Summary
Intracellular cholesterol amounts, distribution, and traffic are tightly regulated to maintain the healthy eukaryotic cell function. However, how intracellular pathogens that require cholesterol, interact with the host cholesterol homeostasis and traffic is not well understood. Anaplasma phagocytophilum is an obligatory intracellular and cholesterol-robbing bacterium, which causes human granulocytic anaplasmosis. Here we found that a subset of cholesterol-binding membrane protein, Niemann–Pick type C1 (NPC1)-bearing vesicles devoid of lysosomal markers were upregulated in HL-60 cells infected with A. phagocytophilum, and trafficked to live bacterial inclusions. The NPC1 localization to A. phagocytophilum inclusions was abolished by low-density lipoprotein (LDL)-derived cholesterol traffic inhibitor U18666A. Studies using NPC1 siRNA and the cell line with cholesterol traffic defect demonstrated that the NPC1 function is required for bacterial cholesterol acquisition and infection. Furthermore, trans-Golgi network-specific soluble N-ethylmaleimide-sensitive factor attachment protein receptors, vesicle-associated membrane protein (VAMP4) and syntaxin 16, which are associated with NPC1 and LDL-derived cholesterol vesicular transport were recruited to A. phagocytophilum inclusions, and VAMP4 was required for bacteria infection. Taken together, A. phagocytophilum is the first example of a pathogen that subverts the NPC1 pathway of intracellular cholesterol transport and homeostasis for bacterial inclusion membrane biogenesis and cholesterol capture.
Keywords: Anaplasma phagocytophilum, NPC1, Inclusion, Cholesterol, LAMP-1, LAMP-2, VAMP4, syntaxin 16
Introduction
Cellular cholesterol level and distribution are tightly regulated by multiple cholesterol sensing/binding proteins via multifarious mechanisms in order to maintain the normal cellular function (Goldstein et al., 2006). Impairment of cellular cholesterol homeostasis is the key element of the pathogenesis of many diseases, including atherosclerosis (Lusis, 2000) and Niemann–Pick type C (NPC) disease (Karten et al., 2009). Mammalian cells acquire cholesterol from two sources: exogenous cholesterol taken up mainly by low-density lipoprotein (LDL) endocytosis via LDL receptors and endogenous biosynthesis in the endoplasmic reticulum (ER) (Chang et al., 2006, Goldstein et al., 2009). In the LDL uptake pathway, after hydrolysis of cholesterol esters in acidic endosomes, LDL-derived free cholesterol (LDL-CHOL) enters the vesicular compartment containing the cholesterol binding protein NPC1; from this compartment the LDL-CHOL moves to the ER. In the ER, excess free cholesterol is esterified and stored as cytoplasmic lipid droplets, or it is transported to the plasma membrane (Chang et al., 2006, Ikonen, 2008). Urano et al. recently demonstrated that a substantial portion of LDL-CHOL from NPC1-containing compartments is transported to the trans-Golgi network (TGN) before its arrival at the ER by vesicular trafficking (Urano et al., 2008).
A. phagocytophilum is an obligatory intracellular bacterium that proliferates in membrane-bound inclusions in granulocytes and endothelial cells of various mammalian species (Chen et al., 1994, Goodman et al., 1996, Munderloh et al., 2004). In humans, A. phagocytophilum causes an emerging and major tick-borne disease called human granulocytic anaplasmosis, an acute febrile disease that is potentially fatal, especially in elderly or immunocompromised individuals (Bakken et al., 2008). A. phagocytophilum is an atypical Gram-negative bacterium, because it contains a substantial amount of cholesterol in its membrane (Lin et al., 2003a). Furthermore, A. phagocytophilum is absolutely dependent on cholesterol, but it lacks genes for cholesterol biosynthesis or modification; thus, it needs to capture cholesterol from host cells (Lin et al., 2003a). Indeed, host cholesterol is a critical factor for A. phagocytophilum infection in vivo (Xiong et al., 2007). Intracellular A. phagocytophilum infection upregulates LDL receptor expression and depends on cholesterol derived from increased LDL taken up by the host cells, but not depends on endogenous cholesterol synthesis (Xiong et al., 2009). However, it has been an enigma how A. phagocytophilum, which replicates within membrane-bound inclusions secluded from endosome-lysosome system (Mott et al., 1999, Niu et al., 2008, Webster et al., 1998), has access to LDL-CHOL to acquire free cholesterol. In this study we investigated whether, where, and how A. phagocytophilum intercepts LDL-CHOL intracellular traffic.
Results
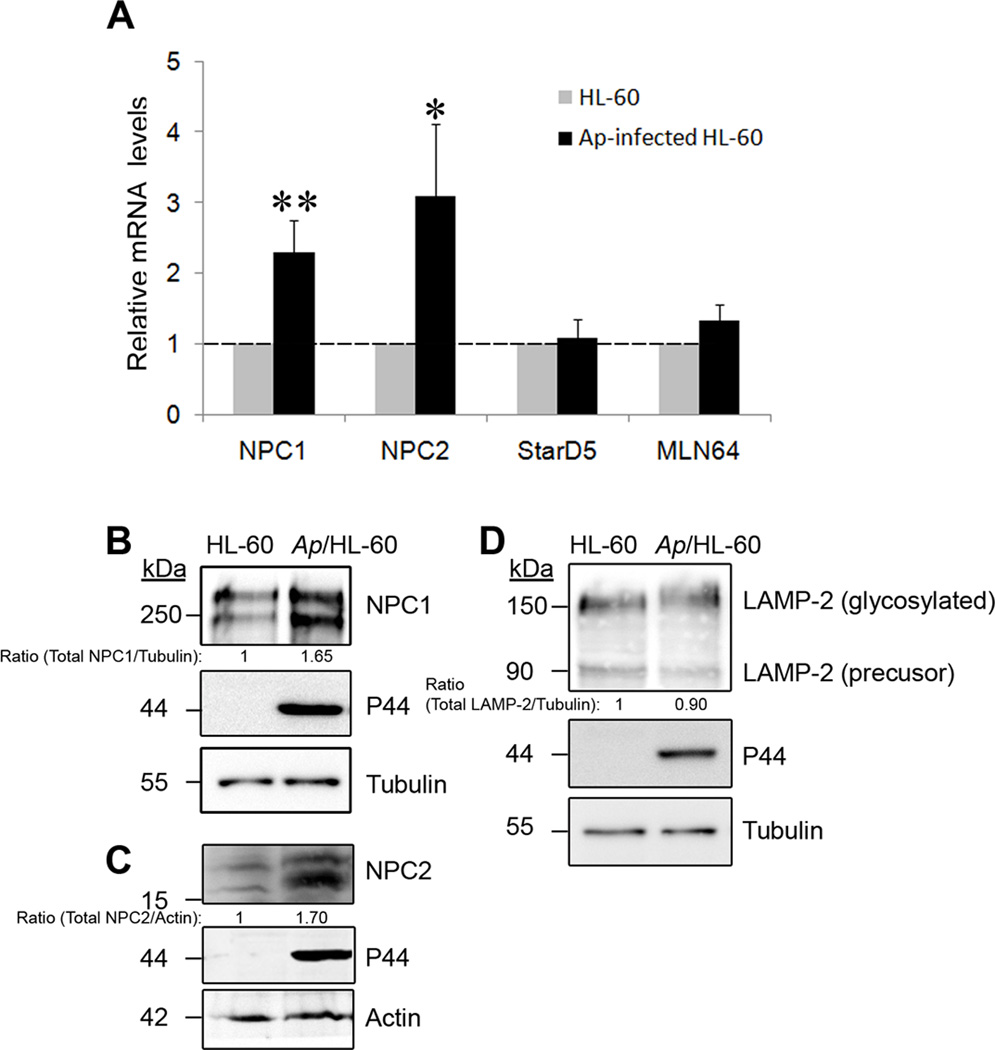
A. phagocytophilum infection upregulates cholesterol transport proteins NPC1 and NPC2, but not STARD5, STARD3/MLN64 or LAMP-2
We first examined influences of A. phagocytophilum infection on expression of cholesterol transport proteins related to LDL-CHOL intracellular trafficking. NPC1 and NPC2 play key roles in regulating the transport of LDL-CHOL from endocytic compartments to other intracellular compartments to maintain intracellular cholesterol distribution and homeostasis (Ikonen, 2008, Karten et al., 2009). NPC1 is a large 13-transmembrane domain-containing cholesterol-binding glycoprotein primarily localized in late endosomes, whereas NPC2 is a small soluble cholesterol-binding glycoprotein localized in late endosomes and lysosomes (Chang et al., 2006). STARD5 and STARD3/MLN64, which contain the steroidogenic acute regulatory protein (StAR)-related lipid transfer domain, specifically bind and traffic free cholesterol between intracellular membranes in mammalian cells (Alpy et al., 2005). Despite lack of cholesterol-binding activity, lysosome-associated membrane protein-2 (LAMP-2), particularly its highly glycosylated luminal domain, was recently reported to play a critical role in endosomal cholesterol transport (Schneede et al., 2011). We found that NPC1 and NPC2 mRNAs were upregulated >2-fold in A. phagocytophilum-infected HL-60 cells compared to uninfected cells. In contrast, the levels of STARD5 and STARD3/MLN64 mRNAs remained unchanged in A. phagocytophilum-infected cells (Fig. 1A). Both NPC1 and NPC2 are glycosylated and the glycosylation is crucial for their biological functions (Chikh et al., 2004, Watari et al., 1999). Western blotting showed that the NPC1 and NPC2 protein levels including their glycosylated forms were also increased (Fig. 1B and 1C) in infected cells, whereas the amounts of LAMP-2 and its glycosylated form did not change (Fig. 1D).
Fig. 1. Cholesterol transport proteins NPC1 and NPC2 are upregulated in A. phagocytophilum–infected HL-60 cells.
A. Total RNA was extracted from uninfected and A. phagocytophilum-infected HL-60 cells at day 2 pi. The mRNA level was quantified using quantitative RT-PCR with specific primers for each gene (NPC1, NPC2, STARD5, and STARD3/MLN64); the level was normalized to human G3PDH in each sample, and the amount of mRNA in uninfected cells was arbitrarily set as 1. Ap, A. phagocytophilum. Results are presented as the mean ± standard deviation of three independent experiments. *, P < 0.05; **, P < 0.01 (unpaired two-tailed t-test).
