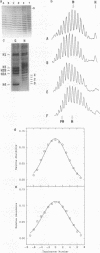
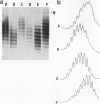
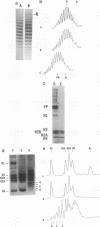
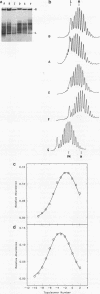
Abstract
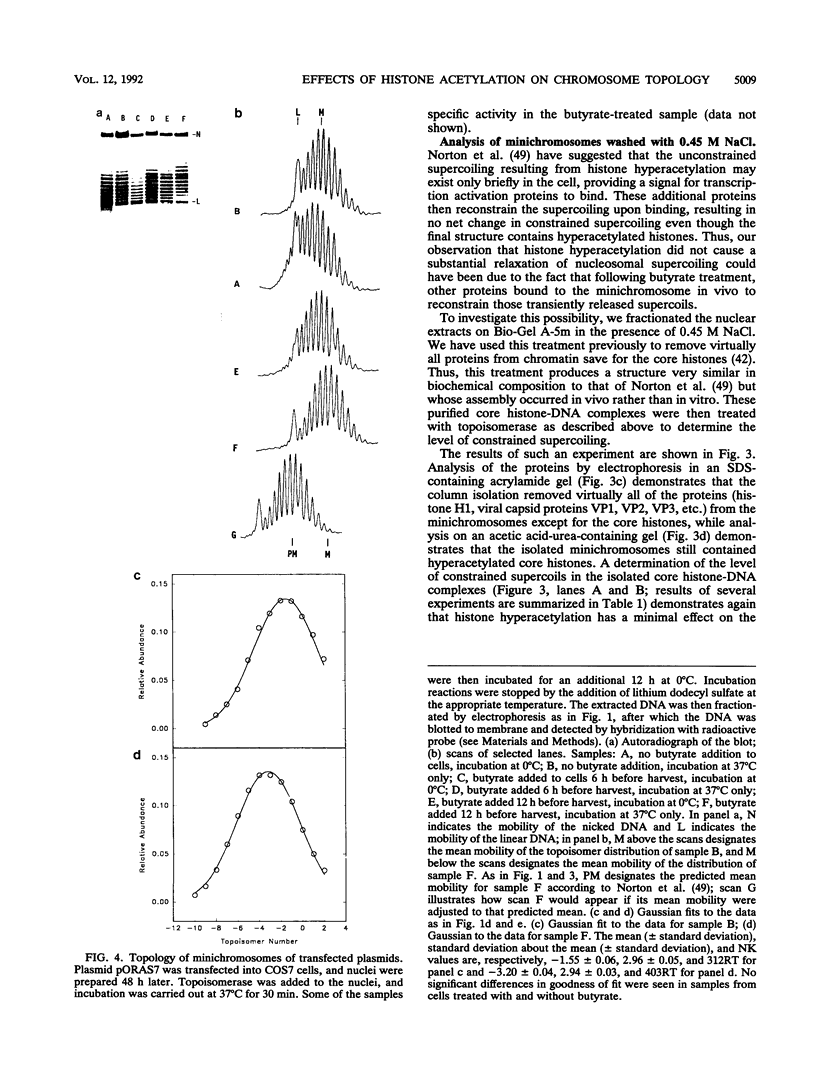
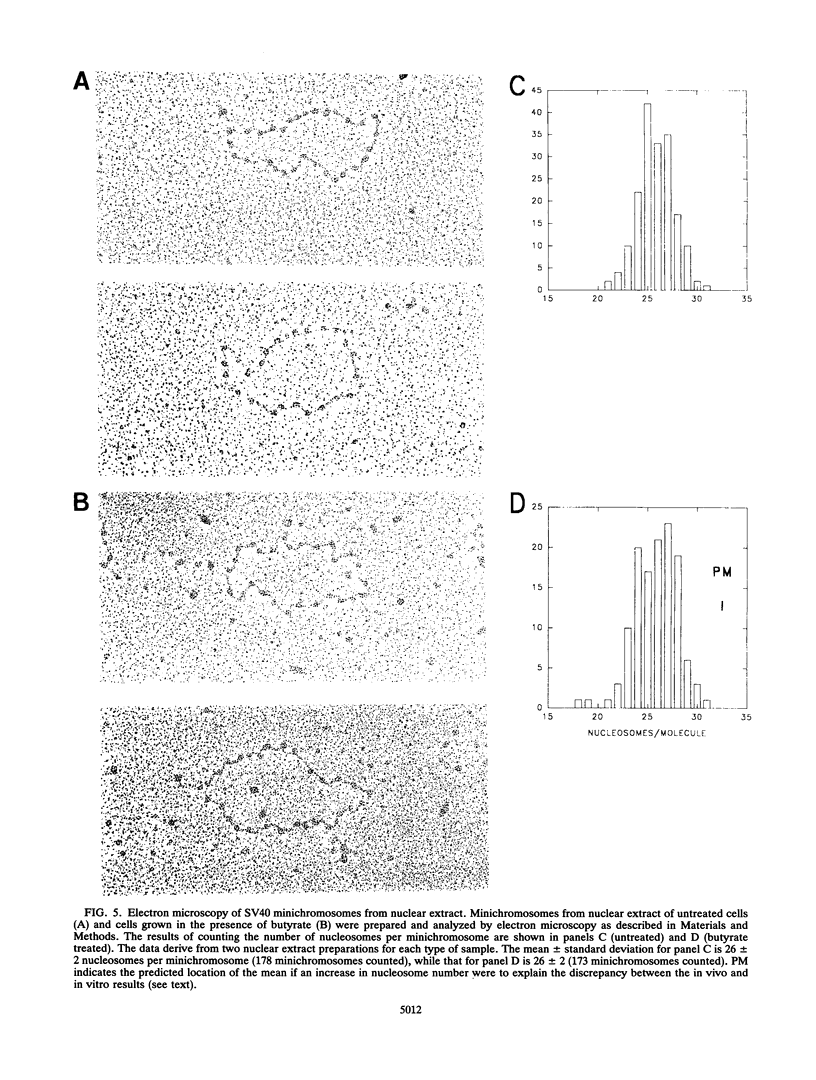
Recently a model for eukaryotic transcriptional activation has been proposed in which histone hyperacetylation causes release of nucleosomal supercoils, and this unconstrained tension in turn stimulates transcription (V. G. Norton, B. S. Imai, P. Yau, and E. M. Bradbury, Cell 57:449-457, 1989; V. G. Norton, K. W. Marvin, P. Yau, and E. M. Bradbury, J. Biol. Chem. 265:19848-19852, 1990). These studies analyzed the effect of histone hyperacetylation on the change in topological linking number which occurs during nucleosome assembly in vitro. We have tested this model by determining the effect of histone hyperacetylation on the linking number change which occurs during assembly in vivo. We find that butyrate treatment of cells infected with simian virus 40 results in hyperacetylation of the histones of the extracted viral minichromosome as expected. However, the change in constrained supercoils of the minichromosome DNA is minimal, a result which is inconsistent with the proposed model. These results indicate that the proposed mechanism of transcriptional activation is unlikely to take place in the cell.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose C., McLaughlin R., Bina M. The flexibility and topology of simian virus 40 DNA in minichromosomes. Nucleic Acids Res. 1987 May 11;15(9):3703–3721. doi: 10.1093/nar/15.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffa L. C., Vidali G., Mann R. S., Allfrey V. G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978 May 25;253(10):3364–3366. [PubMed] [Google Scholar]
- Bonilla P. J., Freytag S. O., Lutter L. C. Enhancer-activated plasmid transcription complexes contain constrained supercoiling. Nucleic Acids Res. 1991 Jul 25;19(14):3965–3971. doi: 10.1093/nar/19.14.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., John S., Berard D. S., LeFebvre P., Hager G. L. Glucocorticoid receptor-dependent disruption of a specific nucleosome on the mouse mammary tumor virus promoter is prevented by sodium butyrate. Proc Natl Acad Sci U S A. 1990 May;87(10):3977–3981. doi: 10.1073/pnas.87.10.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., John S., Hager G. L. Histone hyperacetylation does not alter the positioning or stability of phased nucleosomes on the mouse mammary tumor virus long terminal repeat. Biochemistry. 1991 Apr 9;30(14):3490–3497. doi: 10.1021/bi00228a020. [DOI] [PubMed] [Google Scholar]
- Brill S. J., Sternglanz R. Transcription-dependent DNA supercoiling in yeast DNA topoisomerase mutants. Cell. 1988 Jul 29;54(3):403–411. doi: 10.1016/0092-8674(88)90203-6. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Choder M., Aloni Y. In vitro transcribed SV40 minichromosomes, as the bulk minichromosomes, have a low level of unconstrained negative supercoils. Nucleic Acids Res. 1988 Feb 11;16(3):895–905. doi: 10.1093/nar/16.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M., Aloni Y. RNA polymerase II allows unwinding and rewinding of the DNA and thus maintains a constant length of the transcription bubble. J Biol Chem. 1988 Sep 15;263(26):12994–13002. [PubMed] [Google Scholar]
