Abstract
As an approach to mapping replicons in an extended chromosomal region, the temporal order of DNA replication was analyzed in the murine major histocompatibility gene complex (MHC). Replicating DNA from T-lymphoma and myelomonocyte cell lines was density labeled with bromodeoxyuridine and extracted from cells which had been fractionated into different stages of S phase by centrifugal elutriation. The replicating DNA from each fraction of S phase was separated from nonreplicating DNA on density gradients, blotted, and hybridized with 34 specific MHC probes. The earliest replication occurred in the vicinity of transcribed genes K, HAM1 and HAM2, RD, B144, D, L, T18, and T3. The temporal order of replication of groups of DNA segments suggests the location of five or six replicons within the H-2 complex, some of which appear to be either unidirectional or markedly asymmetric. The rates of replication through each of these apparent replicons appear to be similar. The TL region of the S49.1 T-lymphoma cells, which contains at least three transcribed genes, replicates earlier than the inactive TL region of WEHI-3 myelomonocytic cells. These results provide further evidence of a relationship between transcription and the initiation of DNA replication in mammalian cells. The mouse MHC examined in this study is the largest chromosomal region (> 2,000 kb) measured for timing of replication to date.
Full text
PDF




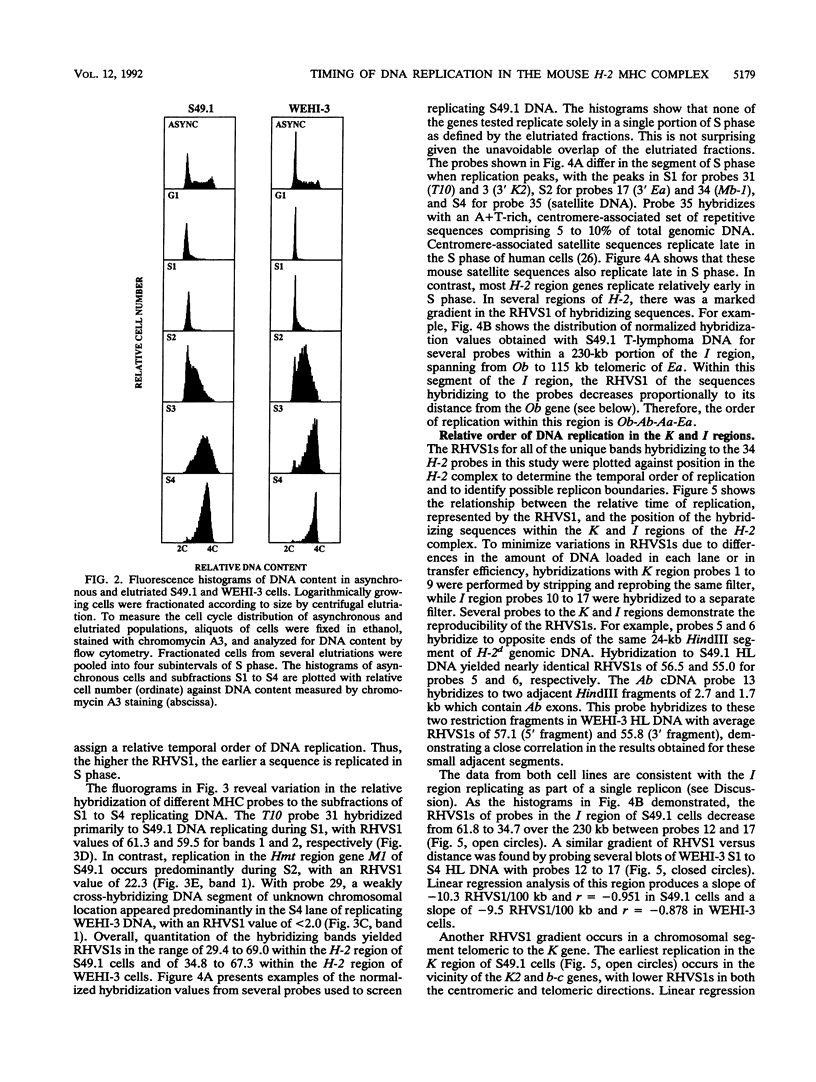









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Wei J. F., Wei F. S., Hsu Y. C., Uehara H., Artzt K., Bennett D. Searching for coding sequences in the mammalian genome: the H-2K region of the mouse MHC is replete with genes expressed in embryos. EMBO J. 1988 Nov;7(11):3441–3449. doi: 10.1002/j.1460-2075.1988.tb03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anachkova B., Hamlin J. L. Replication in the amplified dihydrofolate reductase domain in CHO cells may initiate at two distinct sites, one of which is a repetitive sequence element. Mol Cell Biol. 1989 Feb;9(2):532–540. doi: 10.1128/mcb.9.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Kornberg A. Transcriptional activation of initiation of replication from the E. coli chromosomal origin: an RNA-DNA hybrid near oriC. Cell. 1988 Oct 7;55(1):113–123. doi: 10.1016/0092-8674(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Beck Keeney J., Hedayat M., Myers N. M., Connolly J. M., Hansen T. H. Locus-specific regulation of Kd, Dd, and Ld class I genes in the BALB/c S49 lymphoma sublines. J Immunol. 1989 Oct 1;143(7):2364–2373. [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Brorson K. A., Hunt S. W., 3rd, Hunkapiller T., Sun Y. H., Cheroutre H., Nickerson D. A., Hood L. Comparison of exon 5 sequences from 35 class I genes of the BALB/c mouse. J Exp Med. 1989 Dec 1;170(6):1837–1858. doi: 10.1084/jem.170.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson K. A., Richards S., Hunt S. W., 3rd, Cheroutre H., Lindahl K. F., Hood L. Analysis of a new class I gene mapping to the Hmt region of the mouse. Immunogenetics. 1989;30(4):273–283. doi: 10.1007/BF02421331. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Iqbal M. A., Stuart S., Hatton K. S., Valinsky J., Schildkraut C. L. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987 Jan;7(1):450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans W. C., Vassilev L. T., Caddle M. S., Heintz N. H., DePamphilis M. L. Identification of an origin of bidirectional DNA replication in mammalian chromosomes. Cell. 1990 Sep 7;62(5):955–965. doi: 10.1016/0092-8674(90)90270-o. [DOI] [PubMed] [Google Scholar]
