Abstract
OBJECTIVE
The purpose of this study was to compare the transcriptome between the site of membrane rupture and the chorioamniotic membranes away from the site of rupture.
STUDY DESIGN
The transcriptome of amnion and chorion (n =20 each) from and distal to the site of rupture from women with spontaneous labor and vaginal delivery at term after spontaneous rupture of membranes was profiled with Illumina HumanHT-12 microarrays. Selected genes were validated with the use of quantitative reverse transcription–polymerase chain reaction.
RESULTS
Six hundred seventy-seven genes were differentially expressed in the chorion between the rupture and nonrupture sites (false discovery rate <0.1; fold change >1.5). Quantitative reverse transcription–polymerase chain reaction confirmed the differential expression in 10 of 14 genes. Enriched biological processes included anatomic structure development, cell adhesion and signal transduction. Extracellular matrix–receptor interaction was the most impacted signaling pathway.
CONCLUSION
The transcriptome of fetal membranes after spontaneous rupture of membranes in term labor is characterized by region-and tissue-specific differential expression of genes that are involved in signature pathways, which include extracellular matrix–receptor interactions.
Keywords: chorion, extracellular matrix, inflammation, labor, microarray, parturition, spontaneous rupture of membranes
The common pathway of human parturition is characterized by anatomic, immunologic, and physiologic changes that occur in the mother and/or fetus in both term and preterm parturition.1 In normal pregnancy, the chorioamniotic membranes have important anatomic,2 biochemical,3–5 and antibacterial6,7 properties that serve to protect the fetus and pregnancy. The membranes (chorion and amnion) are intact during gestation and rupture without intervention close to the beginning of the second stage of labor.8
The fetal membranes undergo complex physical and biochemical changes before the onset of labor at term.8–11 In patients at term without labor, the portion of the fetal membranes overlying the cervix contains characteristic histologic and biochemical changes and is thought to be the site at which eventual spontaneous rupture of membranes occurs.2,5,10,12,13 This area has been termed the “zone of altered morphology” (ZAM).2,10 Defined by histologic criteria, the membranes in the ZAM are thinner than in the membranes away from the ZAM. The features of the ZAM include an increase in the thickness of connective tissue of amnion and chorion and decreased thickness of the cytotro-phoblast and decidual layers.10,12 Moreover, El Khwad et al13 demonstrated that the ZAM is a weak zone by using biophysical testing. Several mechanisms have been proposed to contribute to the weakening of the chorioamniotic membranes in the ZAM; these mechanisms include spontaneous separation of fetal membrane layers,14 the exposure of the membranes to cytokines and matrix-degrading enzymes that are present in the vagina15–17 and in amniotic fluid18–25 that are known to increase in normal pregnancy at term and in labor.
The use of high-dimensional biology technology has led to the identification of novel genes, proteins, and pathways that are involved in both term and pre-term labor.26–28 Specifically, transcriptomics (the description of the entire set of genes that are transcribed by a particular tissue at a specific point in time) has been used to examine the differences in global gene expression profiles in intact fetal membranes of women at term with and without labor,29 women in term and preterm labor,30 and in pregnancies that are complicated by preterm labor with and without preterm prelabor rupture of membranes (PROM).31 Gene expression profiling of the chorioamniotic membranes in the context of spontaneous rupture of membranes (SROM) at the site of membrane rupture and away from the site of rupture has not been previously reported.
This study was undertaken to examine the differences in the transcriptome between the site of membrane rupture and a section of the intact chorioamniotic membranes away from the site of rupture in patients at term with spontaneous labor and to gain further insight into the biochemical changes that are associated with the phenomenon of membrane rupture.
Methods
Study group
A prospective study was designed to examine differential gene expression of the chorioamniotic membranes at the gross site of rupture and distant from the site of rupture; samples were obtained from women who were admitted with spontaneous labor at term with SROM (n = 30). The inclusion criteria were (1) gestational age of ≥37 weeks, (2) absence of medical or obstetric complications of pregnancy, (3) no antibiotic administration in labor for group beta streptococcus (GBS) prophylaxis, (4) absence of meconium staining of the amniotic fluid, (5) no prostaglandin administration, (6) no use of amnioinfusion, (7) vaginal birth, and (8) normal pregnancy outcome, including an infant who was of appropriate weight for gestational age without congenital anomalies and Apgar scores >7 at 1 and 5 minutes. All patients provided written informed consent for the collection and use of samples for research purposes under the protocols approved by the institutional review boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Clinical definitions
Labor was defined as the presence of regular uterine contractions accompanied by progressive cervical dilation that led to delivery. SROM was defined as rupture of membranes in the presence of labor and confirmed with sterile speculum examination and a combination of pooling, ferning, and Nitrazine tests. An appropriate-for-gestational-age neonate was defined as a birthweight between the 10th and 90th percentile for the gestational age at birth.32 Histologic chorio-amnionitis was diagnosed on the basis of the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes.33,34 Acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton’s jelly.
Collection of the fetal membranes
Fetal membranes were processed within 30 minutes of delivery. After the identification of the site of rupture, a linear piece of membrane that measured 1 ×4 cm and spanned the gross rupture site was dissected sharply (Figure 1). Similarly, a 1 ×4 cm strip of reflected extraplacental membranes was removed at a site distal to the site of rupture that was approximately one-half the distance between the site of rupture and the placental disk.35,36 The amnion was separated from the chorion, and each tissue was flash-frozen with liquid nitrogen. This method yielded a set of 4 specimens from each patient: (1) amnion from the rupture site, (2) chorion from the rupture site, (3) amnion from the nonrupture site, and (4) chorion from the non-rupture site. In addition, a section of membranes from each patient was obtained for histologic examination with the use of hematoxylineosin–stained histologic sections. The cases with chorioamnionitis, funisitis, meconium-staining, or severe amnion degeneration were excluded from the study.33,34 The sets of specimens were collected prospectively until 30 total sets of fetal membranes were obtained that met all inclusion and histologic criteria. The RNA was extracted and stored at −70°C until it was assayed. The materials were housed at the bank of biologic specimens that was held at the Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
FIGURE 1. Sampling sites of the fetal membranes.
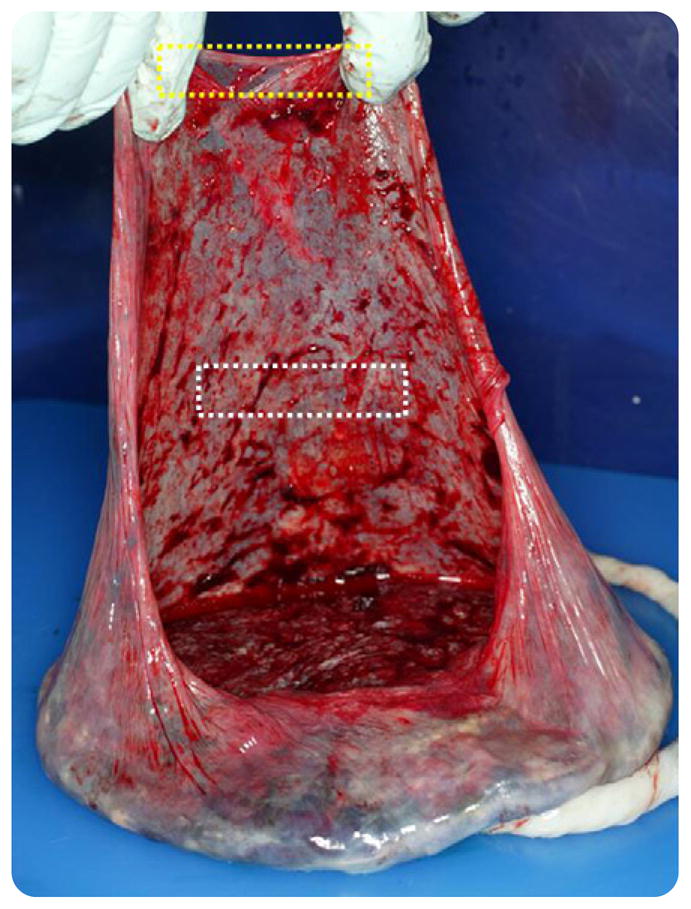
The image of the placenta demonstrates the representative sampling sites of the gross site of rupture (yellow box) and the site away from rupture (white box).
Microarray analysis and real-time quantitative polymerase chain reaction
For microarray analysis, complete sets of chorioamniotic membranes were used (n =20 matched sets of amnion from the rupture site, chorion from the rupture site, amnion from the nonrupture site, and chorion from the nonrupture site from each patient). The Illumina HumanHT-12 expression microarray (version 3; Illumina, San Diego, CA) platform was used to measure the gene expression levels in each specimen, per manufacturer’s instructions. To verify the results from microarray, 14 genes were selected for quantitative polymerase chain reaction (qRT-PCR) assays in a subset of the original sample set (n =10) and in a new set of samples from patients at term with SROM (n =10). The selection criteria for the new specimens were the same as those used to select patients for the microarray analysis, with the exception of 2 patients with mild intermittent asthma without asthma exacerbations or exposure to steroids during the index pregnancy. A detailed description of the method and analysis is available as supplementary material (Supplement 1).