- Coca-Prados M., Vidali G., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. III. Study of histone modifications. J Virol. 1980 Nov;36(2):353–360. doi: 10.1128/jvi.36.2.353-360.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Chalkley R. Hyperacetylated histones facilitate chromatin assembly in vitro. Nucleic Acids Res. 1985 Jan 25;13(2):401–414. doi: 10.1093/nar/13.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens L. S., Gallwitz D., Alberts B. M. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979 Mar 10;254(5):1716–1723. [PubMed] [Google Scholar]
- Csordas A. On the biological role of histone acetylation. Biochem J. 1990 Jan 1;265(1):23–38. doi: 10.1042/bj2650023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bernardin W., Koller T., Sogo J. M. Structure of in-vivo transcribing chromatin as studied in simian virus 40 minichromosomes. J Mol Biol. 1986 Oct 5;191(3):469–482. doi: 10.1016/0022-2836(86)90142-7. [DOI] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F., Sinden R. R. Supercoiling in prokaryotic and eukaryotic DNA: changes in response to topological perturbation of plasmids in E. coli and SV40 in vitro, in nuclei and in CV-1 cells. Nucleic Acids Res. 1987 Jul 10;15(13):5105–5124. doi: 10.1093/nar/15.13.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Lutter L. C., Rhodes D., Brown R. S., Rushton B., Levitt M., Klug A. Structure of nucleosome core particles of chromatin. Nature. 1977 Sep 1;269(5623):29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- Fisher L. M. DNA supercoiling and gene expression. Nature. 1984 Feb 23;307(5953):686–687. doi: 10.1038/307686a0. [DOI] [PubMed] [Google Scholar]
- Freeman L. A., Garrard W. T. DNA supercoiling in chromatin structure and gene expression. Crit Rev Eukaryot Gene Expr. 1992;2(2):165–209. [PubMed] [Google Scholar]
- Futcher B. Supercoiling and transcription, or vice versa? Trends Genet. 1988 Oct;4(10):271–272. doi: 10.1016/0168-9525(88)90166-7. [DOI] [PubMed] [Google Scholar]
- Hadlock K. G., Lutter L. C. T-antigen is not bound to the replication origin of the simian virus 40 late transcription complex. J Mol Biol. 1990 Sep 5;215(1):53–65. doi: 10.1016/S0022-2836(05)80094-4. [DOI] [PubMed] [Google Scholar]
- Hadlock K. G., Quasney M. W., Lutter L. C. Immunoprecipitation of the simian virus 40 late transcription complex with antibody against T-antigen. J Biol Chem. 1987 Nov 15;262(32):15527–15537. [PubMed] [Google Scholar]
- Hagopian H. K., Riggs M. G., Swartz L. A., Ingram V. M. Effect of n-butyrate on DNA synthesis in chick fibroblasts and HeLa cells. Cell. 1977 Nov;12(3):855–860. doi: 10.1016/0092-8674(77)90284-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski W. A., Luchnik A. N. High rotational mobility of DNA in animal cells and its modulation by histone acetylation. Mol Gen Genet. 1991 Dec;231(1):17–21. doi: 10.1007/BF00293816. [DOI] [PubMed] [Google Scholar]