B–D. NPC1 (B), NPC2 (C) and LAMP-2 (D) protein levels in A. phagocytophilum-infected and control HL-60 cells at day 2 pi were analyzed by western blotting using anti-NPC1, anti-NPC2, and monoclonal anti-LAMP-2, respectively. A. phagocytophilum infection was indicated by the presence of bacterial outer membrane protein P44, as determined by western blotting using antibody 5C11. α-Tubulin or actin was used as the protein input control to normalize each sample. Relative density ratios of NPC1 or LAMP-2/tubulin and NPC2/actin bands are shown below each lane. The results are representative of three independent experiments. Numbers on the left of each panel represent molecular sizes.
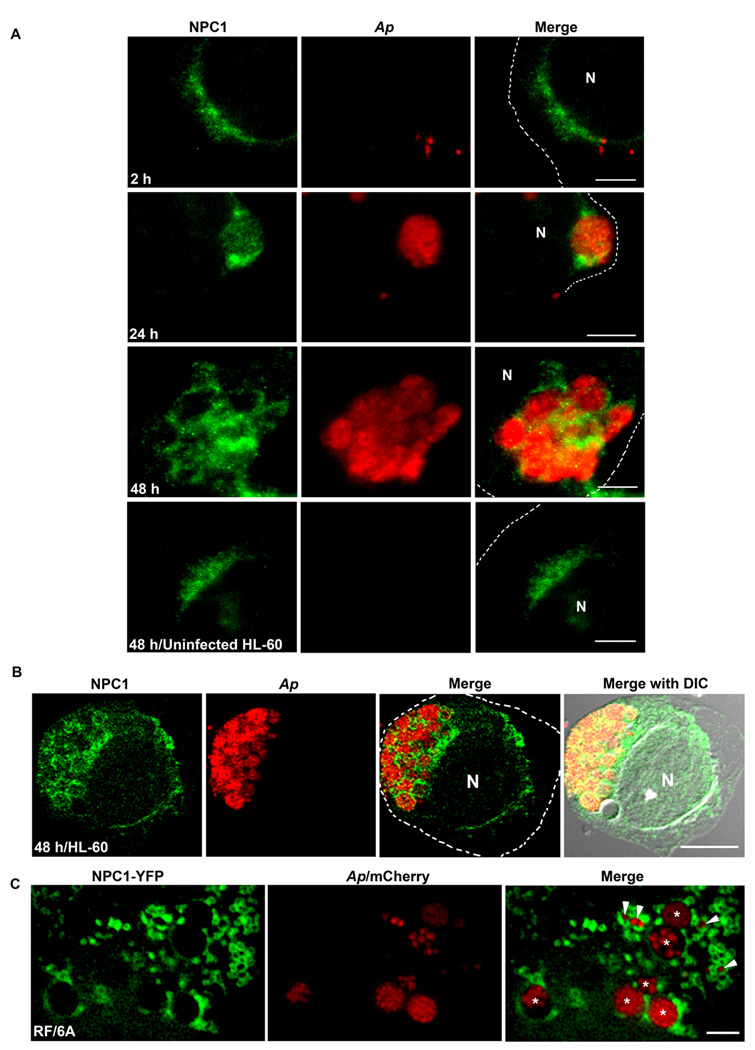
NPC1 and NPC2 localize to A. phagocytophilum inclusions, and NPC1 vesicles target live bacteria inclusions
Since NPC proteins were upregulated, we examined the localization of NPC proteins in A. phagocytophilum-infected cells. By double immunofluorescence labeling, NPC1 expression was progressively upregulated in exponentially growing bacteria, and a large proportion of NPC1 was localized on A. phagocytophilum inclusions (Fig. 2A); large inclusions were ringed by NPC1 in HL-60 cells (Fig. 2A, 24 and 48 h post-infection (pi)) as well as in monkey endothelial RF/6A cells (data not shown). This localization was not evident at 2 h pi (Fig. 2A). NPC1 localization on A. phagocytophilum inclusions was confirmed by confocal microscopy (Fig. 2B). As shown by others (Garver et al., 2000, Higgins et al., 1999) when cells are fixed, NPC1 (Figs. 2A and 2B) or NPC1-YFP (data not shown) appeared as dots and clear NPC1 vesicle structure could not be discerned. However, in live cells, NPC-1-bearing vesicles could be visible when RF/6A cells were transfected with NPC1-YFP as previously reported in NPC1-YFP-transfected CHO cells (Ko et al., 2001). NPC1-YFP-trasfected cells were infected with mCherry A. phagocytophilum and live fluorescence images were captured by deconvolution microscopy. Deconvolution fluorescence microscopy reduces out-of-focus fluorescence by computational processing, thereby promoting the restoration of multiple focal planes into a high-resolution three-dimensional image (McNally et al., 1999). This technique offers another significant advantage over the confocal microscopy by enabling multiple focal-plane imaging of light-sensitive living specimens, because of very low excitation light intensity. The result showed not only the NPC1-YFP protein was on the inclusion membrane, but also multiple NPC1-YFP vesicles were attached to most of A. phagocytophilum inclusions (Fig. 2C), demonstrating that NPC1-YFP vesicles target live bacterial inclusions. NPC1-YFP protein was never found inside of inclusions (Fig. 2C). This localization was specific to A. phagocytophilum, because the NPC1 protein or NPC1 vesicles did not localize to live inclusions of another obligatory intracellular bacterium, Chlamydia trachomatis, in mouse L929 cells (Fig. S1). Unlike A. phagocytophilum, C. trachomatis acquires cholesterol and sphingolipid from the Golgi exocytic pathway (Carabeo et al., 2003) and accumulates lipid droplets in its inclusion (Cocchiaro et al., 2008). Together, these data revealed that NPC1 and NPC1 vesicles traffic to A. phagocytophilum inclusions in host cells. Furthermore, unlike NPC1, NPC2 localized in A. phagocytophilum inclusions at 24 and 48 h pi, suggesting the NPC2 vesicle fusion took place (Fig. S2).
Fig. 2. NPC1 is on A. phagocytophilum inclusions.
A. A. phagocytophilum-infected HL-60 cells were fixed at indicated times pi, stained with anti-NPC1 (green) and mouse anti-A. phagocytophilum P44 antibody 5C11 (red), and analyzed by fluorescence microscopy. The experiment shown is representative of at least four independent experiments. Each dotted line depicts the cell boundary. Bar, 5 µm. Ap, A. phagocytophilum. N, nucleus.
B. A. phagocytophilum-infected HL-60 cells were fixed at 48 h pi, labeled with anti-NPC1 (green) and antibody 5C11 (red), and analyzed by confocal fluorescence microscopy. The experiment shown is representative of at least two independent experiments. Each dotted line depicts the cell boundary. Bar, 5 µm. Ap, A. phagocytophilum. N, nucleus. DIC, differential interference contrast.
C. RF/6A cells were transfected with NPC1-YFP plasmid and inoculated with mCherry-A. phagocytophilum at 8 h post-transfection. At day 2 pi, live cells were observed by DeltaVision fluorescence deconvolution microscopy. Note numerous NPC1 vesicles attaching to A. phagocytophilum inclusions. The experiment shown is representative of at least three independent experiments. Arrows indicate small inclusions, and asterisks indicate larger inclusions. Bar, 5 µm. Ap, A. phagocytophilum. N, nucleus.
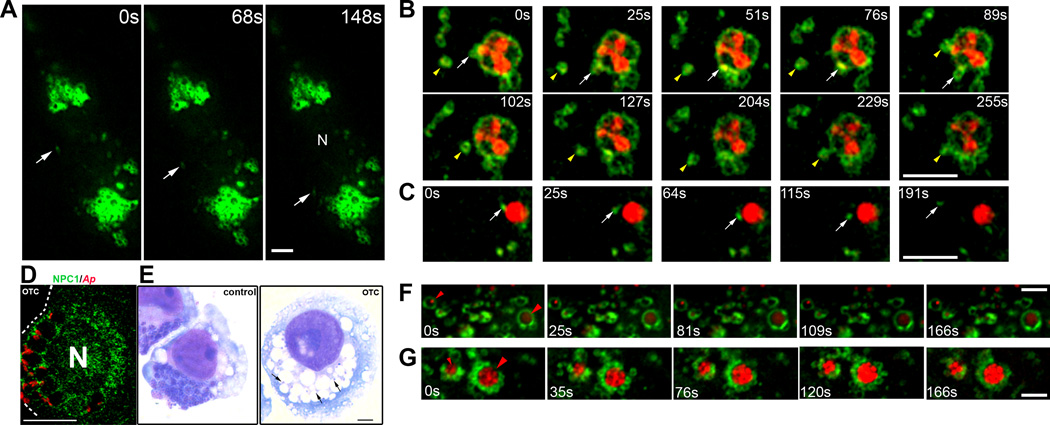
NPC1 vesicles vigorously interact with A. phagocytophilum inclusions
NPC1 vesicles are the most dynamic vesicles in the intracellular transport of LDL-CHOL (Ko et al., 2001). To track NPC1 vesicle movements, NPC1-YFP-transfected RF/6A cells with or without mCherry-A. phagocytophilum infection were examined by time-lapse live fluorescence imaging by deconvolution microscopy. A large number of NPC1 vesicles were found all over the cytoplasm in both infected and uninfected cells. In uninfected cells, numerous NPC1-positive ring-like vesicles (diameter 1.3 ± 0.3 µm; N = 200) showed short (<1 µm) continuous Brownian movement (Fig. 3A and Video S1). We also observed rare smaller (<0.5 µm) NPC1 vesicles that exhibited long-distance (>10 µm) rapid vectorial movement (Fig. 3A, arrow; Video S1 and S1t). In mCherry-A. phagocytophilum-infected cells, NPC1 vesicles vigorously moved and interacted with inclusions. For example, when individual vesicle movement was tracked, some NPC1 vesicles moved along the inclusion membrane and interacted with the inclusion (Fig. 3B, arrow; Video S2 and S2t); others rapidly moved to and attached to the inclusion (76s–89s) and then moved away from the inclusion (Fig. 3B, arrowhead; Video S2 and S2t). Not only the NPC1-YFP protein was on the inclusion membrane, distribution of the fluorescence intensity of NPC1-YFP changed along the inclusion membrane (Fig. 3B). On the other hand, a few smaller NPC1 vesicles attached to the inclusion underwent long-distance (~5 µm) vectorial movement away from the inclusion (Fig. 3C, arrow; Video S3 and S3t). Of note, not all NPC1 vesicles were recruited to and surrounded inclusions. In fact, we did not observe significant change in movements of NPC1 vesicles which were far away from inclusions in A. phagocytophilum -infected cells compared with uninfected cells. Additionally, no movement of NPC1 vesicles other than Brownian movement was seen around C. trachomatis inclusions in L929 cells (Video S4) and the speed of NPC1 vesicle movement around C. trachomatis inclusions was significantly slower compared with those of A. phagocytophilum in RF/6A cells (Table 1).
