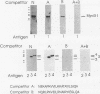
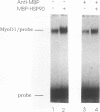
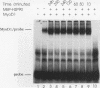
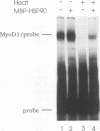
Abstract
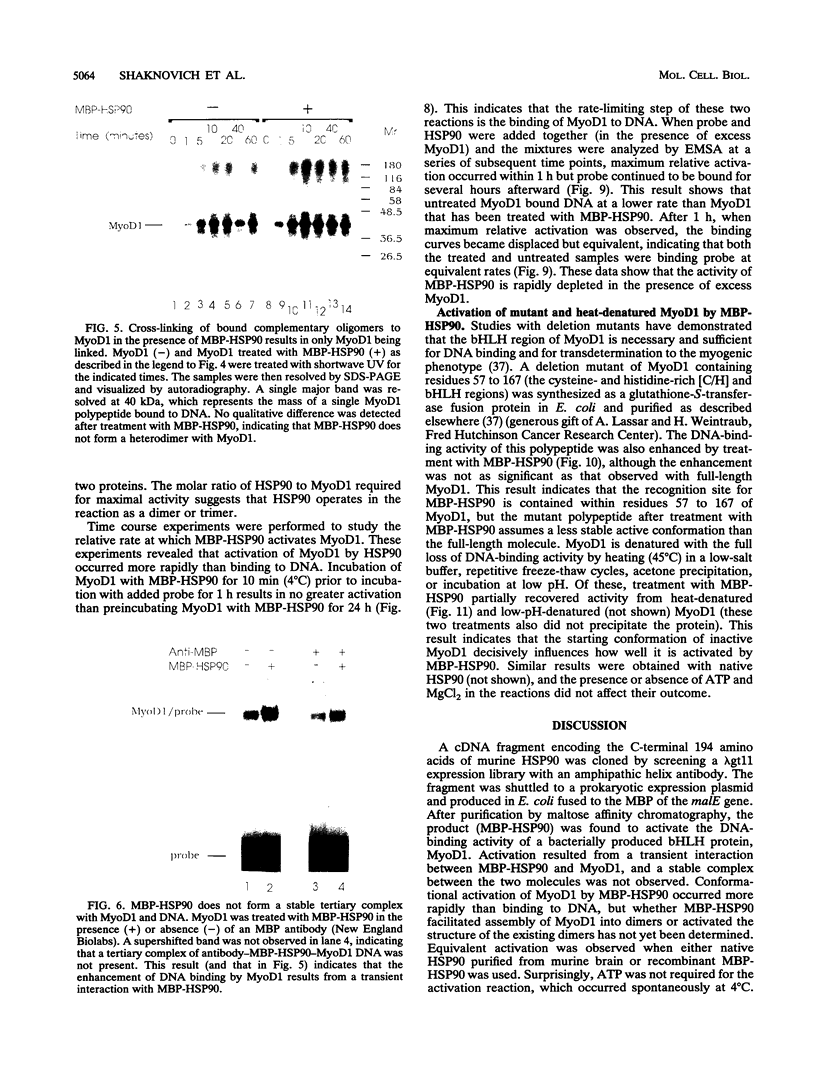
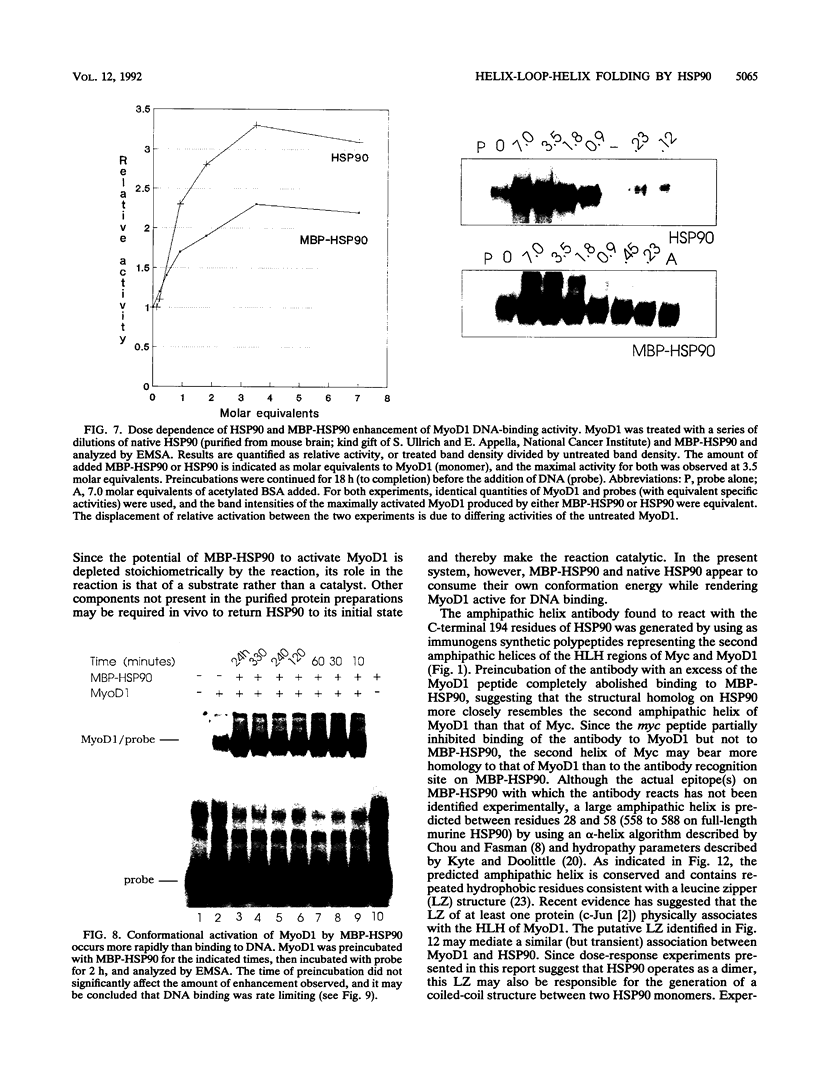
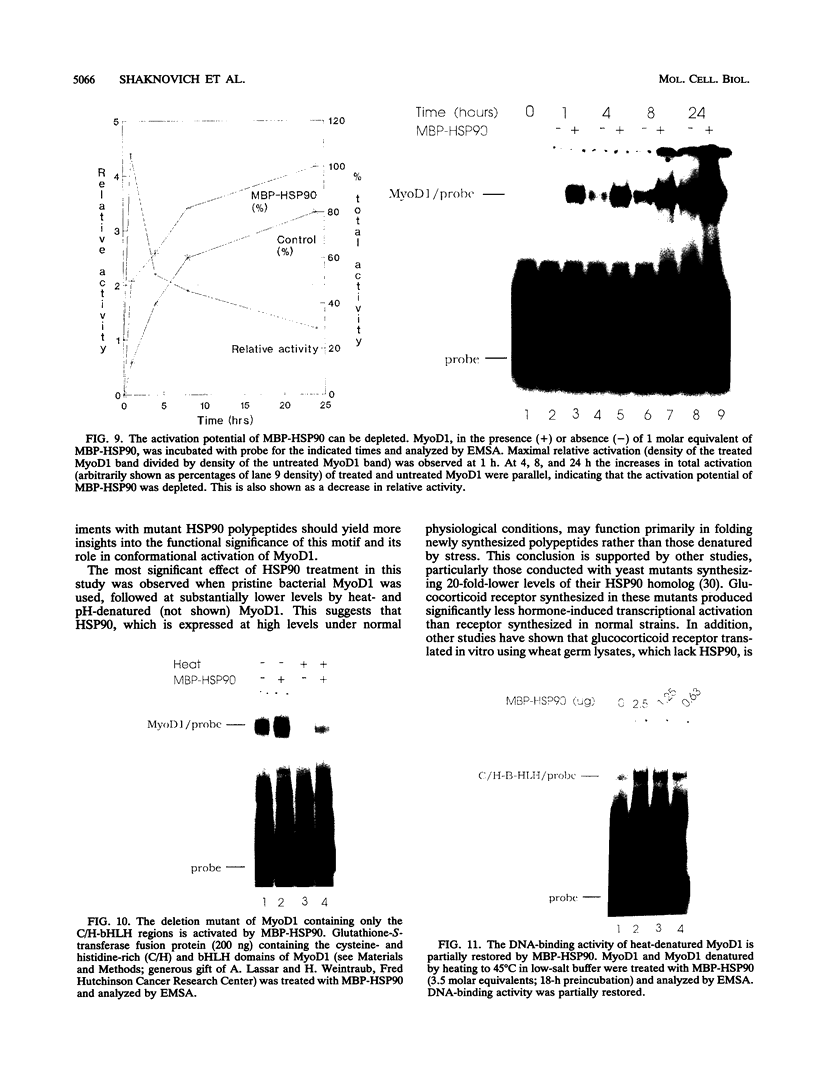
A murine cardiac lambda gt11 expression library was screened with an amphipathic helix antibody, and a recombinant representing the C-terminal 194 residues of murine HSP90 (HSP84) was cloned. Both recombinant and native HSP90s were then found to rapidly convert a basic helix-loop-helix protein (MyoD1) from an inactive to an active conformation, as assayed by sequence-specific DNA binding. The conversion process involves a transient interaction between HSP90 and MyoD1 and does not result in the formation of a stable tertiary complex. Conversion does not require ATP and occurs stoichiometrically in a dose-dependent fashion. HSP90 is an abundant, ubiquitous, and highly conserved protein present in most eukaryotic cells. These results provide direct evidence that HSP90 can affect the conformational structure of a DNA-binding protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengal E., Ransone L., Scharfmann R., Dwarki V. J., Tapscott S. J., Weintraub H., Verma I. M. Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell. 1992 Feb 7;68(3):507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Meselson M. Interspecific nucleotide sequence comparisons used to identify regulatory and structural features of the Drosophila hsp82 gene. J Mol Biol. 1986 Apr 20;188(4):499–515. doi: 10.1016/s0022-2836(86)80001-8. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Lee A. T., Dobson A. W., Conneely O. M., Schrader W. T., O'Malley B. W. Interaction of the chicken progesterone receptor with heat shock protein (HSP) 90. J Steroid Biochem. 1989;34(1-6):1–9. doi: 10.1016/0022-4731(89)90060-5. [DOI] [PubMed] [Google Scholar]
- Chambraud B., Berry M., Redeuilh G., Chambon P., Baulieu E. E. Several regions of human estrogen receptor are involved in the formation of receptor-heat shock protein 90 complexes. J Biol Chem. 1990 Nov 25;265(33):20686–20691. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dalman F. C., Bresnick E. H., Patel P. D., Perdew G. H., Watson S. J., Jr, Pratt W. B. Direct evidence that the glucocorticoid receptor binds to hsp90 at or near the termination of receptor translation in vitro. J Biol Chem. 1989 Nov 25;264(33):19815–19821. [PubMed] [Google Scholar]