- Krajewski W. A., Luchnik A. N. Relationship of histone acetylation to DNA topology and transcription. Mol Gen Genet. 1991 Dec;230(3):442–448. doi: 10.1007/BF00280301. [DOI] [PubMed] [Google Scholar]
- La Bella F., Vesco C. Late modifications of simian virus 40 chromatin during the lytic cycle occur in an immature form of virion. J Virol. 1980 Mar;33(3):1138–1150. doi: 10.1128/jvi.33.3.1138-1150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart P., Koller T. Electron microscope specimen preparation of rat liver chromatin by a modified Miller spreading technique. Eur J Cell Biol. 1981 Jun;24(2):309–316. [PubMed] [Google Scholar]
- Li J. J., Peden K. W., Dixon R. A., Kelly T. Functional organization of the simian virus 40 origin of DNA replication. Mol Cell Biol. 1986 Apr;6(4):1117–1128. doi: 10.1128/mcb.6.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. Eukaryotic genes--are they under torsional stress? Nature. 1983 Sep 22;305(5932):276–277. doi: 10.1038/305276a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis R., Stark G. R. Two deletions within genes for simian virus 40 structural proteins VP2 and VP3 lead to formation of abnormal transcriptional complexes. J Virol. 1981 Apr;38(1):91–103. doi: 10.1128/jvi.38.1.91-103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchnik A. N., Bakayev V. V., Glaser V. M. DNA supercoiling: changes during cellular differentiation and activation of chromatin transcription. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):793–801. doi: 10.1101/sqb.1983.047.01.091. [DOI] [PubMed] [Google Scholar]
- Luchnik A. N., Bakayev V. V., Yugai A. A., Zbarsky I. B., Georgiev G. P. DNAaseI-hypersensitive minichromosomes of SV40 possess an elastic torsional strain in DNA. Nucleic Acids Res. 1985 Feb 25;13(4):1135–1149. doi: 10.1093/nar/13.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchnik A. N., Bakayev V. V., Zbarsky I. B., Georgiev G. P. Elastic torsional strain in DNA within a fraction of SV40 minichromosomes: relation to transcriptionally active chromatin. EMBO J. 1982;1(11):1353–1358. doi: 10.1002/j.1460-2075.1982.tb01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Thermal unwinding of simian virus 40 transcription complex DNA. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8712–8716. doi: 10.1073/pnas.86.22.8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Oudet P., Wasylyk B., Chambon P. Effect of histone acetylation on structure and in vitro transcription of chromatin. Nucleic Acids Res. 1978 Oct;5(10):3523–3547. doi: 10.1093/nar/5.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse R. H., Cantor C. R. Effect of trypsinization and histone H5 addition on DNA twist and topology in reconstituted minichromosomes. Nucleic Acids Res. 1986 Apr 25;14(8):3293–3310. doi: 10.1093/nar/14.8.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse R. H. Topoisomer heterogeneity of plasmid chromatin in living cells. J Mol Biol. 1991 Nov 20;222(2):133–137. doi: 10.1016/0022-2836(91)90198-f. [DOI] [PubMed] [Google Scholar]