Fig. 3. NPC1 vesicles interact with A. phagocytophilum inclusions, which requires bacterial protein synthesis.
A. RF/6A cells were transfected with NPC1-YFP plasmid. At day 2 post-transfection, live images of transfected cells were taken by DeltaVision deconvolution microscopy. Note many large (>1 µm) ring-like NPC1-positive vesicles. The arrow in each panel shows a small (<0.5 µm) NPC1 vesicle undergoing vectorial movement. Video S1 accompanies Fig. 3A. Bar, 5 µm. N, nucleus.
B and C. mCherry-A. phagocytophilum was added to RF/6A cells 1 day post-transfection with NPC1-YFP plasmid. At day 1 pi, live images were taken by DeltaVision deconvolution microscopy. NPC1-YFP (green) and A. phagocytophilum (red) were visualized by sequential photos taken every 15 s. Selected images at different time points are shown. (B) Tracked movements of two large (>1 µm) NPC1 vesicles (white arrow and yellow arrowhead) interacting with the inclusion. (C) Tracked movement of a small NPC1 vesicle (white arrow) showing the beginning of the vectorial movement at 115s. Videos S2 and S3 accompany Figures 3B and 3C, respectively. Bar, 5 µm. N, nucleus.
D and E. A. phagocytophilum-infected HL-60 cells at day 1 pi were treated with oxytetracycline (OTC, 10 µg/ml; control, no OTC) for 24 h. The fixed cells were stained with anti-NPC1 (green) and mouse anti-A. phagocytophilum P44 mAb 5C11 (red) and analyzed by fluorescence microscopy (D). The infected cells were also observed by light microscopy following Diff-Quik staining (E). Note that only a few bacteria remain in the large (otherwise empty) vacuoles. The experiment shown is representative of at least three independent experiments. Dotted line depicts the cell boundary. Bar, 5 µm. Ap, A. phagocytophilum.
F and G. RF/6A cells were transfected with NPC1-YFP plasmid, infected with mCherry-A. phagocytophilum, and treated with OTC (10 µg/ml) starting at 20 h pi for 10 h (F) and no OTC treatment (G). Live images were taken by DeltaVision deconvolution microscopy at 30 h pi. NPC1-YFP (green) and A. phagocytophilum (red) were visualized by sequential photos taken every 15 s. Selected images at different time points are shown. Red arrowheads indicate two bacterial inclusions in F and G. Note no NPC1 vesicle are attached to bacterial inclusions (F), whereas many NPC1 vesicles are attached to bacterial inclusions (G). Videos S5 and S6 accompany Figures 3F and 3G, respectively. Bar, 5 µm. N, nucleus.
Table 1.
Tracked NPC1 vesicle movements around inclusions in A. phagocytophilum (with and without oxytetracycline treatment) and C. trachomatis-infected RF/6A cellsa
| Movement of NPC1 vesicles associated with inclusions |
Ap/CTL (n=98) |
Ap/OTC (n=133) |
Ct (n=100) |
|---|---|---|---|
| Average maximum speed (µm/s) | 0.097±0.034 | 0.051±0.017** | 0.046±0.026** |
| Average speed (µm/s) | 0.046±0.036 | 0.020±0.021** | 0.025±0.016** |
RF/6A cells were transfected with NPC1-YFP plasmid and infected with mCherry-A. phagocytophilum with or without oxytetracycline (OTC) treatment for 10 h or C. trachomatis. Inclusion-bound or -associated vesicles were tracked for 5 min. Data are expressed as mean ± standard deviation (n=98–133). Ap, A. phagocytophilum. Ct, C. trachomatis
P < 0.01 (unpaired two-tailed t-test).
NPC1 vesicle interaction with inclusions requires bacterial protein synthesis
Oxytetracycline (OTC) treatment of A. phagocytophilum-infected cells induces bacterial delivery to lysosomes (Gokce et al., 1999, Huang et al., 2010a). We did not observe a significant reduction of preexisting NPC1 on A. phagocytophilum inclusion membrane after OTC treatment for 1 d (Fig. 3D); the bacteria were cleared after 2 d, resulting in large empty vacuoles in the host cytoplasm (Fig. 3E). These data suggested that bacterial new protein synthesis is not required for retaining NPC1 on inclusions. However, when we tracked NPC1 vesicle movement in live mCherry-A. phagocytophilum-infected NPC1-YFP-transfected RF/6A cells after 10 h of OTC treatment, movement of NPC1 vesicles around A. phagocytophilum inclusions was significantly reduced (Fig. 3F, Video S5 and S5t, and Table 1). Moreover, the number of NPC1 vesicles attached to bacterial inclusions was greatly reduced compared to untreated cells (Fig. 3G and Video S6). Notably, although NPC1 vesicle attachment and movement was compromised, ring-like NPC1-lined inclusions were still observed after OTC treatment (Fig. 3F), which was consistent with the data obtained by immunolabeling of native NPC1 (Fig. 3D). Together, these data indicate that recruitment of new NPC1 vesicles to bacterial inclusions is an active process by the bacteria.
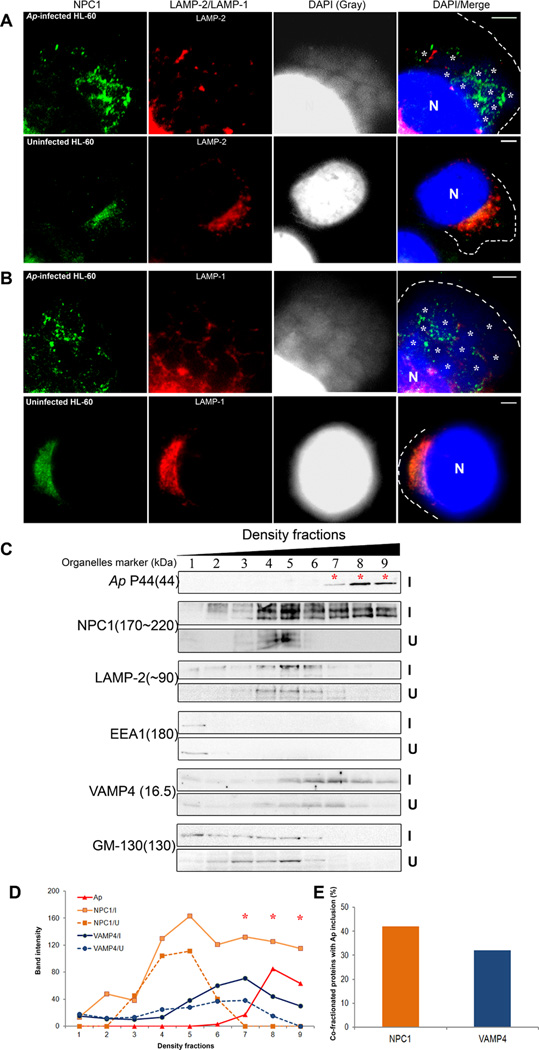
LAMP-1/2-negative NPC1 vesicles are recruited to A. phagocytophilum inclusions
Garver et al. reported that two populations of NPC1 vesicles in murine fibroblasts: one is large (~1 µm) LAMP-1-negative and cholesterol-enriched vesicles, and the other comprises smaller and more diffusely distributed LAMP-1-positive vesicles (Garver et al., 2000). The NPC1 was reported to reside in a novel set of LAMP-2-positive vesicles that differ from LAMP-2-positive lysosomes in human fibroblasts (Neufeld et al., 1999). We found the majority of NPC1 co-localized with LAMP-2 in uninfected HL-60 cells (Fig. 4A). In A. phagocytophilum-infected HL-60 cells, NPC1 was found in both LAMP-2-positive and -negative compartments, and a substantial amount of NPC1 was associated with A. phagocytophilum inclusions. Interestingly, this latter population of NPC1 did not co-localize with LAMP-2 (Fig. 4A). Although some LAMP-2 was found near the inclusions, it did not co-localize with inclusions. Triple fluorescence labeling for NPC1/LAMP-1/DAPI showed similar results (Fig. 4B).
Fig. 4. NPC1 and VAMP4 vesicles recruited to A. phagocytophilum inclusions do not contain LAMP-1/2 or GM-130.
A and B. A. phagocytophilum-infected and control HL-60 cells were fixed at day 2 pi, stained with anti-NPC1 (green), and anti-LAMP-2 (A) or anti-LAMP-1 (B) (red), and analyzed by fluorescence microscopy. A. phagocytophilum inclusions and nuclei were visualized by DAPI staining (blue); the blue/DAPI staining was also shown in gray pseudocolor for clearer viewing of bacterial inclusions. White asterisks indicate individual inclusions. The experiment shown is representative of three independent experiments. Each dotted line depicts the cell boundary. Bar, 5 µm. Ap, A. phagocytophilum. N, nucleus.
C. NPC1 and VAMP4, but not EEA1, LAMP-2, or GM-130, co-fractionate with A. phagocytophilum. A. phagocytophilum-infected and control HL-60 cells were fractionated by density gradient ultracentrifugation (5–25% OptiPrep). An equal volume from each fraction was analyzed by immunoblotting using the indicated antibodies (9 fractions from the top are shown). P44 mAb 5C11 was used to identify fractions containing A. phagocytophilum inclusions (*). The experiment shown is representative of three independent experiments. Ap, A. phagocytophilum. I, A. phagocytophilum-infected HL-60 cells; U, uninfected HL-60 cells. Numbers in parenthesis on the left are molecular sizes of organelle markers: NPC1, NPC1 compartment; LAMP-2, late endosomes-lysosomes; EEA1, early endosomes; VAMP4, trans-Golgi Network SNARE; GM-130, cis-Golgi; and P44, Anaplasma inclusion.