- Dragon E. A., Sias S. R., Kato E. A., Gabe J. D. The genome of Trypanosoma cruzi contains a constitutively expressed, tandemly arranged multicopy gene homologous to a major heat shock protein. Mol Cell Biol. 1987 Mar;7(3):1271–1275. doi: 10.1128/mcb.7.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly F. W., Finkelstein D. B. Complete sequence of the heat shock-inducible HSP90 gene of Saccharomyces cerevisiae. J Biol Chem. 1984 May 10;259(9):5745–5751. [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. A DNA-binding factor specific for xenobiotic responsive elements of P-450c gene exists as a cryptic form in cytoplasm: its possible translocation to nucleus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5859–5863. doi: 10.1073/pnas.85.16.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hapgood J., Cuthill S., Denis M., Poellinger L., Gustafsson J. A. Specific protein-DNA interactions at a xenobiotic-responsive element: copurification of dioxin receptor and DNA-binding activity. Proc Natl Acad Sci U S A. 1989 Jan;86(1):60–64. doi: 10.1073/pnas.86.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Smale G., Lloyd D., Weber L. A. Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol Cell Biol. 1989 Jun;9(6):2615–2626. doi: 10.1128/mcb.9.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991 May 17;252(5008):954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Housley P. R., Sanchez E. R., Danielsen M., Ringold G. M., Pratt W. B. Evidence that the conserved region in the steroid binding domain of the glucocorticoid receptor is required for both optimal binding of hsp90 and protection from proteolytic cleavage. A two-site model for hsp90 binding to the steroid binding domain. J Biol Chem. 1990 Aug 5;265(22):12778–12781. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai B. T., Chin N. W., Stanek A. E., Keh W., Lanks K. W. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984 Dec;4(12):2802–2810. doi: 10.1128/mcb.4.12.