Statistical analyses
The raw gene expression data were normalized by a quantile normalization approach.37 A paired moderated t test was used to test for differential expression with a false discovery rate (FDR)38 threshold of 0.1 in conjunction with a threshold of 1.5 on the fold change to assign gene significance.39 Gene ontology analysis was performed with algorithms that were described previously.40 Pathway analysis was performed on the Kyoto Encyclopedia of Genes and Genomes (KEGG)41 pathway database with an overrepresentation analysis42 and the signaling pathway impact analysis.43,44 The signaling pathway impact analysis differs from the overrepresentation approaches by taking into account the gene-gene signaling interactions and the magnitude and direction of gene expression changes to determine significantly impacted pathways.
Differential expression between experimental regions from qRT-PCR data was performed with a paired t test on −ΔCt values. The Student t, Mann-Whitney U, and χ2 tests were used to identify significant differences in patient demographics between women in the microarray and qRT-PCR groups. SPSS software (version 12.0; SPSS Inc, Chi-cago, IL) was used for statistical analysis of demographic data. A probability value of<.05 was considered statistically significant.
Results
Demographics
Table 1 displays the demographic characteristics of patients who were included in the microarray and qRT-PCR analyses.
TABLE 1.
Patient demographics
| Demographic | Microarray (n =20) | Quantitative reverse transcriptase–polymerase chain reaction (n = 10) | P value |
|---|---|---|---|
| Maternal age, ya | 24.5 (20.3–27.8) | 26 (20–30) | NSb |
| Gravidity, na | 3 (2–4) | 2.5 (2–4.5) | NSc |
| Parity, na | 1 (0–2) | 1 (0–2.8) | NSc |
| Maternal race, % | NSd | ||
| African American | 80 | 70 | |
| White | 10 | 10 | |
| Other | 10 | 20 | |
| Gestational age at delivery, wka | 38.6 (38.2–40.1) | 39 (38.2–40.3) | NSb |
| Rupture of membranes-to-delivery interval, mina | 259 (104–624) | 121 (59–189) | NSc |
| Birthweight, ga | 3285 (3097–3360) | 3335 (3203–3409) | NSb |
NS, not significant.
Values expressed as medians (interquartile range);
Student t test;
Mann-Whitney U test;
χ2 test.
Results of the microarray analysis
Rupture site chorion vs nonrupture site chorion
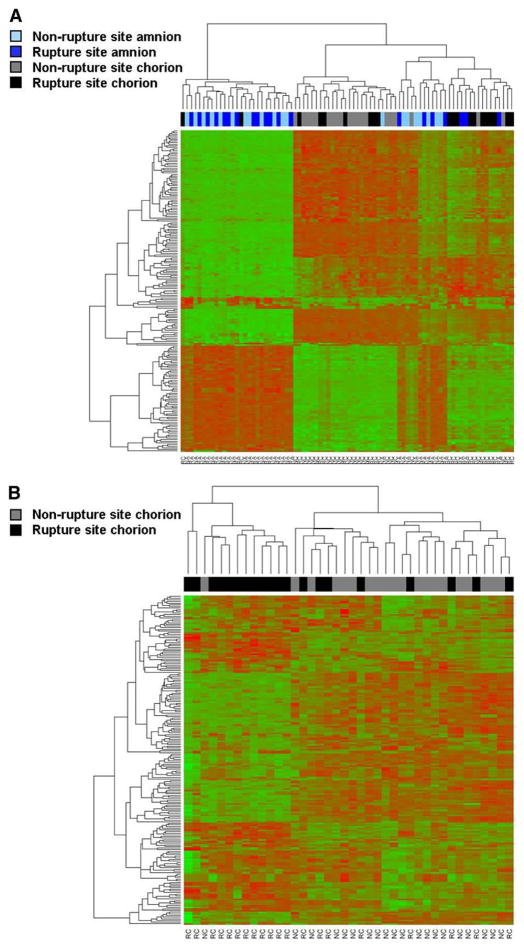
Microarray analysis demonstrated 815 probes that corresponded with 677 unique genes that were expressed differentially in the chorion between the rupture and nonrupture sites (pFDR <0.1; fold change >1.5). A total of 453 genes had decreased expression, and 224 genes had increased expression in the rupture site, compared with the nonrupture site. A “volcano plot” shows the differential expression of all the annotated probes on the Illumina HumanHT-12 v3 array with the log (base 10) of the FDR-adjusted probability values (y-axis) plotted against the log (base 2) fold changes (x-axis) between the rupture site chorion and nonrupture site chorion (Figure 2). A heat map that displays the hierarchical clustering of all samples and 200 probes whose expression had the largest variance across all amnion and chorion samples (both rupture and nonrupture sites) is shown in Figure 3, A. Two main clusters are seen in the heat map that predominantly represent amnion from the rupture and non-rupture site in 1 cluster and chorion from the rupture and nonrupture site in the other cluster. A smaller cluster of mixed samples (both amnion and chorion and rupture vs nonrupture site specimens) is also seen in the heat map and represents specimens that did not fit into the 2 main clusters of specimens. Figure 3, B, shows a hierarchical clustering of the 200 probes with the highest variance across only chorion specimens (both rupture and nonrupture site). A list of the top 100 differentially expressed genes between the rupture site chorion and nonrupture site chorion is presented in Table 2; the complete list of differentially expressed probes is available as supplementary material (Supplement 2).
FIGURE 2. Transcriptomic analysis of chorion specimens.
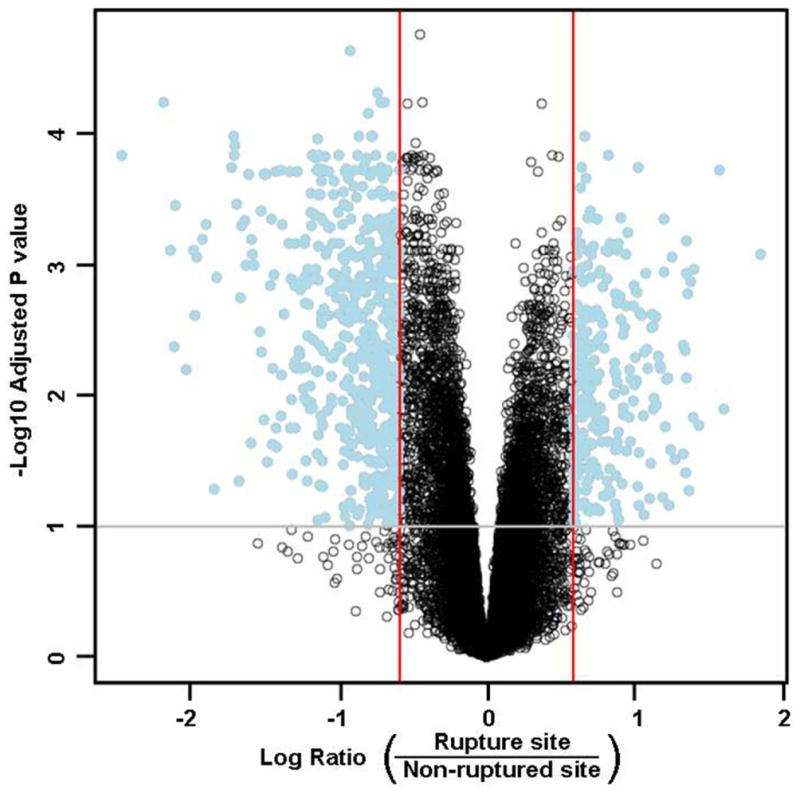
The volcano plot shows probability values of all probes in the microarray plotted against the fold changes. In this Figure, the log (base 10) of the false discovery rate–adjusted probability values are plotted against the log (base 2) ratio of fold changes between ruptured site chorion and nonruptured site chorion. On the Y-axis, values higher than the grey-line threshold represent significant probes with an adjusted probability value of < .1. On the X-axis, values outside the red lines represent fold-changes of ≥1.5 between ruptured site chorion and nonruptured site chorion. The 815 differentially expressed probes are labeled blue in the upper outer quadrants.
FIGURE 3. Heat maps of the clustering of genes.
A, A heat map displays the hierarchical clustering of the 200 probes whose expression had the largest variance across all amnion and chorion specimens. The red and green colors in the heat map correspond with high and low gene expression, respectively. The labels at the top indicate individual patient samples and their membrane region designation. Two main clusters are seen that predominantly represent amnion from the rupture site and nonrupture site on the left and chorion from the rupture site and nonrupture site in the middle. A smaller cluster of mixed samples are on the right. B, A heat map displays the hierarchical clustering of the 200 probes whose expression had the largest variance across chorion specimens. Two separate cluster regions represent the rupture site on the right and non-rupture site on the left.
TABLE 2.