D and E. The amounts of indicated proteins from A. phagocytophilum-infected cells (solid line) and uninfected cells (dotted line) in each fraction were determined by densitometry (D) and % of NPC1 and VAMP4 proteins which were co-fractionated with A. phagocytophilum fractions (7, 8, and 9) are shown (E). The experiment shown is representative of three independent experiments. Ap, A. phagocytophilum. I, A. phagocytophilum-infected HL-60 cells; U, uninfected HL-60 cells.
We next fractionated organelles by OptiPrep density centrifugation. Immunoblot analysis of the resulting fractions showed that NPC1 vesicles co-fractionated with LAMP-2-positive vesicles in uninfected HL-60 cells (Fig. 4C). In infected cells, however, NPC1 level was strikingly increased, especially in the higher-density populations which were negative for both early endosome antigen-1 (EEA1) and LAMP-2. Importantly, this population of NPC1 vesicles (~ 40% of total NPC1) co-fractionated with A. phagocytophilum inclusions (Figs. 4C, 4D, and 4E fractions 7–9). This was selective for NPC1 vesicles, because there was no significant increase or shift of EEA1-positive early endosomes or LAMP-2-positive late endosomes and lysosomes in infected cells compared with uninfected cells (Fig. 4C). Taken together, these results imply that the number of NPC1-bearing vesicles lacking LAMP-1 and/or LAMP-2 was increased upon infection, and this population of NPC1 vesicles, but not LAMP-1 and/or LAMP-2-positive NPC1 vesicles were trafficked to A. phagocytophilum inclusions. This result was in agreement with the lack of fusion of A. phagocytophilum inclusions with early or late endosomes, or lysosomes (Mott et al., 1999, Niu et al., 2008, Webster et al., 1998).
NPC1 is required for A. phagocytophilum infection and cholesterol acquisition by A. phagocytophilum
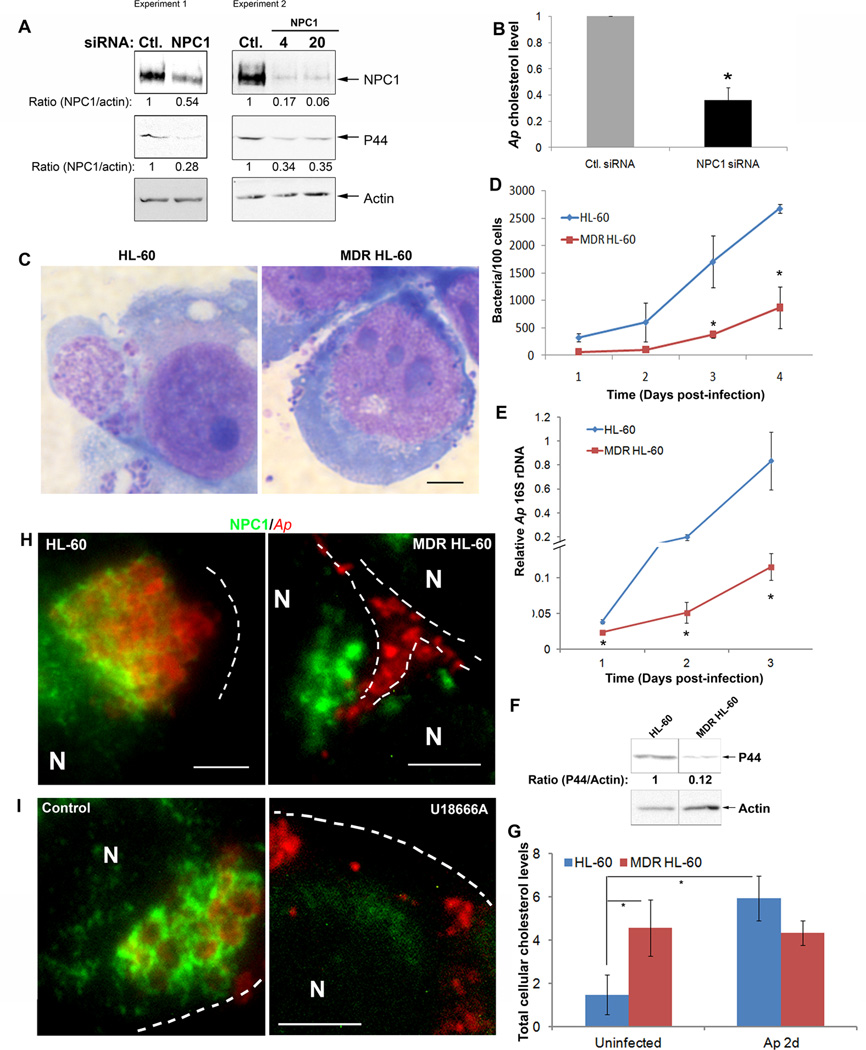
Next we determined whether NPC1 is required for A. phagocytophilum infection using an NPC1 knockdown approach. RF/6A cells were transfected with NPC1 or control siRNA. NPC1 was reduced by ~50–90 % in the cells after siRNA transfection; A. phagocytophilum infection was reduced by ~70% (Fig. 5A). When we purified host cell-free A. phagocytophilum from NPC1 siRNA-transfected cells and measured the cholesterol levels associated with A. phagocytophilum, the cholesterol level per bacterium was significantly reduced by NPC1 siRNA compared with control siRNA transfection (Fig. 5B). These results suggest that NPC1 is required for A. phagocytophilum cholesterol acquisition and infection.
Fig. 5. NPC1 is essential for A. phagocytophilum infection.
A. RF/6A cells were transfected with a control (Ctl.) siRNA or a siRNA specific for NPC1. One day after transfection, A. phagocytophilum was added to the cells and incubated for an additional 2 days. The cell samples were lysed and subjected to western blotting using antibodies against NPC1 and A. phagocytophilum P44. Actin was used as the protein input control to normalize each sample. The amounts of NPC1 and P44 (relative to actin) were determined by densitometry. The values under the bands show the relative ratios of band intensities, with the ratios of those from samples transfected with control siRNA arbitrarily set as 1. Results are representative of four independent experiments. Ap, A. phagocytophilum.
B. RF/6A cells were transfected with siRNA followed by infection with A. phagocytophilum. At day 2 pi, host cell-free bacteria were purified, and bacterial cholesterol was measured. Each cholesterol amount was normalized to the amount of bacterial 16S rDNA as determined by quantitative PCR, and bacterial cholesterol level from control (Ctl.) siRNA-treated cells was arbitrarily set as 1. Results are representative of three independent experiments. Ap, A. phagocytophilum.
C–F. A. phagocytophilum-infected multidrug resistant (MDR) and drug-sensitive HL-60 cells were harvested at the indicated time and observed by light microscopy (C), and bacterial load was determined by counting the number of bacteria in the cells stained by Diff-Quik (D), by measuring bacterial 16S rDNA by quantitative real-time PCR (E), and by western blotting for the outer membrane protein P44 using antibody 5C11 (F). Actin was used as the protein input control to normalize each sample. Data are expressed as mean ± standard deviation (n = 3) and are representative of three independent experiments with similar results. *, P < 0.05; **, P < 0.01 (unpaired two-tailed t-test).
G. Total cellular cholesterol levels in MDR and drug-sensitive HL-60 cells with and without A. phagocytophilum infection were measured and normalized to the total cellular protein levels. Data are expressed as mean ± standard deviation (n = 3) and are representative of three independent experiments with similar results. *, P < 0.05 (unpaired two-tailed t-test).
H and I. A. phagocytophilum-infected MDR and drug-sensitive HL-60 cells at 24 h pi were collected (H) and A. phagocytophilum-infected HL-60 cells at day 1 pi were treated with U18666A (5 µM) for 24 h (I), stained with anti-NPC1 (green) and antibody 5C11 (red), and analyzed by fluorescence microscopy. The data shown are representative of at least three independent experiments. Each dotted line depicts the cell boundary. Bar, 5 µm. Ap, A. phagocytophilum.
NPC1 traffic to A. phagocytophilum inclusions is crucial for infection
NPC1 has homology to the resistance-nodulation-division (RND) family of prokaryotic permeases and may normally function as a transmembrane drug efflux pump (Davies et al., 2000). A multidrug-resistant (MDR) HL-60 cell line is defective in NPC1 regulation of intracellular cholesterol traffic even though NPC1 mRNA and protein expression levels are higher than those in normal drug-sensitive HL-60 cells. The phenotype of MDR HL-60 cell line, including cholesterol traffic, NPC1 expression is consistent with a loss of NPC1 activity (Gong et al., 2006). We confirmed NPC1 dysfunction in the MDR HL-60 cells by western blotting for NPC1 and filipin staining for nonesterified cholesterol accumulation (data not shown). Because A. phagocytophilum can infect HL-60 cells, we took advantage of MDR HL-60 cells to examine whether NPC1 function is required for A. phagocytophilum infection. We observed a striking difference between infection profiles of MDR HL-60 cells and drug-sensitive cells. At 60 h pi, large inclusions were absent and bacteria were dispersed at the periphery of MDR HL-60 cells (Fig. 5C). The overall infection level in MDR HL-60 was much lower compared with HL-60 cells determined by morphological observation after Diff-Quik staining (Fig. 5D) and quantitative PCR by measuring A. phagocytophilum 16S rDNA gene (Fig. 5E). The result was also confirmed by western blotting of A. phagocytophilum P44 major outer membrane protein (Fig. 5F). MDR HL-60 cells had higher cellular cholesterol level compared with sensitive HL-60 cells (Fig. 5G). Although A. phagocytophilum infection greatly upshifted cellular cholesterol level in sensitive HL-60(Xiong et al., 2009), but not in MDR HL-60 cells (Fig. 5G), suggesting increased cellular cholesterol alone is not sufficient for A. phagocytophilum infection. Importantly, unlike drug-sensitive cells, NPC1 did not localize to A. phagocytophilum inclusions in MDR HL-60 cells (Fig. 5H).