- Caddle M. S., Dailey L., Heintz N. H. RIP60, a mammalian origin-binding protein, enhances DNA bending near the dihydrofolate reductase origin of replication. Mol Cell Biol. 1990 Dec;10(12):6236–6243. doi: 10.1128/mcb.10.12.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Chaplin D. D., Woods D. E., Whitehead A. S., Goldberger G., Colten H. R., Seidman J. G. Molecular map of the murine S region. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6947–6951. doi: 10.1073/pnas.80.22.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Attaya M., Brown M. G., Monaco J. J. A cluster of transcribed sequences between the Pb and Ob genes of the murine major histocompatibility complex. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5197–5201. doi: 10.1073/pnas.88.12.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. Correlations between sister chromatid exchange frequencies and replicon sizes. A model for the mechanism of SCE production. Exp Cell Res. 1981 Nov;136(1):27–30. doi: 10.1016/0014-4827(81)90034-3. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Cohen D. I., Nielsen E. A., Steinmetz M., Paul W. E., Hood L. Cell-type-specific cDNA probes and the murine I region: the localization and orientation of Ad alpha. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2194–2198. doi: 10.1073/pnas.81.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Dhar V., Mager D., Iqbal A., Schildkraut C. L. The coordinate replication of the human beta-globin gene domain reflects its transcriptional activity and nuclease hypersensitivity. Mol Cell Biol. 1988 Nov;8(11):4958–4965. doi: 10.1128/mcb.8.11.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel P. A., Vaughn J. P., Hamlin J. L. Mapping of replication initiation sites in mammalian genomes by two-dimensional gel analysis: stabilization and enrichment of replication intermediates by isolation on the nuclear matrix. Mol Cell Biol. 1991 Aug;11(8):3850–3859. doi: 10.1128/mcb.11.8.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Estess P., Begovich A. B., Koo M., Jones P. P., McDevitt H. O. Sequence analysis and structure-function correlations of murine q, k, u, s, and f haplotype I-A beta cDNA clones. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3594–3598. doi: 10.1073/pnas.83.11.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falus A., Wakeland E. K., McConnell T. J., Gitlin J., Whitehead A. S., Colten H. R. DNA polymorphism of MHC III genes in inbred and wild mouse strains. Immunogenetics. 1987;25(5):290–298. doi: 10.1007/BF00404421. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Hunt S. W., 3rd, Hood L. Structure of a gene encoding a murine thymus leukemia antigen, and organization of Tla genes in the BALB/c mouse. J Exp Med. 1985 Aug 1;162(2):528–545. doi: 10.1084/jem.162.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst A., Brown E. H., Braunstein J. D., Schildkraut C. L. alpha-Globulin sequences are located in a region of early-replicating DNA in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1023–1027. doi: 10.1073/pnas.78.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Handeli S., Klar A., Meuth M., Cedar H. Mapping replication units in animal cells. Cell. 1989 Jun 16;57(6):909–920. doi: 10.1016/0092-8674(89)90329-2. [DOI] [PubMed] [Google Scholar]
- Hanson I. M., Trowsdale J. Colinearity of novel genes in the class II regions of the MHC in mouse and human. Immunogenetics. 1991;34(1):5–11. doi: 10.1007/BF00212306. [DOI] [PubMed] [Google Scholar]
- Hatton K. S., Dhar V., Brown E. H., Iqbal M. A., Stuart S., Didamo V. T., Schildkraut C. L. Replication program of active and inactive multigene families in mammalian cells. Mol Cell Biol. 1988 May;8(5):2149–2158. doi: 10.1128/mcb.8.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist G. P. Evolution of chromosome bands: molecular ecology of noncoding DNA. J Mol Evol. 1989 Jun;28(6):469–486. doi: 10.1007/BF02602928. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Klein J., Figueroa F., David C. S. H-2 haplotypes, genes and antigens: second listing. II. The H-2 complex. Immunogenetics. 1983;17(6):553–596. doi: 10.1007/BF00366126. [DOI] [PubMed] [Google Scholar]
- Kreuzer K. N., Alberts B. M. A defective phage system reveals bacteriophage T4 replication origins that coincide with recombination hot spots. Proc Natl Acad Sci U S A. 1985 May;82(10):3345–3349. doi: 10.1073/pnas.82.10.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel P. L., Pelletier A. J., Hill T. M. Tus and the terminators: the arrest of replication in prokaryotes. Cell. 1989 Nov 17;59(4):581–583. doi: 10.1016/0092-8674(89)90001-9. [DOI] [PubMed] [Google Scholar]