List of the top 100 differentially expressed probes in chorion between the rupture site and nonruptured site
| Rank | Name | Entreza | Symbolb | Fold changec | P value |
|---|---|---|---|---|---|
| 1 | Methyltransferase like 7A | 25840 | METTL7A | −1.9 | .000023 |
| 2 | Epoxide hydrolase 1, microsomal (xenobiotic) | 2052 | EPHX1 | −1.7 | .000048 |
| 3 | Arylacetamide deacetylase-like 2 | 344752 | AADACL2 | −4.5 | .000057 |
| 4 | KIT ligand | 4254 | KITLG | −1.6 | .000057 |
| 5 | Glutathione S-transferase alpha 4 | 2941 | GSTA4 | −1.6 | .000057 |
| 6 | Killer cell lectin-like receptor subfamily C, member 1 | 3821 | KLRC1 | −1.7 | .000069 |
| 7 | Chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1) | 6387 | CXCL12 | −1.7 | .000100 |
| 8 | Basic helix-loop-helix family, member e40 | 8553 | BHLHE40 | 1.6 | .000100 |
| 9 | Aldehyde oxidase 1 | 316 | AOX1 | −3.3 | .000100 |
| 10 | Meis homeobox 1 | 4211 | MEIS1 | −1.8 | .000100 |
| 11 | Complement factor I | 3426 | CFI | −1.7 | .000100 |
| 12 | Tetraspanin 7 | 7102 | TSPAN7 | −2.2 | .000110 |
| 13 | Phosphoglucomutase 5 | 5239 | PGM5 | −3.3 | .000120 |
| 14 | G protein-coupled receptor, family C, group 5, member A | 9052 | GPRC5A | 1.8 | .000140 |
| 15 | Arylacetamide deacetylase (esterase) | 13 | AADAC | −5.5 | .000140 |
| 16 | EGF-containing fibulin-like extracellular matrix protein 1 | 2202 | EFEMP1 | −2.2 | .000140 |
| 17 | Chromosome 12 open reading frame 48 | 55010 | C12orf48 | −1.8 | .000140 |
| 18 | B-cell CLL/lymphoma 2 | 596 | BCL2 | −2.3 | .000140 |
| 19 | Regulator of G-protein signaling 5 | 8490 | RGS5 | −2.0 | .000140 |
| 20 | BTB (POZ) domain containing 3 | 22903 | BTBD3 | −2.2 | .000140 |
| 21 | Phosphoribosyl pyrophosphate synthetase 2 | 5634 | PRPS2 | −1.7 | .000140 |
| 22 | Phosphoglucomutase 5 | 5239 | PGM5 | −3.3 | .000140 |
| 23 | Primary ciliary dyskinesia protein 1 | 200373 | hCG_17324 | −1.6 | .000150 |
| 24 | Killer cell lectin-like receptor subfamily C, member 1 | 3821 | KLRC1 | −2.1 | .000150 |
| 25 | Gtpase, IMAP family member 6 | 474344 | GIMAP6 | −2.2 | .000150 |
| 26 | Transmembrane 4 L 6 family member 18 | 116441 | TM4SF18 | −1.8 | .000150 |
| 27 | Interleukin 15 | 3600 | IL15 | −1.8 | .000160 |
| 28 | Lectin, galactoside-binding, soluble, 9 | 3965 | LGALS9 | −1.8 | .000170 |
| 29 | Plasticity related gene 1 | 9890 | LPPR4 | −1.6 | .000180 |
| 30 | Endo/exonuclease (5′-3′), endonuclease G-like | 9941 | EXOG | 2.0 | .000180 |
| 31 | CD34 molecule | 947 | CD34 | −3.3 | .000180 |
| 32 | MAP6 domain containing 1 | 79929 | MAP6D1 | 1.6 | .000180 |
| 33 | Calcitonin receptor-like | 10203 | CALCRL | −1.9 | .000180 |
| 34 | Cathepsin W | 1521 | CTSW | −2.2 | .000180 |
| 35 | Toll-like receptor 7 | 51284 | TLR7 | −1.5 | .000190 |
| 36 | G protein-coupled receptor 34 | 2857 | GPR34 | −1.8 | .000190 |
| 37 | Keratin 14 | 3861 | KRT14 | 3.0 | .000190 |
| 38 | Acetyl-coenzyme A acetyltransferase 1 | 38 | ACAT1 | −1.6 | .000190 |
| 39 | C-type lectin domain family 14, member A | 161198 | CLEC14A | −2.2 | .000190 |
| 40 | Similar to Complement C3 precursor | 653879 | LOC653879 | −2.6 | .000190 |
| 41 | Phosphoglucomutase 5 | 5239 | PGM5 | −2.2 | .000190 |
| 42 | Chemokine (C-C motif) ligand 18 (pulmonary and activation- regulated) | 6362 | CCL18 | −2.2 | .000190 |
| 43 | LIM domain binding 2 | 9079 | LDB2 | −2.1 | .000190 |
| 44 | GRB2-related adaptor protein | 10750 | GRAP | −1.9 | .000190 |
| 45 | B-cell CLL/lymphoma 2 | 596 | BCL2 | −1.9 | .000190 |
| 46 | Olfactomedin-like 1 | 283298 | OLFML1 | −1.6 | .000190 |
| 47 | CHK2 checkpoint homolog (S. Pombe) | 11200 | CHEK2 | −1.6 | .000190 |
| 48 | Gtpase, IMAP family member 7 | 168537 | GIMAP7 | −2.4 | .000190 |
| 49 | Chromosome 5 open reading frame 4 | 10826 | C5orf4 | −1.9 | .000190 |
| 50 | Sterile alpha motif domain containing 3 | 154075 | SAMD3 | −1.6 | .000190 |
| 51 | Src kinase associated phosphoprotein 1 | 8631 | SKAP1 | −1.7 | .000190 |
| 52 | Tetraspanin 7 | 7102 | TSPAN7 | −2.5 | .000190 |
| 53 | Teashirt zinc finger homeobox 2 | 128553 | TSHZ2 | −2.1 | .000190 |
| 54 | Transcobalamin II; macrocytic anemia | 6948 | TCN2 | −1.9 | .000190 |
| 55 | Purinergic receptor P2Y, G-protein coupled, 14 | 9934 | P2RY14 | −2.7 | .000190 |
| 56 | Family with sequence similarity 150, member B | 285016 | FAM150B | −3.1 | .000200 |
| 57 | Carboxypeptidase B1 (tissue) | 1360 | CPB1 | −2.8 | .000200 |
| 58 | Regulator of G-protein signaling 22 | 26166 | RGS22 | −2.8 | .000200 |
| 59 | Uracil-DNA glycosylase | 7374 | UNG | −1.6 | .000210 |
| 60 | Phospholipid scramblase 4 | 57088 | PLSCR4 | −1.7 | .000210 |
| 61 | Dynein, cytoplasmic 2, light intermediate chain 1 | 51626 | DYNC2LI1 | −1.6 | .000220 |
| 62 | Chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1) | 6387 | CXCL12 | −1.9 | .000220 |
| 63 | Uridine phosphorylase 1 | 7378 | UPP1 | 1.6 | .000220 |
| 64 | Pyruvate dehydrogenase kinase, isozyme 4 | 5166 | PDK4 | −2.2 | .000230 |
| 65 | Nuclear factor I/B | 4781 | NFIB | −2.0 | .000230 |
| 66 | Regulator of G-protein signaling 5 | 8490 | RGS5 | −1.9 | .000250 |
| 67 | Chromosome 21 open reading frame 34 | 388815 | C21orf34 | −1.8 | .000250 |
| 68 | Pannexin 1 | 24145 | PANX1 | 1.6 | .000250 |
| 69 | Acetyl-coenzyme A carboxylase beta | 32 | ACACB | −1.6 | .000270 |
| 70 | CHK2 checkpoint homolog (S. Pombe) | 11200 | CHEK2 | −1.6 | .000280 |
| 71 | Family with sequence similarity 5, member B | 57795 | FAM5B | −2.0 | .000280 |
| 72 | Integral membrane protein 2A | 9452 | ITM2A | −2.3 | .000280 |
| 73 | Chemokine (C-C motif) ligand 15 | 6359 | CCL15 | −2.2 | .000290 |
| 74 | ATP-binding cassette, sub-family A (ABC1), member 8 | 10351 | ABCA8 | −2.2 | .000290 |
| 75 | Lectin, galactoside-binding, soluble, 9 | 3965 | LGALS9 | −2.0 | .000290 |
| 76 | Phosphoinositide-3-kinase, regulatory subunit 1 (alpha) | 5295 | PIK3R1 | −1.7 | .000290 |
| 77 | Gap junction protein, alpha 4, 37 kd | 2701 | GJA4 | −1.6 | .000290 |
| 78 | Sorting nexin family member 21 | 90203 | SNX21 | −1.6 | .000300 |
| 79 | Phosphoribosyl pyrophosphate synthetase 2 | 5634 | PRPS2 | −1.5 | .000300 |
| 80 | LRRN4 C-terminal like | 221091 | LRRN4CL | −1.8 | .000310 |
| 81 | Transmembrane protein 27 | 57393 | TMEM27 | −2.0 | .000340 |
| 82 | CD34 molecule | 947 | CD34 | −3.2 | .000340 |
| 83 | Von Willebrand factor | 7450 | VWF | −4.3 | .000350 |
| 84 | Major histocompatibility complex, class II, DO alpha | 3111 | HLA-DOA | −1.9 | .000360 |
| 85 | Granzyme A (granzyme 1) | 3001 | GZMA | −2.9 | .000380 |
| 86 | Transforming growth factor, beta-induced, 68 kd | 7045 | TGFBI | 1.6 | .000390 |
| 87 | Nuclear factor I/B | 4781 | NFIB | −2.0 | .000390 |
| 88 | Galactosidase, beta 1-like 2 | 89944 | GLB1L2 | −2.3 | .000390 |
| 89 | Endoplasmic reticulum metallopeptidase 1 | 79956 | ERMP1 | −1.5 | .000400 |
| 90 | Serum deprivation response (phosphatidylserine binding protein) | 8436 | SDPR | −2.2 | .000410 |
| 91 | ABI family, member 3 | 51225 | ABI3 | −1.6 | .000420 |
| 92 | Sterol-C4-methyl oxidase-like | 6307 | SC4MOL | 1.6 | .000430 |
| 93 | Solute carrier family 7 (cationic amino acid transporter, y3 system), member 5 | 8140 | SLC7A5 | 1.9 | .000430 |
| 94 | Src homology 2 domain containing E | 126669 | SHE | −1.5 | .000440 |
| 95 | Similar to Complement C3 precursor | 653879 | LOC653879 | −2.2 | .000440 |
| 96 | Ras homolog gene family, member J | 57381 | RHOJ | −1.9 | .000440 |
| 97 | Membrane-spanning 4-domains, subfamily A, member 4 | 51338 | MS4A4A | −1.7 | .000440 |
| 98 | Hypothetic protein LOC100133666 | 100133666 | LOC100133666 | −1.6 | .000440 |
| 99 | Frizzled homolog 8 (Drosophila) | 8325 | FZD8 | 1.7 | .000440 |
| 100 | Osteoglycin | 4969 | OGN | −2.7 | .000440 |
ABI, Abelson-interactor; ATP, adenosine triphospate; BTB, Broad-complex, Tramtrack and Bric-abrac; CD34, cluster of differentiation 34; CHK2, checkpoint kinase 2; CLL, chronic lymphocytic leukemia; EGF, epidermal growth factor; GRB2, growth factor receptor-bound protein 2; IMAP, immunity-associated protein; KIT, c-Kit; LRRN4, leucine rich repeat neuronal 4; MAP6, micortubule-associated protein 6; POZ, poxvirus and zinc finger.