The compound (3β)-3-[2-(diethylamino)ethoxy]androst-5-en-17-one dihydrochloride (U18666A) is a cell-permeable, amphiphilic amino-steroid that causes an NPC1-deficient phenotype by blocking cholesterol egress from the endocytic system, resulting in intravacuolar accumulation of nonesterified cholesterol (Cenedella, 2009). U18666A effectively blocks A. phagocytophilum infection in host cells (Xiong et al., 2009). We found that U18666A treatment for 24 h almost completely abolished the co-localization of NPC1 with bacterial inclusions in HL-60 cells (Fig. 5I). These results suggested that NPC1 vesicle trafficking to the A. phagocytophilum inclusion is crucial for A. phagocytophilum infection of host cells and that A. phagocytophilum recruits NPC1 vesicles downstream of the U18666A target site in the LDL-CHOL intracellular traffic.
SNAREs of the TGN are recruited to A. phagocytophilum inclusions
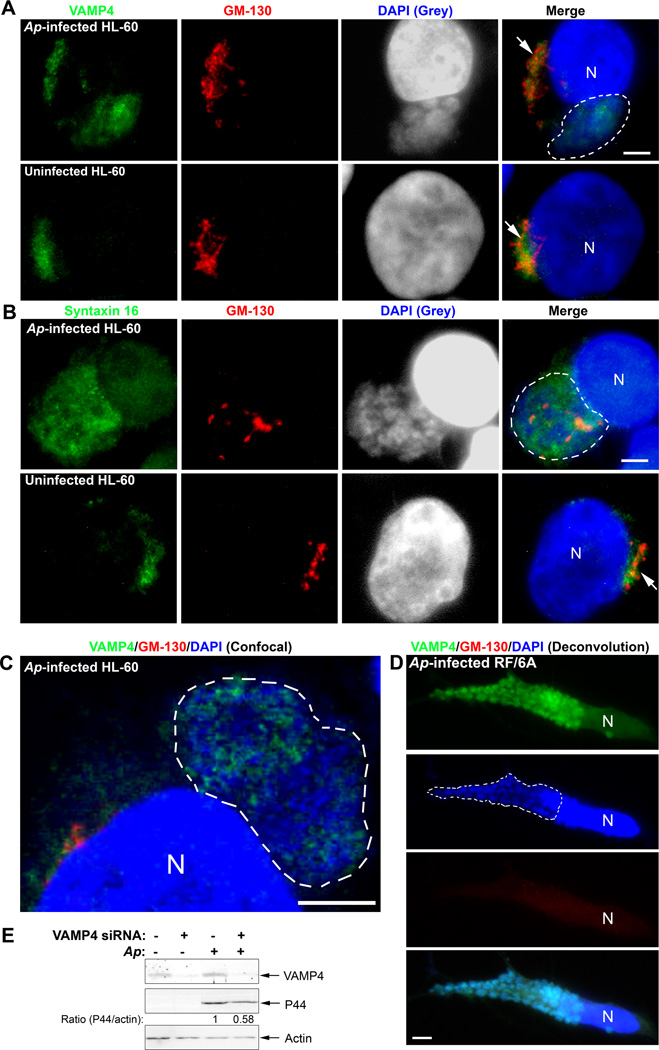
A substantial portion of LDL-CHOL from NPC1-containing compartments is transported by vesicular trafficking to TGN before its arrival at the ER, and soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) proteins: vesicle associated membrane protein (VAMP4), syntaxin 6, and syntaxin 16 are involved in this process (Urano et al., 2008). We determined the subcellular localization of native VAMP4 and syntaxin 16 in A. phagocytophilum-infected cells by triple fluorescence labeling using respective antibodies in conjunction with anti-GM-130 (stains cis-Golgi) and DAPI (stains DNA). In uninfected cells, VAMP4 and syntaxin 16 were associated with GM-130, indicating their localization on Golgi (Fig. 6A and 6B, arrow); however, a large portion of VAMP4 and syntaxin 16, but not GM-130, co-localized to A. phagocytophilum inclusions in HL-60 cells (Fig. 6A and 6B, inclusions outlined). The colocalization of VAMP4 with A. phagocytophilum inclusions in HL-60 cells (Fig. 6C, inclusion outlined) and RF/6A cells (Fig. 6D, inclusion outlined and Fig. S4) were confirmed by confocal fluorescence microscopy and deconvolution fluorescence microscopy, respectively. Of note, Golgi was greatly dispersed and even became invisible in A. phagocytophilum highly infected cells (Figs. 6D and S4). We also examined the localization of VAMP4 and syntaxin 6 with bacterial inclusions in VAMP4-GFP and syntaxin 6-GFP-transfected RF/6A cells. As expected, VAMP4-GFP was found on the inclusion membrane; however, syntaxin 6-GFP did not localize to A. phagocytophilum inclusions at all (data not shown). As a negative control, VAMP4 did not localize to C. trachomatis inclusions as shown by immunofluorescence labeling with anti-VAMP4 (Fig. S3), which is in agreement with the previous report using a VAMP4-GFP plasmid (Delevoye et al., 2008). Our organelle fractionation by Optiprep density gradient also showed that the VAMP4 fractions were shifted and partially co-fractionated (around 30%, Fig. 4E) with A. phagocytophilum inclusion in infected HL-60 (Figs. 4C, 4D, and 4E), while cis-Golgi marker GM-130 fractions were remained unchanged in both infected and uninfected cells and not cofractionated with bacterial inclusions (Fig. 4C). Additionally, we determined the requirement of VAMP4 for A. phagocytophilum infection by siRNA knockdown in RF/6A cells; the level of VAMP4 protein was reduced by >90%, resulting in ~50% reduction in A. phagocytophilum infection (Fig. 6E).
Fig. 6. SNARE proteins VAMP4 and syntaxin 16 are recruited to A. phagocytophilum inclusions.
A–C. A. phagocytophilum-infected and control HL-60 cells were fixed at day 2 pi, stained with anti-VAMP4 (A) or anti-syntaxin 16 (B) (green) and anti-GM-130 (red), and analyzed by fluorescence microscopy (A, B) or confocal microscopy (C). A. phagocytophilum inclusions were visualized by DAPI staining (blue). The experiment shown is representative of two independent experiments. Each Dotted line encircles a cluster of bacterial inclusions. Bar, 5 µm. Ap, A. phagocytophilum. N, nucleus.
D. A. phagocytophilum-infected RF/6A cells at day 2 pi were stained with anti-VAMP4 (green), anti-GM-130 (red), and DAPI (blue), analyzed by DeltaVision deconvolution microscopy. Note highly dispersed and hardly visible GM-130 signal. The experiment shown is representative of two independent experiments. Dotted lines encircle bacterial inclusions. Bar, 5 µm. Ap, A. phagocytophilum. N, nucleus.
E. RF/6A cells were transfected with a control siRNA or a siRNA specific for VAMP4. One day after transfection, A. phagocytophilum was added to the cells and incubated for an additional 2 days. The cells were lysed and subjected to western blotting using antibodies against VAMP4 and A. phagocytophilum P44. Actin was used as the protein input control to normalize each sample. The amounts of VAMP4 and P44 (relative to actin) were determined by densitometry. The values under the bands show the relative ratios of band intensities, with the ratios of those from samples transfected with control siRNA arbitrarily set as 1. Results are representative of three independent experiments. Ap, A. phagocytophilum.
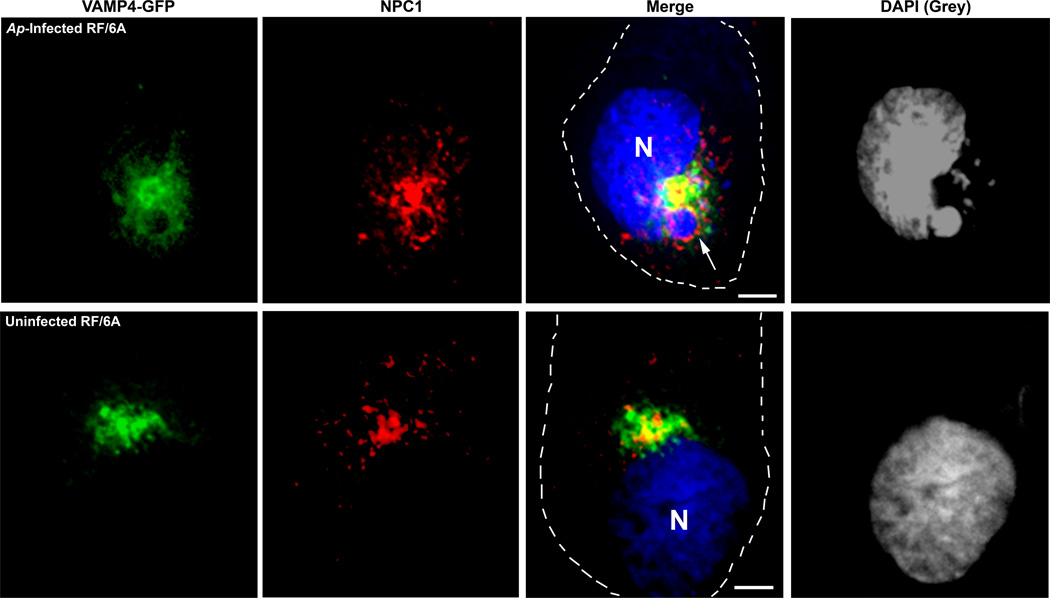
Since both NPC1 and VAMP4 are recruited and co-localized to A. phagocytophilum inclusions, we further examined the localization of NPC1 and VAMP4 in VAMP4-GFP transfected RF/6A cells with a combination of NPC1 antibody and DAPI staining. In uninfected cells, VAMP4-GFP showed intense signals at perinuclar Golgi region and NPC1 was partially colocalized with VAMP4 (Fig. 7) which is in agreement with co-localization study by Higgins et al. using NPC1 antibody and Rab 11 antibody as a TGN marker (Higgins et al., 1999) as well as organelle fractionation study by Urano et al. using NPC1 antibody and syntaxin 6 antibody as a TGN marker (Urano et al., 2008). In A. phagocytophilum-infected RF/6A cells, it was found that NPC1 and VAMP4 colocalize to the same Anaplasma inclusion. However, VAMP4-GFP did not completely overlap with NPC1 around Anaplasma inclusion (Fig. 7).
Fig. 7. VAMP4 and NPC1 are co-localized to A. phagocytophilum inclusions.