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Cutt J. R., Brugge J. S. Association of the transforming proteins of Rous, Fujinami, and Y73 avian sarcoma viruses with the same two cellular proteins. Mol Cell Biol. 1982 Jul;2(7):875–880. doi: 10.1128/mcb.2.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. K., Kozak C., Robinson E. A., Ullrich S. J., Appella E. Cloning and nucleotide sequence of the murine hsp84 cDNA and chromosome assignment of related sequences. Gene. 1987;56(1):29–40. doi: 10.1016/0378-1119(87)90155-7. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nishida E., Koyasu S., Sakai H., Yahara I. Calmodulin-regulated binding of the 90-kDa heat shock protein to actin filaments. J Biol Chem. 1986 Dec 5;261(34):16033–16036. [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Sep 25;263(27):13802–13805. [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Rebbe N. F., Ware J., Bertina R. M., Modrich P., Stafford D. W. Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family. Gene. 1987;53(2-3):235–245. doi: 10.1016/0378-1119(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Rose D. W., Wettenhall R. E., Kudlicki W., Kramer G., Hardesty B. The 90-kilodalton peptide of the heme-regulated eIF-2 alpha kinase has sequence similarity with the 90-kilodalton heat shock protein. Biochemistry. 1987 Oct 20;26(21):6583–6587. doi: 10.1021/bi00395a003. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Meshinchi S., Tienrungroj W., Schlesinger M. J., Toft D. O., Pratt W. B. Relationship of the 90-kDa murine heat shock protein to the untransformed and transformed states of the L cell glucocorticoid receptor. J Biol Chem. 1987 May 25;262(15):6986–6991. [PubMed] [Google Scholar]
- Sanchez E. R., Redmond T., Scherrer L. C., Bresnick E. H., Welsh M. J., Pratt W. B. Evidence that the 90-kilodalton heat shock protein is associated with tubulin-containing complexes in L cell cytosol and in intact PtK cells. Mol Endocrinol. 1988 Aug;2(8):756–760. doi: 10.1210/mend-2-8-756. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Catelli M. G., Joab I., Moncharmont B. Association of the heat shock protein hsp90 with steroid hormone receptors and tyrosine kinase oncogene products. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1298–1307. doi: 10.1016/s0006-291x(86)80424-7. [DOI] [PubMed] [Google Scholar]