Entrez gene identification;
Symbol was taken from the gene database and corresponds to official Human Genome Organization Gene Nomenclature Committee symbols;
fold change (the number of times the average expression level in the ruptured sites is different from the average expression level in the nonruptured sites) refers to probe expression change in ruptured chorion relative to nonruptured chorion; positive values (no sign) indicate increased expression, and negative values (minus sign) indicate decreased expression.
Gene ontology metaanalysis of the significantly up- and down-regulated genes was performed to identify gene ontology terms that were represented by the differentially expressed genes. In this analysis, 130 biologic processes were enriched (pFDR, <0.05); the top 20 processes are presented in Table 3.
TABLE 3.
Top 20 biological processes with enrichment in chorion between the rupture site and the nonrupture site
| Rank | Biological process | Genes in differentially expressed list, n | Genes in reference array, n | Adjusted P value |
|---|---|---|---|---|
| 1 | Anatomic structure development | 151 | 1785 | < .0001 |
| 2 | Multicellular organismal process | 202 | 2681 | < .0001 |
| 3 | Immune response | 61 | 520 | < .0001 |
| 4 | Multicellular organismal development | 152 | 1919 | < .0001 |
| 5 | Response to external stimulus | 66 | 596 | < .0001 |
| 6 | Epidermis development | 25 | 118 | < .0001 |
| 7 | Antigen processing and presentation of peptide or polysaccharide antigen via major histocompatibility complex class II | 10 | 16 | < .0001 |
| 8 | Cell adhesion | 65 | 591 | < .0001 |
| 9 | Biologic adhesion | 65 | 591 | < .0001 |
| 10 | Developmental process | 196 | 2720 | < .0001 |
| 11 | Organ development | 98 | 1074 | < .0001 |
| 12 | Cell communication | 211 | 3006 | < .0001 |
| 13 | Ectoderm development | 26 | 132 | < .0001 |
| 14 | Signal transduction | 194 | 2739 | < .0001 |
| 15 | Anatomic structure morphogenesis | 89 | 971 | < .0001 |
| 16 | System development | 122 | 1496 | < .0001 |
| 17 | Response to wounding | 47 | 381 | < .0001 |
| 18 | Response to stimulus | 157 | 2100 | < .0001 |
| 19 | Immune system process | 72 | 753 | < .0001 |
| 20 | Locomotion | 42 | 346 | < .0001 |
Pathway analysis of the significant genes was undertaken with an overrepresentation method and a signaling pathway impact analysis method. Using the overrepresentation method, 14 KEGG pathways were significant (pFDR, <0.05) in the comparison between rupture site chorion and nonrupture site chorion (Table 4). The 5 most significant pathways were (1) cell junctions, (2) graft-vs-host disease, (3) complement and coagulation cascades, (4) extracellular matrix–receptor interaction, and (5) allograft rejection. Impact analysis of the KEGG signaling pathways also identified 14 significant pathways (Figure 4), although the ranking of the most impacted pathways were different than the ranking of the pathways from the overrepresentation method. The most impacted pathway was the extracellular matrix–receptor interaction. Moreover, the signaling pathway impact method identified 4 pathways that were not identified by the overrepresentation method: cytokine-cytokine receptor interaction, gap junction, pathogenic Escherichia coli infection, and adipocytokine signaling.
TABLE 4.
Significant pathways with overrepresentation analysis in chorion between rupture site and nonrupture site
| Rank | Map name | Kyoto Encyclopedia of Genes and Genomes identification number | Genes in differentially expressed list, n | Genes in reference array, n | Adjusted P value |
|---|---|---|---|---|---|
| 1 | Cell junctions | 1430 | 30 | 108 | < .0001 |
| 2 | Graft-vs-host disease | 5332 | 17 | 38 | < .0001 |
| 3 | Complement and coagulation cascades | 4610 | 17 | 53 | < .0001 |
| 4 | Extracellular matrix–receptor interaction | 4512 | 20 | 73 | < .0001 |
| 5 | Allograft rejection | 5330 | 13 | 32 | < .0001 |
| 6 | Cell adhesion molecules | 4514 | 25 | 115 | < .0001 |
| 7 | Type I diabetes mellitus | 4940 | 13 | 38 | < .0001 |
| 8 | Asthma | 5310 | 10 | 23 | < .0001 |
| 9 | Focal adhesion | 4510 | 30 | 184 | < .0001 |
| 10 | Autoimmune thyroid disease | 5320 | 12 | 40 | .0001 |
| 11 | Antigen processing and presentation | 4612 | 16 | 74 | .0002 |
| 12 | Hematopoietic cell lineage | 4640 | 15 | 70 | .0003 |
| 13 | Systemic lupus erythematosus | 5322 | 16 | 101 | .0064 |
| 14 | Metabolism of xenobiotics by cytochrome P450 | 980 | 9 | 43 | .0130 |
FIGURE 4. Significantly impacted pathways in chorion specimens.
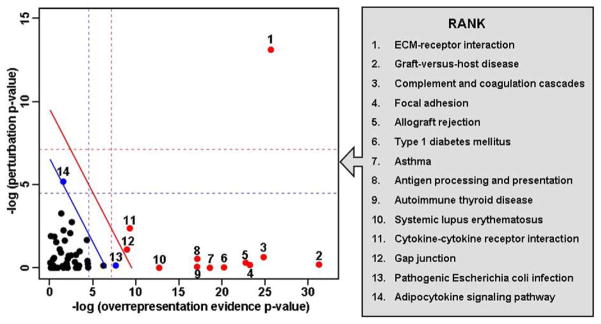
Two-dimensional plot illustrates the relationship between the 2 types of evidence considered by signaling pathway impact analysis. The X-axis shows the overrepresentation evidence (−log[P value]); the Y-axis shows the perturbation evidence (−log[P value]). Each pathway is represented by a point. Pathways above the oblique red line (red dots) are significant at 5% after Bonferroni correction; the pathways (blue dots and red dots) above the oblique blue line are significant at 5% after false discovery rate correction. The vertical and horizontal thresholds represent the same corrections for the 2 types of evidence considered individually.
Rupture site chorion vs rupture site amnion
In the comparison between rupture site chorion and rupture site amnion, transcriptional profiling demonstrated 2279 differentially expressed probes that corresponded with 1981 unique genes (pFDR <0.1; fold change >1.5). Gene ontology metaanalysis of the significant genes revealed the enrichment of 172 biological processes that include developmental process, anatomic structure development, cell adhesion, biologic adhesion, and multicellular organismal development. The over-representation analysis of genes identified 6 significant KEGG pathways: (1) cell junctions, (2) cell adhesion molecules, (3) complement and coagulation cascades, (4) extracellular matrix–receptor interaction, (5) focal adhesion, and (6) asthma. In contrast, signaling pathway impact analysis identified 12 significantly impacted pathways. The data are available as supplementary material (Supplement 3).
Nonrupture site chorion vs nonrupture site amnion
In the comparison between chorion and amnion from the nonruptured site, 3427 probes that represented 2849 genes were differentially expressed (pFDR <0.1; fold change >1.5, data not shown). There were 217 biological processes that were enriched; the top 5 biological processes were anatomic structure development, developmental process, cell adhesion, biologic adhesion, and organ development. Twelve overrepresented KEGG pathways and 17 significantly impacted pathways were identified in this comparison and are available as supplementary material (Supplement 3).
Rupture site amnion vs nonrupture site amnion
There were no significant regional differences in the amnion transcriptome (rupture vs nonrupture).