RF/6A cells were transfected with VAMP4-GFP plasmid and inoculated with host cell free A. phagocytophilum at 4h post transfection. At day 2 pi, the cells were fixed and stained with anti-NPC1 antibody and DAPI. VAMP4-GFP (green), NPC1 (red) and DAPI (blue) were visualized by DeltaVision deconvolution microscopy. A. phagocytophilum inclusions were visualized by DAPI staining (blue); the blue/DAPI staining was transformed to gray pseudocolor for clearer viewing of bacterial inclusions. The experiment shown is representative of three independent experiments. Bar, 5 µm. Ap, A. phagocytophilum. N, nucleus.
Discussion
Herein, we reported that NPC1 vesicles are recruited to A. phagocytophilum inclusions and that the recruitment is induced by and required for the infection of host cells by this cholesterol-dependent bacterium. To our knowledge, this is the first example of NPC1 vesicle trafficking and NPC1 localization on a pathogen-associated membrane. Interestingly, a recent study demonstrated that intracellular cholesterol trafficking pathways mediated by NPC1 are needed for efficient HIV-1 production during virus assembly and budding (Tang et al., 2009). Brucella abortus infection in mice requires NPC1, although how NPC1 is involved in its infection is unknown (Watarai et al., 2002).
A. phagocytophilum hijacks NPC1 vesicles containing free cholesterol. What is important from our study is that this is not simple capture of preexisting NPC1 vesicles by the bacterium, but hijacking a subpopulation of NPC1 vesicles which are highly expanded by the infection. This population of NPC1 vesicles are similar to the larger, LAMP-1-negative, cholesterol-rich and caveolin-1-associated vesicles described by Garver et al. (Garver et al., 2000). The result is in agreement with our previous data that A. phagocytophilum enters host cell through cholesterol-rich lipid rafts, and caveolin-1 is colocalized to both early and replicative A. phagocytophilum inclusions (Lin et al., 2003b). This mechanism prevents NPC1 vesicles from delivering lysosomal digestive enzymes to inclusions during A. phagocytophilum cholesterol capture. Conservation of normal NPC1 vesicle population in infected cells is expected to reduce negative impacts of intracellular cholesterol robbery by Anaplasma on host cells which must be kept alive for obligatory intracellular infection.
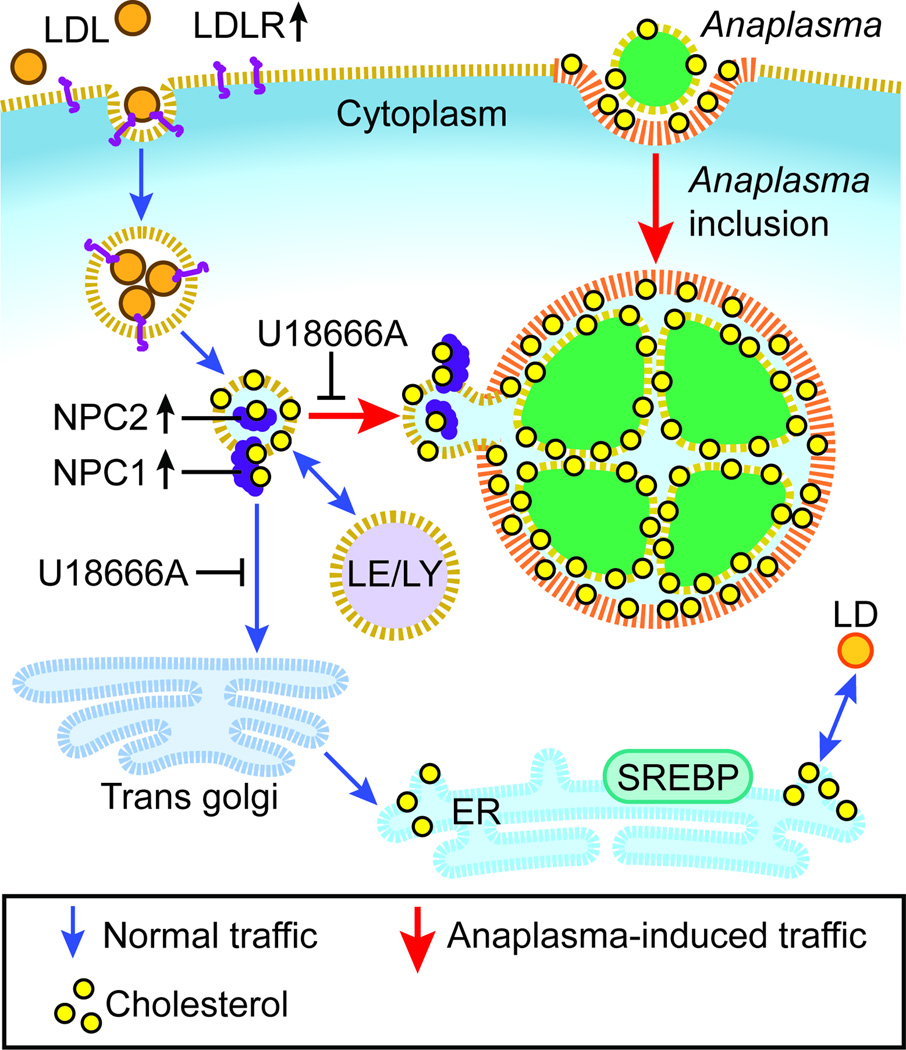
LDLR is significantly upregulated by A. phagocytophilum infection, which leads to enhanced uptake of LDL and increased cellular cholesterol (Xiong, et al., 2009). Despite the increase in intracellular cholesterol in A. phagocytophilum-infected cells, ER-residing sterol regulatory element–binding proteins, the key transcription factors that regulate the expression of genes encoding cholesterol uptake and synthesis (Goldstein et al., 2006) are not activated (Xiong et al., 2009), suggesting that A. phagocytophilum snatches LDL-CHOL before it reaches the ER. Based on our previous and current data, our proposed model of intracellular transport of LDL-CHOL to Anaplasma inclusions is depicted in Fig. 8. Our current data suggest the new NPC1 vesicle traffic is formed toward A. phagocytophilum (Fig. 8). Higgins et al. originally proposed dynamic and transient interaction of NPC1 vesicles with lysosomes and the TGN to assist in cholesterol exit from lysosomes based on immunofluorescence labeling of fixed cells (Higgins et al., 1999). More recently, Urano et al. (Urano et al., 2008) showed that a substantial portion of LDL-CHOL is transported to the TGN from NPC1 compartments before its arrival at the ER by vesicular trafficking by using 3H-LDL-CHOL. There has been only a single report on live image analysis of NPC1-containing vesicles, which showed the interaction between NPC1 vesicles and the ER (Ko et al., 2001). In the current live-cell imaging study, we found not only vigorous interaction of NPC1-containing vesicles with A. phagocytophilum inclusions, but also NPC1 localizes on A. phagocytophilum inclusion membrane, suggesting the NPC1 vesicle fusion.
Fig. 8. Proposed model of intracellular transport of LDL-CHOL to Anaplasma inclusions.
A. phagocytophilum hijacks free cholesterol from NPC1 vesicles on the way to Trans-Golgi network. Blue arrows indicate vesicular and cholesterol traffic pathways in uninfected cells. Red arrows indicate vesicular and cholesterol traffic pathways induced by Anaplasma infection. Yellow dot, non-esterified cholesterol; orange ball (esterified cholesterol) in LDL (low-density lipoprotein) and LD (lipid droplet); LDLR, low-density lipoprotein receptor; EE, early endosome; LE, late endosome; LY, lysosome; ER, endoplasmic reticulum; SREBP, sterol regulatory element-binding protein.
Urano et al. demonstrated the involvement of TGN SNAREs (VAMP4, syntaxin 6, and syntaxin 16)-mediated vesicular trafficking in transporting LDL-CHOL from the NPC1-containing compartment to the TGN; however, because single VAMP4, syntaxin 6, or syntaxin 16 knockdown only partially reduces LDL-CHOL transport from NPC1 compartment to the ER, they also suggested unknown alternative pathways or mechanisms (Urano et al., 2008). In our study a nearly 90% knockdown of VAMP4 resulted in only a 42% decrease in bacterial infection and we could not see significant decrease in cholesterol in bacteria purified from VAMP4-knocked down cells (data not shown) perhaps due to low levels of inhibition of LDL-CHOL transport. VAMP4 is localized on A. phagocytophilum inclusions and crucial for bacteria infection; however, currently we do not have direct evidence that VAMP4 is required for bacterial cholesterol acquisition. Also, we could not deny the possibility of other important functions of the VAMP4 recruitment by A. phagocytophilum, such as membrane biogenesis and nutrient acquisition. Further experiments are ongoing to address these questions.
VAMP4 is enriched in the TGN, and Tran et al. elucidated that VAMP4 cycles from the cell surface to the TGN via sorting and recycling endosomes (Tran et al., 2007). It is possible that A. phagocytophilum inclusions recruit VAMP4 from the TGN. However, GTPases Rab10 and Rab14, which are associated with recycling endosomes, both localize to inclusions, and this co-localization is not changed by brefeldin A treatment, even though brefeldin A treatment of cells causes Golgi fragmentation; this suggests that an intact Golgi is not required for A. phagocytophilum infection and recruitment of Rabs (Huang et al., 2010a). Together with our previous data obtained from brefeldin A-treated cells and NBD-ceramide labeling (Mott et al., 1999), we propose an alternative hypothesis: A. phagocytophilum recruits VAMP4 by hijacking VAMP4-containing vesicles from recycling endosomes. Interaction of v-SNARE VAMP4 and the TGN-localized putative t-SNAREs syntaxin 6 and syntaxin 16, was reported (Mallard et al., 2002). We also found that syntaxin 16 is associated with A. phagocytophilum inclusions.
In this study, for the first time, we clearly observed the Golgi fragmentation or dispersal in A. phagocytophilum highly infected cells. It is currently unknown whether this Golgi fragmentation benefits A. phagocytophilum growth or infection by providing protein and lipid sources for A. phagocytophilum or its inclusions in the cell. Interestingly, C. trachomatis also induces Golgi fragmentation to generate Golgi ministacks surrounding the bacterial inclusion, which is required for infection and this may be one of lipid acquisition mechanisms to ensure bacteria intracellular growth (Heuer et al., 2009). A. phagocytophilum induces Golgi fragmentation, but unlike C. trachomatis, Golgi is dispersed throughout the cytoplasm. Taken together with differences in NPC1 vesicle traffic between Anaplasma and Chlamydia, our data support the notion of distinct cholesterol acquisition mechanisms by these two obligatory intracellular bacteria.