- North G. Eukaryotic topoisomerases come into the limelight. Nature. 1985 Aug 1;316(6027):394–395. doi: 10.1038/316394a0. [DOI] [PubMed] [Google Scholar]
- Norton V. G., Imai B. S., Yau P., Bradbury E. M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989 May 5;57(3):449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- Norton V. G., Marvin K. W., Yau P., Bradbury E. M. Nucleosome linking number change controlled by acetylation of histones H3 and H4. J Biol Chem. 1990 Nov 15;265(32):19848–19852. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Petryniak B., Lutter L. C. Topological characterization of the simian virus 40 transcription complex. Cell. 1987 Jan 30;48(2):289–295. doi: 10.1016/0092-8674(87)90432-6. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Campos A., Shimamura A., Worcel A. Assembly and properties of chromatin containing histone H1. J Mol Biol. 1989 Sep 5;209(1):135–150. doi: 10.1016/0022-2836(89)90177-0. [DOI] [PubMed] [Google Scholar]
- Roman A. Alteration in the simian virus 40 maturation pathway after butyrate-induced hyperacetylation of histones. J Virol. 1982 Dec;44(3):958–962. doi: 10.1128/jvi.44.3.958-962.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Structure of the two distinct types of minichromosomes that are assembled on DNA injected in Xenopus oocytes. Cell. 1985 Apr;40(4):923–932. doi: 10.1016/0092-8674(85)90352-6. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Deficiency in histone acetylation in nontransforming host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1092–1096. doi: 10.1073/pnas.73.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Shelton E. R., Wassarman P. M., DePamphilis M. L. Structure, spacing, and phasing of nucleosomes on isolated forms of mature simian virus 40 chromosomes. J Biol Chem. 1980 Jan 25;255(2):771–782. [PubMed] [Google Scholar]
- Simpson R. T., Thoma F., Brubaker J. M. Chromatin reconstituted from tandemly repeated cloned DNA fragments and core histones: a model system for study of higher order structure. Cell. 1985 Oct;42(3):799–808. doi: 10.1016/0092-8674(85)90276-4. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Stein A. DNA wrapping in nucleosomes. The linking number problem re-examined. Nucleic Acids Res. 1980 Oct 24;8(20):4803–4820. doi: 10.1093/nar/8.20.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen B., Bendixen C., Westergaard O. Histone hyperacetylation is accompanied by changes in DNA topology in vivo. Eur J Biochem. 1991 Oct 1;201(1):107–111. doi: 10.1111/j.1432-1033.1991.tb16262.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H. A dominant role for DNA secondary structure in forming hypersensitive structures in chromatin. Cell. 1983 Apr;32(4):1191–1203. doi: 10.1016/0092-8674(83)90302-1. [DOI] [PubMed] [Google Scholar]