845–2854. doi: 10.1111/j.1365-2958.1991.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Venkov P. V., Hadjiolov A. A., Battaner E., Schlessinger D. Saccharomyces cerevisiae: sorbitol-dependent fragile mutants. Biochem Biophys Res Commun. 1974 Feb 4;56(3):599–604. doi: 10.1016/0006-291x(74)90646-9. [DOI] [PubMed] [Google Scholar]
- de la Fuente J. M., Alvarez A., Nombela C., Sanchez M. Flow cytometric analysis of Saccharomyces cerevisiae autolytic mutants and protoplasts. Yeast. 1992 Jan;8(1):39–45. doi: 10.1002/yea.320080104. [DOI] [PubMed] [Google Scholar]