A. phagocytophilum actively modifies its host cell-derived inclusion membrane by translocating its own proteins to that membrane or to the host cell cytoplasm for subsequent targeting host cell organelles. Besides the known Type IV secretion substrates such as AnkA and Ats-1 secreted into the host cell cytoplasm (Rikihisa et al., 2010), some A. phagocytophilum proteins targeted to the inclusion membrane, such as APH_0032 (Huang et al., 2010b), APH_1387 (Huang et al., 2010c), and AptA (Sukumaran et al., 2011), have been identified. AptA is involved in the activation of ERK1/2 during A. phagocytophilum infection (Sukumaran et al., 2011), but the functions of APH_0032 and APH_1387 have not been characterized. Whether these known or other bacterial proteins are involved in recruiting NPC1 or TGN SNAREs to inclusions awaits further investigation. During exponential growth, A. phagocytophilum inclusions resemble early autophagosomes (Niu et al., 2008), and they selectively recruit a set of Rabs primarily associated with recycling endosomes (Huang et al., 2010a). The present study support the concept that the biogenesis of A. phagocytophilum inclusions requires the recruitment of membrane vesicles from multiple intracellular compartments (Rikihisa, 2011).
The present study identified the critical site and proteins for diversion of the host LDL-CHOL vesicular traffic for A. phagocytophilum infection. Identified NPC1 and VAMP4 pathways provide new targets for a future investigation toward development of intervention strategies against HGA. In addition, the knowledge obtained through our investigation on cholesterol-robbing bacteria contributes fundamentally to advances of the field of intracellular cholesterol regulation.
Experimental procedures
Chemicals, antibodies, plasmid constructs, and cell lines
Oxytetracycline, imipramine, U18666A and OptiPrep (Axis-Shield) were obtained from Sigma (St. Louis, MO). 4′,6-diamidino-2-phenylindole, dilactate (DAPI) was from Molecular Probes (Eugene, OR). Horse anti-A. phagocytophilum antiserum (EQ005 at day 31 post inoculation) was previously described (Wang et al., 2004). The mouse mAb 5C11 recognizing the N-terminal conserved region of P44 of A. phagocytophilum has been described (Kim et al., 1998). The following primary and second antibodies were used: rabbit anti-NPC1 (Novus Biologicals, Littleton, CO), rabbit anti-NPC2 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse mAb anti-GM-130 (BD, Sparks, MD), rabbit anti-VAMP4 and rabbit anti-Syntaxin 16 sera (Synaptic Systems, Goettingen, Germany), mouse mAb anti-LAMP-1 (H4B4) and LAMP-2 (H4A3) (Iowa hydridoma bank, Iowa City, IA), mouse mAb anti-EEA1 (BD), mouse mAb anti-α-tubulin (Santa Cruz), rabbit anti-actin (Sigma). Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 555-conjugated goat anti-mouse IgG (Molecular Probes), DyLight 488 AffiniPure goat anti-horse IgG and DyLight 549 AffiniPure goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), and Peroxidase-conjugated secondary antibodies (KPL, Gaithersburg, MD). Normal mouse IgG was purchased from Santa Cruz. NPC1, VAMP4, and negative control siRNAs were purchased from Applied Biosystems/Ambion (Austin, TX). mCherry-A. phagocytophilum was provided by Dr. Ulrike Munderloh at University of Minnesota. The human isolate Chlamydia trachomatis was a gift from Dr. Preeti Pancholi at The Ohio State University Medical Center. The following plasmids were generous gifts from various sources: NPC1-YFP (Dr. Matthew P. Scott at Stanford University); VAMP4-GFP (Dr. Wanjin Hong at Institute of Molecular and Cell Biology, Singapore); syntaxin 6-GFP (Dr. Jeffrey Pessin at Albert Einstein College of Medicine). MDR and drug-sensitive HL-60 cell lines were provided by Dr. Jeffrey Krise at University of Kansas, KS.
Cultivation of organisms and host cells
A. phagocytophilum HZ strain and mCherry-A. phagocytophilum were cultivated in HL-60 cells as previously described (Rikihisa et al., 1997) and in RF/6A in advanced MEM with 10% FBS. Host cell-free A. phagocytophilum was prepared and added to the cells as previously described (Xiong et al., 2009). The degree of bacterial infection in host cells and the number of A. phagocytophilum organisms was estimated as previously described (Park et al., 1992).
Chlamydia trachomatis was cultivated in L929 or RF/6A in DMEM with 10% FBS, containing 2 µg/ml cycloheximide. Cells were inoculated with C. trachomatis isolated from highly infected L929 cells by centrifugation at 500 × g for 30 min at room temperature.
Subcellular fractionation by Optiprep gradient
The method described by Urano et al. (Urano et al., 2008) was used with some modification. Briefly, 2×107 A. phagocytophilum-infected and uninfected HL-60 cells were harvested at day 2 pi, washed twice with PBS, once with homogenization buffer (HB, 250 mM sucrose, 20 mM Tris-HCl, pH 7.4, 1 mM EDTA), and resuspended in 1 ml of HB with protease inhibitor cocktail III (Calbiochem, San Diego, CA). The cell suspension was homogenized by using a tight-fitted (label as “B”) Dounce homogenizer until about 90% of cells have been broken as determined by the phase contrast microscopy or by Diff-Quik staining of cytocentrifuged specimens. The homogenate was centrifuged at 500 × g for 5 min to precipitate the nucleus and unbroken cells to obtain the post nuclear supernatant. The supernatant was placed onto the top of an 11-ml linear 5–25% OptiPrep gradient in HB. The gradient was centrifuged at 200,000 × g for 3 h in a Beckman SW41 rotor; 12 fractions (1 ml each) were collected from the top. Equal aliquots from each fraction were analyzed by immunoblotting using antibodies against various organelle markers as indicated.
Immunofluorescence microscopy
Cells were fixed in methanol: acetone (80:20) at −20°C for 20 min and blocked in blocking buffer (PBS containing 2% bovine serum albumin, 0.2% gelatin) for 30 min at room temperature. Then the cells were incubated with primary antibodies and horse anti-A. phagocytophilum antiserum or mouse mAb 5C11 in blocking buffer for 60 min at 37°C followed by incubation with fluorescence-conjugated secondary antibodies (1:300 dilution) for 1 h. Normal mouse or rabbit antibodies were used as negative controls. Alternatively, the cells were fixed in 4% paraformaldehyde at room temperature for 15 min and blocked in PGS buffer (PBS containing 0.1% gelatin and 0.3% saponin). For triple fluorescence labeling, the cells were further counterstained for the nucleus of host cells and A. phagocytophilum with DAPI for 5 min. Cells were then washed and observed under a Nikon Eclipse E400 fluorescence microscope with a xenon-mercury light source (Nikon Instruments, Melville, NY). The confocal images were captured by Olympus Flowview FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan). The deconvolution images were obtained using DeltaVision microscopy system (Applied Precision, Issaquah, WA) and the data were processed using SoftWoRX software (Applied Precision) and Adobe Photoshop software CS2 (Adobe Systems, Mountain View, CA).
Western blot analysis
Samples were separated by SDS-PAGE with 6 – 15% polyacrylamide resolving gels, transferred to a nitrocellulose membrane or a PVDF membrane, and then probed with primary antibodies and subsequently with peroxidase-conjugated secondary antibodies as previously described (Xiong et al., 2009). Immunoreactive bands were visualized with enhanced chemiluminescence using LAS3000 image documentation system (FUJIFILM Medical Systems USA, Stamford, CT) and band intensities were determined by densitometry using Multi Gauge software (FUJIFILM).
Quantitative RT-PCR and PCR
A. phagocytophilum-infected and control HL-60 cells were harvested, and RNA was isolated and reverse transcribed as previously described (Xiong et al., 2009). Quantitative PCR (total volume 20 µl) was set up with 1 µl (corresponding 0.2–0.4 µg of total RNA) of cDNA, 0.25 µM of each primer, and Stratagene SYBR Green PCR kit reagents (Stratagene, La Jolla, CA) and performed in the instrument Mx3000P (Stratagene). NPC1, NPC2, STARD5, STARD3/MLN64 and G3PDH primer sequences are available upon request. To measure the bacterial load in HL-60 cells, DNA was extracted from infected cells using a QIAamp blood kit (QIAGEN), and the bacterial infection was assessed by quantitative real-time PCR using A. phagocytophilum 16S rDNA primers, and host cell G3PDH primers to normalize host cell host DNA input.
siRNA and plasmid transfection
RF/6A cells were transfected with siRNAs and plasmids using lipofectamine 2000 transfection regent (Invitrogen, Carlsbad, CA) and HD FuGene (Roche, Indianapolis, IN), respectively. Host cell-free A. phagocytophilum was added to cells 8–24 h after transfection, and incubated for additional 1–2 days. Cells were harvested and subjected to Western blotting using antibodies against NPC1 or VAMP4 and A. phagocytophilum P44 outer membrane protein (mAb 5C11) (Kim et al., 1998).
Cholesterol Assay of Purified Bacteria and host cells
Host cell–free bacteria were purified from NPC1 siRNA-transfected and control siRNA-transfected RF/6A cells in four wells each of 6-well plates, and cholesterol levels of purified bacteria were measured with an Amplex Red cholesterol assay kit (Molecular Probes) as described (Lin et al., 2003a), but without further Percoll density gradient centrifugation. DNA was extracted from equal portions of transfected cells using a QIAamp blood kit (QIAGEN), and the bacterial infection was assessed by real-time PCR as described above using A. phagocytophilum 16S rDNA primers. Total cholesterol content was normalized to the amount of bacterial 16S rDNA. Total cellular cholesterol levels in host cells were measured and normalized by total protein levels as previously described (Xiong et al., 2009).
Live-Cell Imaging and Analysis
RF/6A cells were grown in chambered glass bottom dish (WillCo Wells, The Netherlands) and were transfected by plasmids followed by inoculation with host cell-free mCherry-A. phagocytophilum purified from HL-60 cells. Before imaging, the medium was changed to phenol red–free advanced MEM. Cells were imaged using DeltaVision deconvolution microscopy. Temperature was maintained at 37°C. Time-lapse imaging for NPC1-YFP was performed using an approximately 15s interval for less than10 min. Fluorescence imaging does not affect the movement or localization of NPC1-YFP vesicles within 10 min according to Ko et al. (Ko et al., 2001). NPC1-YFP signals were adjusted into green color with SoftWoRX software (Applied Precision). Stacks of time-lapse images were analyzed using Image J software (NIH, Bethesda, MD). The movies were processed using Image J software. Movement of NPC1 vesicles was tracked with the manual tracking plug-in (NIH).