Augmentation of spontaneous labor
In spontaneous labor at term, there was no difference in the transcriptome of the chorion of patients who underwent augmentation with oxytocin, when compared with patients without augmentation. In contrast, 4 genes were expressed differentially when amnion was compared with and without exposure to oxytocin augmentation: (1) AGA (1.5-fold decrease; pFDR <0.01); (2) RRAGD (1.8-fold increase; pFDR <0.01); (3) LGALS7B (4.2-fold increase; pFDR <0.01); and (4) SORD2 (1.8-fold increase; pFDR <0.01). No significant biological processes or pathways were identified between the amnion groups. These data were not validated with qRT-PCR.
qRT-PCR analysis
Rupture site chorion vs nonrupture site chorion
The results of qRT-PCR of a random subset of the original specimens (n =10) confirmed the differential expression of 11 of 14 genes that were found to be significant on the microarray analysis (Table 5). In a new set of specimens (n =10), qRT-PCR validated the microarray results in 10 of 14 genes (Figure 5). Specifically, qRT-PCR confirmed increased expression of IL6 and PTGS2. Consistent with the microarray analysis, qRT-PCR revealed decreased expression of PAEP, GNLY, CXCL12, CXCL14, IGFBP2, IGFBP4, CNR1, and ADAMTS5 in the rupture site, compared with the nonrupture site. Combining the 2 sets of patients (n =20) for analysis, qRT-PCR validated both the direction of fold change and the significance of 13 of 14 of the differentially expressed genes selected from the microarray analysis.
TABLE 5.
Comparison of microarray data to quantitative reverse transcriptase–polymerase chain reaction results of selected genes in original study samples and in a new replication set in chorion between rupture site and nonrupture site
| Gene symbol | Original sample set: array fold change (n = 20) | Quantitative reverse transcriptase–polymerase chain reaction
|
|||||
|---|---|---|---|---|---|---|---|
| Original sample set (n = 10)a
|
New replication set (n = 10)
|
Combined sample set (n = 20)
|
|||||
| Fold change | P value | Fold change | P value | Fold change | P value | ||
| ADAMTS5 | −3.17 | −2.81 | .0239 | −2.20 | .0816 | −2.46 | .0084 |
|
| |||||||
| BCL2 | −2.26 | −5.47 | .0190 | −2.48 | NS | −3.54 | .0116 |
|
| |||||||
| CNR1 | −2.26 | −2.64 | .0681 | −2.77 | .0824 | −2.71 | .0188 |
|
| |||||||
| IL1RN | 3.05 | 2.37 | .0965 | 3.71 | NS | 3.03 | .0480 |
|
| |||||||
| CXCL12 | −1.72 | −15.60 | .0009 | −10.58 | .0139 | −12.72 | .0001 |
|
| |||||||
| IL-6 | 2.71 | 2.66 | NS | 13.25 | .0233 | 6.44 | .0105 |
|
| |||||||
| CXCL14 | −2.32 | −6.14 | .0205 | −2.83 | .0545 | −4.01 | .0037 |
|
| |||||||
| PTGS2 | 2.10 | 1.61 | NS | 3.45 | .0213 | 2.45 | .0090 |
|
| |||||||
| GNLY | −4.40 | −6.27 | .0050 | −6.31 | .0123 | −6.29 | .0003 |
|
| |||||||
| PAEP | −3.93 | −24.15 | .0072 | −30.97 | .0218 | −27.53 | .0008 |
|
| |||||||
| IGF2 | 1.57 | NA | NA | −1.00 | NS | −1.00 | NS |
|
| |||||||
| IGFBP2 | −2.97 | −3.51 | .0156 | −3.29 | .0323 | −3.39 | .0020 |
|
| |||||||
| IGFBP4 | −1.50 | −2.71 | .0420 | −3.07 | .0166 | −2.90 | .0022 |
|
| |||||||
| IGFBP6 | −2.30 | −3.13 | .0394 | −1.09 | NS | −1.74 | .0711 |
NA, not available; NS, not significant.
Of the 20 original specimens, 10 specimens were selected randomly for quantitative reverse transcriptase–polymerase chain reaction.
FIGURE 5. Quantitative reverse transcriptase–polymerase chain reaction results.
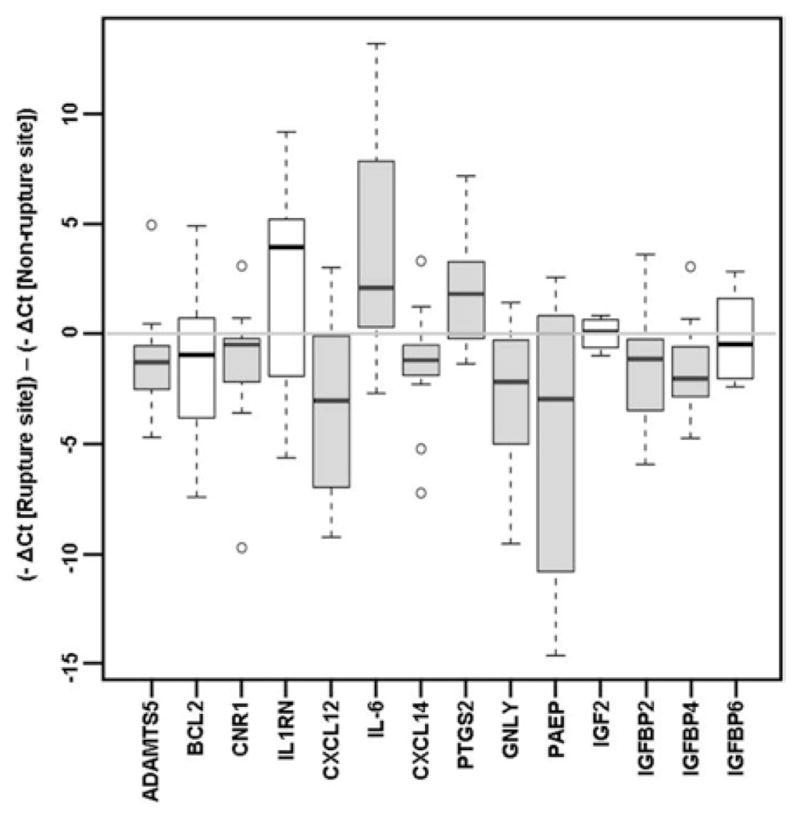
Results of quantitative reverse transcriptase–polymerase chain reaction assay of selected genes in a new set of specimens. The shaded boxes represent genes that are statistically significant (P< .05); the white boxes represent nonsignificant genes. The data are presented as the difference between the −ΔCt of rupture site chorion and the −ΔCt of non-rupture site chorion: positive values correlate with increased expression and negative values correlate with decreased expression of the gene in the rupture site chorion compared with the nonrupture site chorion. The boxes encompass 50% of the data from the 1st quartile to the 3rd quartile. The middle line represents the median value (50%) quartile. The whiskers extend to the most extreme data point but do not exceed values of >1.5 times the inter-quartile range from the box. The circles represent outliers.
Nonrupture site chorion vs nonrupture site amnion
In the comparison between nonrupture site chorion and nonrupture site amnion, gene expression was found to correspond with the microarray data for PTGS2 (9.4-fold decrease; P=.0044), IL1RN (69.9-fold decrease; P=.0039), and CXCL14 (76.4-fold increase; P=.002).
Rupture site chorion vs rupture site amnion
In the comparison between rupture site chorion and rupture site amnion, significant fold changes in messenger RNA (mRNA) expression were detected for PTGS2 (2.9-fold decrease; P=.011), IL1RN (30.2-fold decrease; P=.002), and CXCL14 (88-fold increase; P=.002).
Rupture site amnion vs nonrupture site amnion
Corresponding with the microarray analysis, PTGS2, IL1RN, and CXCL14 PCR results were not statistically significant in the comparisons between rupture site amnion and nonrupture site amnion.
Comment
Microarray analysis revealed 677 differentially expressed genes in the chorion of the fetal membranes between the site of rupture and the site away from rupture in patients with term labor and SROM. Biological processes with enrichment in the chorion included anatomic structure development, cell adhesion, signal transduction, and cell communication. The extracellular matrix–receptor interaction, graft vs host disease, and complement and coagulation cascades were among the most impacted signaling pathways by the presence of rupture in the chorion. There were no significant regional gene expression differences (rupture vs nonrupture) in the amnion transcriptome. Stereotypic impacted pathways and biological processes with enrichment were represented in the comparisons between amnion and chorion in the rupture and nonrupture sites.
This study describes, for the first time, the transcriptome of the amnion and chorion at the site of SROM and at the site distal to the site of rupture in patients with spontaneous labor at term. Previous investigations about gene expression,45–47 protein immunolocalization and quantification,4,12,13,45–50 and enzymatic activity4 at the site of rupture have used a targeted approach. The development of high-throughput technology and computational biology makes possible the use of high-dimensional biological techniques to characterize the global changes that are associated with a particular phenomenon.26,28 This is the first study to use transcriptomics to identify changes in gene expression in amnion and chorion at the site of membrane rupture.
Malak, Bell, McLaren, McParland, and Taylor made classic contributions by demonstrating that the fetal membranes overlying the cervix undergo morphologic2,10,12 and biochemical4,12,48 changes before labor. They referred to this area as the ZAM.10 The morphologic changes include decreased thickness of the trophoblast and decidua and increased thickness of the connective tissue of amnion and chorion.2,10,12,13 The biochemical changes that were observed in this area included an increase in pro–matrix metalloproteinase (MMP)-9 (92 kd) protein concentration and enzymatic activity determined by enzyme-linked immunosorbent assay and zymography, respectively; no significant regional differences of pro–MMP-2 (72 kd) activity were detected in prelabor specimens.4 In the same study, increased MMP-9 protein concentration was found in specimens that were obtained after labor. Subsequently, El Khwad et al13 reported a zone of weakness on biophysical testing; this weak zone had increased MMP-9 and decreased tissue metallopeptidase inhibitor-3 (TIMP-3) protein by Western blot analysis. This study found that TIMP-1 was barely detectable in the membranes; TIMP-2 and TIMP-4 were detectable but did not change as a function of membrane strength. Moreover, another study from the same group demonstrated that the incubation of membranes in vitro with interleukin (IL)-1β and tumor necrosis factor alpha (TNFα) was able to induce the biophysical changes, increase MMP-9 protein (both 92 kd and 83 kd bands), and decrease TIMP-3.25 These observations are consistent with an increase in amniotic fluid MMPs (MMP-1, MMP-3, MMP-7, MMP-8, MMP-9),19,20,22–24,51–56 neutrophil elastase,57 IL-1β, and TNFα18,20,;58–61 in spontaneous labor at term and/or preterm PROM.