- Lader E., Clark B. T., Jhanwar S. C., Chaganti R. S., Bennett D. Definitive chromosomal location of the H-2 complex by in situ hybridization to pachytene chromosomes. Immunogenetics. 1985;22(1):49–54. doi: 10.1007/BF00430593. [DOI] [PubMed] [Google Scholar]
- Lai E., Wilson R. K., Hood L. E. Physical maps of the mouse and human immunoglobulin-like loci. Adv Immunol. 1989;46:1–59. doi: 10.1016/s0065-2776(08)60650-1. [DOI] [PubMed] [Google Scholar]
- Leffak M., James C. D. Opposite replication polarity of the germ line c-myc gene in HeLa cells compared with that of two Burkitt lymphoma cell lines. Mol Cell Biol. 1989 Feb;9(2):586–593. doi: 10.1128/mcb.9.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens M. H., Huberman J. A. The two faces of higher eukaryotic DNA replication origins. Cell. 1990 Sep 7;62(5):845–847. doi: 10.1016/0092-8674(90)90258-g. [DOI] [PubMed] [Google Scholar]
- Lugo M. H., Rauchfuss H. S., Zakour H. R., Allen J. W., Hozier J. C. Evidence for chromosomal replicons as units of sister chromatid exchanges. Chromosoma. 1989 Jun;98(1):69–76. doi: 10.1007/BF00293337. [DOI] [PubMed] [Google Scholar]
- Ma C., Leu T. H., Hamlin J. L. Multiple origins of replication in the dihydrofolate reductase amplicons of a methotrexate-resistant chinese hamster cell line. Mol Cell Biol. 1990 Apr;10(4):1338–1346. doi: 10.1128/mcb.10.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Leu T. H., Hamlin J. L. Multiple origins of replication in the dihydrofolate reductase amplicons of a methotrexate-resistant chinese hamster cell line. Mol Cell Biol. 1990 Apr;10(4):1338–1346. doi: 10.1128/mcb.10.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Benoist C. O., Williams V. E., 2nd, Kanter M. R., McDevitt H. O. The murine E alpha immune response gene. Cell. 1983 Mar;32(3):745–754. doi: 10.1016/0092-8674(83)90060-0. [DOI] [PubMed] [Google Scholar]
- Mengle-Gaw L., McDevitt H. O. Isolation and characterization of a cDNA clone for the murine I-E beta polypeptide chain. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7621–7625. doi: 10.1073/pnas.80.24.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco J. J., Cho S., Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990 Dec 21;250(4988):1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- Müller U., Stephan D., Philippsen P., Steinmetz M. Orientation and molecular map position of the complement genes in the mouse MHC. EMBO J. 1987 Feb;6(2):369–373. doi: 10.1002/j.1460-2075.1987.tb04764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Sepich D. S. Genes for murine fourth complement component (C4) and sex-limited protein (Slp) identified by hybridization to C4- and Slp-specific cDNA. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4908–4911. doi: 10.1073/pnas.81.15.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Shreffler D. C., Sepich D. S., Lilly S. P. cDNA clone spanning the alpha-gamma subunit junction in the precursor of the murine fourth complement component (C4). Proc Natl Acad Sci U S A. 1983 Aug;80(16):5061–5065. doi: 10.1073/pnas.80.16.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulnock-King D., Sizer K. C., Freund Y. R., Jones P. P., Parnes J. R. Coordinate induction of Ia alpha, beta, and Ii mRNA in a macrophage cell line. J Immunol. 1985 Jul;135(1):632–636. [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radic M. Z., Lundgren K., Hamkalo B. A. Curvature of mouse satellite DNA and condensation of heterochromatin. Cell. 1987 Sep 25;50(7):1101–1108. doi: 10.1016/0092-8674(87)90176-0. [DOI] [PubMed] [Google Scholar]
- Richards S., Bucan M., Brorson K., Kiefer M. C., Hunt S. W., 3rd, Lehrach H., Lindahl K. F. Genetic and molecular mapping of the Hmt region of mouse. EMBO J. 1989 Dec 1;8(12):3749–3757. doi: 10.1002/j.1460-2075.1989.tb08551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. H., Willison K. R. A major rearrangement in the H-2 complex of mouse t haplotypes. Nature. 1983 Aug 11;304(5926):549–552. doi: 10.1038/304549a0. [DOI] [PubMed] [Google Scholar]