Statistical Analysis
Statistical analyses were performed by paired, 2-tailed Student’s t-test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank Dr. Jeffrey P. Krise from University of Kansas for providing the MDR and drug-sensitive HL-60 cell lines; Dr. Ulrike Munderloh from University of Minnesota for providing mCherry-A. phagocytophilum; Dr. Preeti Pancholi from the Ohio State University for providing C. trachomatis; Dr. Matthew P. Scott from Stanford University for the NPC1-YFP plasmid; Dr. Wan-jing Hong from Institute of Molecular and Cell Biology, Singapore for the VAMP4-GFP plasmid; and Dr. Jeffrey Pessin from Albert Einstein College of Medicine for the syntaxin 6-GFP plasmid. We also acknowledge Dr. Ta-Yuan Chang from Dartmouth Medical School for his helpful advice and critical reading of the manuscript; Mr. Tim Vojt for assistance in preparing Fig. 8; and Dr. Mingqun Lin for assistance in using confocal microscopy. This project was supported by National Institutes of Health grant R01 AI030010.
Footnotes
Supporting Information
Supplemental information includes 4 figures and 10 videos.
References
- Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- Bakken JS, Dumler S. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2008;22:433–448. doi: 10.1016/j.idc.2008.03.011. viii. [DOI] [PubMed] [Google Scholar]
- Carabeo RA, Mead DJ, Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci U S A. 2003;100:6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenedella RJ. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009;44:477–487. doi: 10.1007/s11745-009-3305-7. [DOI] [PubMed] [Google Scholar]
- Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikh K, Vey S, Simonot C, Vanier MT, Millat G. Niemann-Pick type C disease: importance of N-glycosylation sites for function and cellular location of the NPC2 protein. Mol Genet Metab. 2004;83:220–230. doi: 10.1016/j.ymgme.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A. 2008;105:9379–9384. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science. 2000;290:2295–2298. doi: 10.1126/science.290.5500.2295. [DOI] [PubMed] [Google Scholar]
- Delevoye C, Nilges M, Dehoux P, Paumet F, Perrinet S, Dautry-Varsat A, Subtil A. SNARE protein mimicry by an intracellular bacterium. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000022. e1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver WS, Heidenreich RA, Erickson RP, Thomas MA, Wilson JM. Localization of the murine Niemann-Pick C1 protein to two distinct intracellular compartments. J Lipid Res. 2000;41:673–687. [PubMed] [Google Scholar]
- Gokce HI, Ross G, Woldehiwet Z. Inhibition of phagosome-lysosome fusion in ovine polymorphonuclear leucocytes by Ehrlichia (Cytoecetes) phagocytophila. J Comp Pathol. 1999;120:369–381. doi: 10.1053/jcpa.1998.0287. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Gong Y, Duvvuri M, Duncan MB, Liu J, Krise JP. Niemann-Pick C1 protein facilitates the efflux of the anticancer drug daunorubicin from cells according to a novel vesicle-mediated pathway. J Pharmacol Exp Ther. 2006;316:242–247. doi: 10.1124/jpet.105.089482. [DOI] [PubMed] [Google Scholar]
- Goodman JL, Nelson C, Vitale B, Madigan JE, Dumler JS, Kurtti TJ, Munderloh UG. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- Heuer D, Rejman Lipinski A, Machuy N, Karlas A, Wehrens A, Siedler F, et al. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- Higgins ME, Davies JP, Chen FW, Ioannou YA. Niemann-Pick C1 is a late endosome-resident protein that transiently associates with lysosomes and the trans-Golgi network. Mol Genet Metab. 1999;68:1–13. doi: 10.1006/mgme.1999.2882. [DOI] [PubMed] [Google Scholar]
- Huang B, Hubber A, McDonough JA, Roy CR, Scidmore MA, Carlyon JA. The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes. Cell Microbiol. 2010a;12:1292–1307. doi: 10.1111/j.1462-5822.2010.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Troese MJ, Howe D, Ye S, Sims JT, Heinzen RA, et al. Anaplasma phagocytophilum APH_0032 is expressed late during infection and localizes to the pathogen-occupied vacuolar membrane. Microb Pathog. 2010b;49:273–284. doi: 10.1016/j.micpath.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Troese MJ, Ye S, Sims JT, Galloway NL, Borjesson DL, Carlyon JA. Anaplasma phagocytophilum APH_1387 is expressed throughout bacterial intracellular development and localizes to the pathogen-occupied vacuolar membrane. Infect Immun. 2010c;78:1864–1873. doi: 10.1128/IAI.01418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- Karten B, Peake KB, Vance JE. Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim Biophys Acta. 2009;1791:659–670. doi: 10.1016/j.bbalip.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Kim HY, Rikihisa Y. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J Clin Microbiol. 1998;36:3278–3284. doi: 10.1128/jcm.36.11.3278-3284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko DC, Gordon MD, Jin JY, Scott MP. Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events. Mol Biol Cell. 2001;12:601–614. doi: 10.1091/mbc.12.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003a;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Rikihisa Y. Obligatory intracellular parasitism by Ehrlichia chaffeensis and Anaplasma phagocytophilum involves caveolae and glycosylphosphatidylinositol-anchored proteins. Cell Microbiol. 2003b;5:809–820. doi: 10.1046/j.1462-5822.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–664. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JG, Karpova T, Cooper J, Conchello JA. Three-dimensional imaging by deconvolution microscopy. Methods. 1999;19:373–385. doi: 10.1006/meth.1999.0873. [DOI] [PubMed] [Google Scholar]
- Mott J, Barnewall RE, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munderloh UG, Lynch MJ, Herron MJ, Palmer AT, Kurtti TJ, Nelson RD, Goodman JL. Infection of endothelial cells with Anaplasma marginale and A. phagocytophilum. Vet Microbiol. 2004;101:53–64. doi: 10.1016/j.vetmic.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Neufeld EB, Wastney M, Patel S, Suresh S, Cooney AM, Dwyer NK, et al. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J Biol Chem. 1999;274:9627–9635. doi: 10.1074/jbc.274.14.9627. [DOI] [PubMed] [Google Scholar]
- Niu H, Yamaguchi M, Rikihisa Y. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cell Microbiol. 2008;10:593–605. doi: 10.1111/j.1462-5822.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Park J, Rikihisa Y. L-arginine-dependent killing of intracellular Ehrlichia risticii by macrophages treated with gamma interferon. Infect Immun. 1992;60:3504–3508. doi: 10.1128/iai.60.9.3504-3508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y. Mechanisms of Obligatory Intracellular Infection with Anaplasma phagocytophilum. Clin Microbiol Rev. 2011;24:469–489. doi: 10.1128/CMR.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Lin M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol. 2010;13:59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Zhi N, Wormser GP, Wen B, Horowitz HW, Hechemy KE. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York state. J Infect Dis. 1997;175:210–213. doi: 10.1093/infdis/175.1.210. [DOI] [PubMed] [Google Scholar]
- Schneede A, Schmidt CK, Holtta-Vuori M, Heeren J, Willenborg M, Blanz J, et al. Role for LAMP-2 in endosomal cholesterol transport. J Cell Mol Med. 2011;15:280–295. doi: 10.1111/j.1582-4934.2009.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran B, Mastronunzio JE, Narasimhan S, Fankhauser S, Uchil PD, Levy R, et al. Anaplasma phagocytophilum AptA modulates Erk1/2 signalling. Cell Microbiol. 2011;13:47–61. doi: 10.1111/j.1462-5822.2010.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Leao IC, Coleman EM, Broughton RS, Hildreth JE. Deficiency of niemann-pick type C-1 protein impairs release of human immunodeficiency virus type 1 and results in Gag accumulation in late endosomal/lysosomal compartments. J Virol. 2009;83:7982–7995. doi: 10.1128/JVI.00259-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Zeng Q, Hong W. VAMP4 cycles from the cell surface to the trans-Golgi network via sorting and recycling endosomes. J Cell Sci. 2007;120:1028–1041. doi: 10.1242/jcs.03387. [DOI] [PubMed] [Google Scholar]
- Urano Y, Watanabe H, Murphy SR, Shibuya Y, Geng Y, Peden AA, et al. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proc Natl Acad Sci U S A. 2008;105:16513–16518. doi: 10.1073/pnas.0807450105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rikihisa Y, Lai TH, Kumagai Y, Zhi N, Reed SM. Rapid sequential changeover of expressed p44 genes during the acute phase of Anaplasma phagocytophilum infection in horses. Infect Immun. 2004;72:6852–6859. doi: 10.1128/IAI.72.12.6852-6859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai M, Makino S, Michikawa M, Yanagisawa K, Murakami S, Shirahata T. Macrophage plasma membrane cholesterol contributes to Brucella abortus infection of mice. Infect Immun. 2002;70:4818–4825. doi: 10.1128/IAI.70.9.4818-4825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari H, Blanchette-Mackie EJ, Dwyer NK, Watari M, Neufeld EB, Patel S, et al. Mutations in the leucine zipper motif and sterol-sensing domain inactivate the Niemann-Pick C1 glycoprotein. J Biol Chem. 1999;274:21861–21866. doi: 10.1074/jbc.274.31.21861. [DOI] [PubMed] [Google Scholar]
- Webster P, JW IJ, Chicoine LM, Fikrig E. The agent of Human Granulocytic Ehrlichiosis resides in an endosomal compartment. J Clin Invest. 1998;101:1932–1941. doi: 10.1172/JCI1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Lin M, Rikihisa Y. Cholesterol-dependent Anaplasma phagocytophilum exploits the low-density lipoprotein uptake pathway. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000329. e1000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Wang X, Rikihisa Y. High-cholesterol diet facilitates Anaplasma phagocytophilum infection and up-regulates macrophage inflammatory protein-2 and CXCR2 expression in apolipoprotein E-deficient mice. J Infect Dis. 2007;195:1497–1503. doi: 10.1086/514819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.