In a study of chorioamniotic membranes from patients with SROM or artificial rupture of membranes who delivered vaginally at term, El Khwad et al49 used topographic mapping to identify a 10-cm (diameter) zone of weakness that surrounded the site of rupture where a decreased rupture threshold was observed. Moreover, Strohl et al14 recently demonstrated the increased separation and decreased adherence of the amnion and choriodecidua in specimens that were obtained from term deliveries, when compared with preterm membranes, which was an effect that was more pronounced in specimens from women in labor.62 However, there is a paucity of evidence about whether the gene expression profile of the chorion and amnion is different at the site of rupture or away from the site of rupture.
One study has examined the change in the transcriptome of the chorioamniotic membranes that were obtained from women not in labor at term and in women at term.29 Importantly, membranes of women with subclinical inflammation (histologic chorioamnionitis) were excluded. The key finding of the study was that labor was associated with up-regulation of genes that are involved in the inflammatory response. These findings, which were derived originally from microarray studies, were confirmed with quantitative PCR. Therefore, spontaneous labor is associated with a signature gene expression that is consistent with inflammation of the chorioamniotic membranes, even in the absence of histopathologic evidence of such processes. Targeted studies have yielded findings that are consistent with these observations. For example, Osman et al47 reported a higher mRNA expression for IL-1β, −6, and −8 in women in labor than in women not in labor. Such findings are consistent with previous observations that the concentrations of these cytokines are increased in the amniotic fluid of women in labor (term and preterm). 18,58,59,61,63–71 Of interest, Osman et al did not find regional differences in cytokine expression in the ZAM compared with specimens of membranes that were obtained distal to this site.
Our microarray results demonstrated higher expression of IL-6 and IL-8 in the chorion at the site of rupture than in the chorion from the nonrupture site. These results were confirmed by qRT-PCR for IL-6 (13.2-fold increase). The difference in study design may explain the different results between these studies: Osman et al47 examined specimens from the ZAM in women (n =8) in labor with a cervical dilation that ranged from 4–9 cm who underwent cesarean delivery for fetal distress (no information was provided regarding the rupture status or mode of membrane rupture at the time of cesarean delivery). In contrast, the specimens that were used in our study were collected from 20 patients with confirmed SROM who underwent successful spontaneous vaginal delivery.
It is possible that the inflammatory changes at the site of rupture are due to exposure to vaginal fluid and its content, which includes microbial products, microorganisms, and inflammatory mediators. Microbial invasion of the amniotic cavity is identified with standard culture techniques in 18% of patients at term in labor with intact membranes72 and in 30% of patients with PROM at term.73 Because bacteria can cross the chorioamniotic membranes,74 it has been proposed that the source of the organisms is from ascending microbial invasion from the vagina. In the absence of microbial invasion of the amniotic cavity, fetal membranes that are exposed to the vagina have been thought to respond to stimuli that is present in the vaginal fluid with the production of proinflammatory cytokines and prostaglandins.75 Concentrations of IL-1β secreted by regional decidua and the prostaglandins PGE2 and PGF2α were higher in cervical/vaginal lavage specimens from women in labor than that of women not in labor.76 Moreover, in vitro studies with fetal membranes demonstrate reduction of elasticity and bursting tension after exposure to bacteria and products of activated neutrophils.77
The changes in the gene expression at the rupture site may be associated with alterations in the concentration of inflammatory mediators in the amniotic fluid adjacent to the site of impending rupture. In humans, amniotic fluid prostaglandin concentrations increase before the onset of spontaneous term labor and may play a role in the initiation of labor.78–84 Moreover, rupture of membranes at term is accompanied by increased concentrations of prostaglandins in amniotic fluid in patients with and without labor, when compared with women with intact membranes without labor.85 The concentrations of eicosanoids, protein-degrading enzymes, and chemokines, which include tissue plasminogen activator, urokinase plasminogen activator, plasminogen activator inhibitor-2,86 MMP-9,21 MMP-9/TIMP-1 ratios,21 and the prostaglandins PGF2α, PGFM, and PGE2,87,88 are higher in the fluid in direct contact with the ZAM (fore-water) than in fluid from the upper compartment (hindwater) in women with term labor. The changes of the concentrations of serine proteases (tissue plasminogen activator and urokinase plasminogen activator) were thought to be a result of exposure to cytokines that are present in the vagina. Moreover, in the case of prostaglandins, the concentrations increase with advancing cervical dilation.88 Further evidence of topographic differences in amniotic fluid markers is the increased concentration of IL-6 concentration in the forewater than in the hindwater in both term89 and preterm18 labor. The source for the higher regional concentrations of prostaglandins and IL-6 in the forewater is unclear but may also be related to exposure to cervical and vaginal stimuli or a result of increased production by regional fetal membranes or decidua.
The results of the microarray reported herein support the concept that the fetal membranes at the site of rupture have a different pattern of gene expression than the chorioamniotic membranes away from the site of rupture. The expression of prostaglandin endoperoxidase synthase 2 gene (PTGS2), also known as cyclooxygenase 2 (COX2), was also increased in chorion from the site of membrane rupture when compared with chorion from the site away from rupture (validated by qRT-PCR). PTGS2 may be regulated by nuclear factor kappa-B (NF-κB) and is produced predominantly in amnion at term90–92 and significantly increased in labor.93 Our microarray results do not demonstrate increased PTGS2 expression in the amnion at the site of rupture when compared with amnion at the site away from rupture; however, the increase in the chorion at the site of rupture suggests that the prostaglandin biosynthesis pathway may be subject to a spatial regulation (amnion vs chorion).
ZAM is associated with a reduction in the cellularity of the cytotrophoblast and decidual layers. Apoptotic bodies (detected by electron microscopy) are increased in all regions of the fetal membranes, but specifically in the ZAM rather than in the distal regions of the membranes.48 In addition, there was no substantial immunoreactivity for B-cell lymphoma protein 2 (BCL2), an anti-apoptotic protein, but strong for BCL2-associated X protein, a proapoptotic protein, in the decidual layer. This is consistent with observations that apoptosis occurs in the rat amnion.94,95
Further evidence in support of this concept is the observation that there is increased cleaved caspase-3 and caspase-9 (apoptotic markers) and decreased BCL2 in the ZAM.50 Immunohistochemical staining of BCL2 in earlier work by McLaren et al. did not demonstrate a significant difference in BCL2 expression between the ZAM and a site distal to this site.48 Interestingly, BCL2 was one of the most significant differentially expressed genes in our microarray experiments and was decreased in the chorion at the site of rupture, compared with the nonruptured site. Further studies are required to validate these findings at the mRNA and protein levels.
As discussed earlier, there is extensive evidence of extracellular matrix remodeling at the site of rupture that is demonstrated by the increased expression of MMP-9 in the membranes before spontaneous labor. We did not find a significant difference in MMP-9 mRNA expression at the site of rupture in either the chorion or amnion, when compared with the nonrupture site. The difference in these findings may be due to the different assays. Microarrays assess changes in mRNA expression; immunoassays detect protein, and zymography detects enzymatic activity.
The PAEP gene encodes for glycodelin, which previously was also known as progestagen-associated endometrial protein or placental protein-14, which is a major progesterone-regulated lipocalin that has high carbohydrate content.96–98 Three differentially glycosylated isoforms of glycodelin are expressed in different tissues. Glycode-lin-A is the most abundant glycoprotein in the uterus in early pregnancy. It has angiogenic,99 immunosuppressive,100 and paracrine effects on gestational tissues and has been detected in human serum,101,102 amniotic fluid,101,103–105 endometrium (mainly secretory),106 decidua,107 and cervix.108 Glycodelin-A concentrations in maternal serum and decidua increase from conception to 6–10 weeks and then decrease with advancing gestational age.101 Moreover, glycodelin-A concentrations in amniotic fluid are detected at 10 weeks, peak at 15–16 weeks, and decline with advancing gestational age.101,104,105
Perturbations of glycodelin-A expression have been identified in abnormal pregnancies. It has been found to be increased in the decidua of molar pregnancies109 and decreased in decidua from pregnancies that are complicated by spontaneous abortion,109 ectopic implantation,110 intrauterine growth restriction,107 and HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome.107 In vitro studies have demonstrated that recombinant or immunopurified glycodelin-A induces apoptosis in monocytes and inhibits their NF-κB activation and inhibits T-cell proliferation and IL-2 secretion, which are roles that support immunotolerance during pregnancy.100,111 Moreover, glycodelin-A suppresses MMP-2 mRNA and protein expression and MMP-2, MMP-9, and urokinase plasminogen activator enzyme activity in a first-trimester extravillous cytotropho-blast cell line.112
We report the decreased expression of PAEP in microarray experiments (3.9-fold) in the chorion at the rupture site, which was confirmed with qRT-PCR (31-fold). It is possible that the decreased PAEP expression is associated with the release of inhibition of the NF-κB signaling pathway or MMP activity. Indeed, increases in NF-κB signaling proteins5 and MMP-9 activity4,49 have been described previously in supracervical membranes before the onset of labor, when compared with membranes distal to the cervix.