- Sargent C. A., Dunham I., Campbell R. D. Identification of multiple HTF-island associated genes in the human major histocompatibility complex class III region. EMBO J. 1989 Aug;8(8):2305–2312. doi: 10.1002/j.1460-2075.1989.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig S., Okumura K., Ward D. C., Cedar H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J. 1992 Mar;11(3):1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D. S., Hare J., Golding H., Flaherty L., Rudikoff S. Characterization of a new subfamily of class I genes in the H-2 complex of the mouse. Immunogenetics. 1988;28(1):13–21. doi: 10.1007/BF00372524. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Strominger J. L. Human major histocompatibility complex contains a minimum of 19 genes between the complement cluster and HLA-B. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8955–8958. doi: 10.1073/pnas.86.22.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Malissen M., Hood L., Orn A., Maki R. A., Dastoornikoo G. R., Stephan D., Gibb E., Romaniuk R. Tracts of high or low sequence divergence in the mouse major histocompatibility complex. EMBO J. 1984 Dec 1;3(12):2995–3003. doi: 10.1002/j.1460-2075.1984.tb02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Stephan D., Fischer Lindahl K. Gene organization and recombinational hotspots in the murine major histocompatibility complex. Cell. 1986 Mar 28;44(6):895–904. doi: 10.1016/0092-8674(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Stephan D., Sun H., Lindahl K. F., Meyer E., Hämmerling G., Hood L., Steinmetz M. Organization and evolution of D region class I genes in the mouse major histocompatibility complex. J Exp Med. 1986 May 1;163(5):1227–1244. doi: 10.1084/jem.163.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Hagen K. G., Gilbert D. M., Willard H. F., Cohen S. N. Replication timing of DNA sequences associated with human centromeres and telomeres. Mol Cell Biol. 1990 Dec;10(12):6348–6355. doi: 10.1128/mcb.10.12.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge I., Shen F. W., Steinmetz M., Boyse E. A. A gene in the H-2S:H-2D interval of the major histocompatibility complex which is transcribed in B cells and macrophages. Immunogenetics. 1987;26(6):378–380. doi: 10.1007/BF00343709. [DOI] [PubMed] [Google Scholar]
- Uehara H., Abe K., Park C. H., Shin H. S., Bennett D., Artzt K. The molecular organization of the H-2K region of two t-haplotypes: implications for the evolution of genetic diversity. EMBO J. 1987 Jan;6(1):83–90. doi: 10.1002/j.1460-2075.1987.tb04722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umek R. M., Linskens M. H., Kowalski D., Huberman J. A. New beginnings in studies of eukaryotic DNA replication origins. Biochim Biophys Acta. 1989 Jan 23;1007(1):1–14. doi: 10.1016/0167-4781(89)90123-1. [DOI] [PubMed] [Google Scholar]
- Vassilev L., Johnson E. M. Mapping initiation sites of DNA replication in vivo using polymerase chain reaction amplification of nascent strand segments. Nucleic Acids Res. 1989 Oct 11;17(19):7693–7705. doi: 10.1093/nar/17.19.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. P., Dijkwel P. A., Hamlin J. L. Replication initiates in a broad zone in the amplified CHO dihydrofolate reductase domain. Cell. 1990 Jun 15;61(6):1075–1087. doi: 10.1016/0092-8674(90)90071-l. [DOI] [PubMed] [Google Scholar]
- Winoto A., Steinmetz M., Hood L. Genetic mapping in the major histocompatibility complex by restriction enzyme site polymorphisms: most mouse class I genes map to the Tla complex. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3425–3429. doi: 10.1073/pnas.80.11.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerer E. J., Passmore H. C. Structural and genetic properties of the Eb recombinational hotspot in the mouse. Immunogenetics. 1991;33(2):132–140. doi: 10.1007/BF00210827. [DOI] [PubMed] [Google Scholar]