A disintegrin and metallopeptidase with thrombospondin type-1 motif-5 (ADAMTS-5), also known as aggre-canase-2, ADAMTS11, and implantin, is a proteinase that is known to cleave aggrecan, which is a large proteoglycan that is found in the extracellular matrix of the cartilage and cervix.113 Members of the ADAM metallopeptidase with throm-bospondin motif (ADAMTS) family are secreted molecules that can interact with the extracellular matrix. The ADAMTS-5 aggrecanase is expressed in the uterus, cervix, ovary, and placenta.114 In mice, ADAMTS-5 mRNA expression has been detected in the decidua in the dam in the periimplantation period, although its highest expression was detected in the placenta and is thought to contribute to extracellular matrix–remodeling at the maternal-fetal interface.115,116 The role of ADAMTS-5 in unknown in humans. In vitro studies have described that ADAMTS-5 expression is altered by cytokines; IL-1β increases and TGF-β1 decreases the expression of ADAMTS-5 in human decidual stromal cell cultures.116 Other members of the ADAMTS family of aggrecanases have similar functions, although the distribution varies. For instance, in the cervix after spontaneous labor and delivery at term, ADAMTS-9 (an aggrecanase that cleaves both aggrecan and versican) is up-regulated.117
In both the microarray and qRT-PCR analysis, ADAMTS-5 was found to have decreased expression at the rupture site chorion, when compared with the non-rupture site chorion. We propose that, at the ZAM and the subsequent site of rupture, the maintenance of the maternal-fetal interface is no longer required and that ADAMTS-5 is rendered inconsequential. In support of this view, examination of the microarray data reveals that ADAMTS-9 is also decreased significantly in the rupture site chorion. Furthermore, our results identify the activation of the graft-vs-host disease and the allograft rejection pathways, both of which are among the top 5 most impacted and significant pathways.
Microarray analysis did not reveal a significant difference in gene expression profile of the amnion samples that were obtained from the rupture site and non-rupture site. This finding was unexpected. We expected differences in gene expression in this fetal membrane based on its role in membrane integrity and previous observations in the literature.5,10,36,118–120
The lack of detectable difference may be a consequence of the large variance among patients. It is also possible that the anatomic location of the amnion precludes regional differences in the amnion at the rupture site and the nonrupture site. Indeed, the thinning of the fetal membranes in the ZAM is mostly a consequence of thinning of decidual and trophoblast layers and not the amnion.2,10 In addition, the studies that have evaluated membrane strength indicate that the weakness in fetal membranes is predominantly due to the changes that have occurred in the chorion and less because of the amnion.13 Recently, our group has reported that the amnion overlying the placenta is distinctly different from the reflected amnion of the extraplacental membranes.36 Using microarray analysis and tissue culture and immunoblotting techniques, we have reported that, in term fetal membranes, labor-associated switching to a proinflammatory signature is localized to placental amnion and not reflected amnion. Indeed, regional perturbations of gene expression and regulation in the amnion may occur between reflected and placental amnion but may not be evident at the site of membrane rupture.
Global analyses of mRNA expression of a particular tissue in a particular condition have been useful in the understanding of the physiologic and pathologic evidence of parturition. In spontaneous term labor, the transcriptome of the chorioamniotic membranes has an acute inflammatory gene expression signature, even in the absence of histologic chorioamnionitis.29 Indeed, inflammation plays an important role in normal term labor,121 as evidenced by activation of the inflammatory pathways described in the cervix,117,122 myometrium,122 and fetal membranes29,122 of women in labor. Although different genes and specific pathways are represented in each different gestational tissue type, the core response is the activation of the inflammatory pathway. The transcriptome of the chorion at the site of rupture reported herein also supports the role of inflammation in SROM. The graft-vs-host disease, complement and coagulation cascades, and allograft rejection pathways are participants in inflammation.
The inflammatory pathway is not the only pathway of interest. In this study, the most significant and impacted pathways represented by SROM were the extracellular matrix–receptor interaction pathway and other pathways that involve cell adhesion, angiogenesis, apoptosis, and immunotolerance. Two of the top 5 most impacted pathways that have been identified in the chorion are graft-vs-host disease and allograft rejection, which sustains the role of regional withdrawal of pregnancy support at the site of rupture. Moreover, the signature pathways that have been identified were both stereotypic and novel and support the notion that human parturition and SROM are complex interactions of many biological processes.
This study describes the transcriptome of a set of matched amnion and chorion from both the site of rupture and the site away from rupture. The strengths of the study are its prospective design, inclusion of a large sample number of normal pregnant women (n =20) with SROM and vaginal delivery, exclusion of maternal diseases and specimens with documented GBS colonization of the genital tract, clinical or histologic chorioamnionitis, and meconium staining. A limitation of the study is that the differential expression of some of the genes reported herein may be due to the mechanical stress and biochemical changes that are associated with the act of rupture of membranes. To characterize the genes that are expressed differentially as a result of exposure to labor processes and/or mechanical/physiologic stress of rupture of membranes, the gene expression profile of the ZAM should be investigated. Future studies of fetal membranes may include the validation of gene regulation and the processes and pathways that have been identified to be significant and were impacted in this study.
The transcriptome of fetal membranes in spontaneous rupture in term labor is characterized by region- and tissue-specific differential expression of genes that are involved in signal transduction, cell communication, the complement and coagulation cascades, and graft-vs-host disease. In accordance with previous global studies of the transcriptome of other tissues (such as the uterine cervix) after spontaneous term parturition, genes from the extracellular matrix–receptor interaction pathway and those involved in inflammation appear to be involved in parturition. These regional differences were detected in the chorion but not in the amnion. Our findings suggest that the site of rupture in chorionic membranes has a region- and tissue-specific gene expression signature in spontaneous labor at term.
Acknowledgments
Supported by the Division of Intramural Research, Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. 0002-9378/$36.00
We thank Dr Susan Land and Dan Lott at the Applied Genomics Technology Center of Wayne State University for performing the mi-croarrays and the nursing staff of the Perinatol-ogy Research Branch and Detroit Medical Center for their contribution to the study.
Footnotes
Presented orally at the 30th Annual Meeting of the Society for Maternal-Fetal Medicine, Chicago, IL, Feb. 1–6, 2010. The racing flag logo above indicates that this article was rushed to press for the benefit of the scientific community.
References
- 1.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14:237–41. doi: 10.1093/humrep/14.1.237. [DOI] [PubMed] [Google Scholar]
- 3.Sawdy RJ, Dennes WJ, Allport V, et al. Region and labour-dependent synthesis of prostaglandin E2 by human fetal membranes. Placenta. 1999;20:181–4. doi: 10.1053/plac.1998.0372. [DOI] [PubMed] [Google Scholar]
- 4.McLaren J, Taylor DJ, Bell SC. Increased concentration of pro-matrix metalloproteinase 9 in term fetal membranes overlying the cervix before labor: implications for membrane remodeling and rupture. Am J Obstet Gynecol. 2000;182:409–16. doi: 10.1016/s0002-9378(00)70232-8. [DOI] [PubMed] [Google Scholar]
- 5.Lappas M, Odumetse TL, Riley C, et al. Pre-labour fetal membranes overlying the cervix display alterations in inflammation and NF-kappaB signaling pathways. Placenta. 2008;29:995–1002. doi: 10.1016/j.placenta.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–8. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 7.Kjaergaard N, Hein M, Hyttel L, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–9. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 8.Santolaya-Forgas J, Romero R, Espinoza J, et al. Prelabor rupture of the membranes. In: Reece EA, Hobbins JC, editors. Clinical obstetrics: the fetus and mother. Malden, (MA): Black-well Publishing; 2007. pp. 1130–88. [Google Scholar]
- 9.Artal R, Sokol RJ, Neuman M, Burstein AH, Stojkov J. The mechanical properties of prematurely and non--prematurely ruptured membranes: methods and preliminary results. Am J Obstet Gynecol. 1976;125:655–9. doi: 10.1016/0002-9378(76)90788-2. [DOI] [PubMed] [Google Scholar]
- 10.Malak TM, Bell SC. Structural characteristics of term human fetal membranes: a novel zone of extreme morphological alteration within the rupture site. BJOG. 1994;101:375–86. doi: 10.1111/j.1471-0528.1994.tb11908.x. [DOI] [PubMed] [Google Scholar]
- 11.Bell SC, McParland PC. Fetal membrane rupture. In: Critchley HO, Bennett P, Tibshirani R, editors. Preterm birth. London: Royal College of Obstetricians and Gynaecologists Press; 2004. pp. 195–212. [Google Scholar]
- 12.McParland PC, Taylor DJ, Bell SC. Mapping of zones of altered morphology and chorionic connective tissue cellular phenotype in human fetal membranes (amniochorion and decidua) overlying the lower uterine pole and cervix before labor at term. Am J Obstet Gynecol. 2003;189:1481–8. doi: 10.1067/s0002-9378(03)00585-4. [DOI] [PubMed] [Google Scholar]
- 13.El Khwad M, Stetzer B, Moore RM, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72:720–6. doi: 10.1095/biolreprod.104.033647. [DOI] [PubMed] [Google Scholar]
- 14.Strohl A, Kumar D, Novince R, et al. Decreased adherence and spontaneous separation of fetal membrane layers—amnion and choriodecidua—a possible part of the normal weakening process. Placenta. 2010;31:18–24. doi: 10.1016/j.placenta.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGregor JA, Lawellin D, Franco-Buff A, Todd JK, Makowski EL. Protease production by microorganisms associated with reproductive tract infection. Am J Obstet Gynecol. 1986;154:109–14. doi: 10.1016/0002-9378(86)90404-7. [DOI] [PubMed] [Google Scholar]
- 16.McGregor JA, French JI, Lawellin D, Franco-Buff A, Smith C, Todd JK. Bacterial protease-induced reduction of chorioamniotic membrane strength and elasticity. Obstet Gynecol. 1987;69:167–74. [PubMed] [Google Scholar]
- 17.McGregor JA, Schoonmaker JN, Lunt BD, Lawellin DW. Antibiotic inhibition of bacterially induced fetal membrane weakening. Obstet Gynecol. 1990;76:124–8. [PubMed] [Google Scholar]
- 18.Cox SM, Casey ML, MacDonald PC. Accumulation of interleukin-1beta and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum Reprod Update. 1997;3:517–27. doi: 10.1093/humupd/3.5.517. [DOI] [PubMed] [Google Scholar]
- 19.Athayde N, Edwin SS, Romero R, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Ob-stet Gynecol. 1998;179:1248–53. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 20.Maymon E, Ghezzi F, Edwin SS, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142–8. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 21.Maymon E, Edwin S, Gonzalez R, et al. Topographical differences in the bioavailability of matrix metalloproteinases within the uterine cavity: a possible mechanism or the development of a regional area of membrane weakness predisposing to rupture. Am J Obstet Gynecol. 1999;180(suppl):S1444. [Google Scholar]
- 22.Maymon E, Romero R, Pacora P, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 23.Maymon E, Romero R, Pacora P, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–16. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 24.Park KH, Chaiworapongsa T, Kim YM, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31:12–22. doi: 10.1515/JPM.2003.002. [DOI] [PubMed] [Google Scholar]
- 25.Kumar D, Fung W, Moore RM, et al. Proinflammatory cytokines found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured human fetal membranes. Biol Reprod. 2006;74:29–34. doi: 10.1095/biolreprod.105.045328. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Espinoza J, Gotsch F, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG. 2006;113(suppl 3):118–35. doi: 10.1111/j.1471-0528.2006.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero R, Tromp G. High-dimensional biology in obstetrics and gynecology: functional genomics in microarray studies. Am J Obstet Gynecol. 2006;195:360–3. doi: 10.1016/j.ajog.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Tarca AL, Tromp G. Insights into the physiology of childbirth using transcriptomics. PLoS Med. 2006;3:e276. doi: 10.1371/journal.pmed.0030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394. e1–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod. 2002;8:399–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 31.Tromp G, Kuivaniemi H, Romero R, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1331–8. doi: 10.1016/j.ajog.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 33.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–48. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 34.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation: a workshop report. Placenta. 2005;26(suppl A):S114–7. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Benirschke K, Kaufmann P, Baergen R. Anatomy and pathology of the placental membranes. In: Benirschke K, Kaufmann P, Baergen R, editors. Pathology of the human placenta. New York: Springer; 2006. pp. 321–79. [Google Scholar]
- 36.Han YM, Romero R, Kim JS, et al. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod. 2008;79:954–61. doi: 10.1095/biolreprod.108.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 38.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 39.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Draghici S, Khatri P, Tarca AL, et al. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–45. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khatri P, Draghici S, Ostermeier GC, Krawetz SA. Profiling gene expression using onto-express. Genomics. 2002;79:266–70. doi: 10.1006/geno.2002.6698. [DOI] [PubMed] [Google Scholar]
- 43.Tarca AL, Romero R, Draghici S. Analysis of microarray experiments of gene expression profiling. Am J Obstet Gynecol. 2006;195:373–88. doi: 10.1016/j.ajog.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarca AL, Draghici S, Khatri P, et al. A novel signaling pathway impact analysis. Bioinformatics. 2009;25:75–82. doi: 10.1093/bioinformatics/btn577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osman I, Young A, Ledingham MA, et al. Leukocyte density and proinflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–5. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 46.Osman I, Crawford M, Jordan F, Young A, Norman J, Thomson A. Expression and localization of cell adhesion molecules in human fetal membranes during parturition. J Reprod Immunol. 2004;63:11–21. doi: 10.1016/j.jri.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig. 2006;13:97–103. doi: 10.1016/j.jsgi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 48.McLaren J, Taylor DJ, Bell SC. Increased incidence of apoptosis in non-labour-affected cytotrophoblast cells in term fetal membranes overlying the cervix. Hum Reprod. 1999;14:2895–900. doi: 10.1093/humrep/14.11.2895. [DOI] [PubMed] [Google Scholar]
- 49.El Khwad M, Pandey V, Stetzer B, et al. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13:191–5. doi: 10.1016/j.jsgi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Reti NG, Lappas M, Riley C, et al. Why do membranes rupture at term? Evidence of increased cellular apoptosis in the supracervical fetal membranes. Am J Obstet Gynecol. 2007;196:484–10. doi: 10.1016/j.ajog.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Athayde N, Romero R, Gomez R, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med. 1999;8:213–9. doi: 10.1002/(SICI)1520-6661(199909/10)8:5<213::AID-MFM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 52.Fortunato SJ, Menon R, Lombardi SJ. MMP/TIMP imbalance in amniotic fluid during PROM: an indirect support for endogenous pathway to membrane rupture. J Perinat Med. 1999;27:362–8. doi: 10.1515/JPM.1999.049. [DOI] [PubMed] [Google Scholar]
- 53.Maymon E, Romero R, Pacora P, et al. Evidence for the participation of interstitial collage-nase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–20. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 54.Maymon E, Romero R, Pacora P, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Ob-stet Gynecol. 2000;183:887–94. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 55.Maymon E, Romero R, Pacora P, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1545–53. doi: 10.1067/mob.2000.107652. [DOI] [PubMed] [Google Scholar]
- 56.Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149–55. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 57.Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–46. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 58.Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 59.Romero R, Parvizi ST, Oyarzun E, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–8. [PubMed] [Google Scholar]
- 60.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–87. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 61.Opsjln SL, Wathen NC, Tingulstad S, et al. Tumor necrosis factor, interleukin-1, and inter-leukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993;169:397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 62.Kumar D, Novince R, Strohl A, et al. A new methodology to measure strength of adherence of the fetal membrane components, amnion and the choriodecidua. Placenta. 2009;30:560–3. doi: 10.1016/j.placenta.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor: association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 65.Santhanam U, Avila C, Romero R, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991;3:155–63. doi: 10.1016/1043-4666(91)90037-e. [DOI] [PubMed] [Google Scholar]
- 66.Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 67.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleu-kin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167–83. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 68.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–51. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 69.Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleu-kin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 70.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–10. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 71.Figueroa R, Garry D, Elimian A, Patel K, Sehgal PB, Tejani N. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2005;18:241–7. doi: 10.1080/13506120500223241. [DOI] [PubMed] [Google Scholar]
- 72.Romero R, Nores J, Mazor M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543–8. [PubMed] [Google Scholar]
- 73.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Infection and labor: VII, microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 74.Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol. 1984;148:915–28. doi: 10.1016/0002-9378(84)90534-9. [DOI] [PubMed] [Google Scholar]
- 75.MacDonald PC, Koga S, Casey ML. Decidual activation in parturition: examination of amniotic fluid for mediators of the inflammatory response. Ann N Y Acad Sci. 1991;622:315–30. doi: 10.1111/j.1749-6632.1991.tb37877.x. [DOI] [PubMed] [Google Scholar]
- 76.Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1 beta, -1 alpha, and -6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab. 1993;77:805–15. doi: 10.1210/jcem.77.3.8370702. [DOI] [PubMed] [Google Scholar]
- 77.Schoonmaker JN, Lawellin DW, Lunt B, McGregor JA. Bacteria and inflammatory cells reduce chorioamniotic membrane integrity and tensile strength. Obstet Gynecol. 1989;74:590–6. [PubMed] [Google Scholar]
- 78.Keirse MJ, Mitchell MD, Turnbull AC. Changes in prostaglandin F and 13,14-dihydro-15-ketoprostaglandin F concentrations in amniotic fluid at the onset of and during labour. BJOG. 1977;84:743–6. doi: 10.1111/j.1471-0528.1977.tb12484.x. [DOI] [PubMed] [Google Scholar]
- 79.Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157:1461–7. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 80.Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Increased amniotic fluid leukotriene C4 concentration in term human parturition. Am J Obstet Gynecol. 1988;159:655–7. doi: 10.1016/s0002-9378(88)80028-0. [DOI] [PubMed] [Google Scholar]
- 81.Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1988;34:141–5. doi: 10.1016/0952-3278(88)90137-8. [DOI] [PubMed] [Google Scholar]
- 82.Romero R, Wu YK, Sirtori M, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in pre-term labor. Prostaglandins. 1989;37:149–61. doi: 10.1016/0090-6980(89)90038-5. [DOI] [PubMed] [Google Scholar]
- 83.Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids. 1996;54:187–91. doi: 10.1016/s0952-3278(96)90015-0. [DOI] [PubMed] [Google Scholar]
- 84.Lee SE, Romero R, Park IS, Seong HS, Park CW, Yoon BH. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Matern Fetal Neonatal Med. 2008;21:89–94. doi: 10.1080/14767050701830514. [DOI] [PubMed] [Google Scholar]
- 85.Romero R, Baumann P, Gomez R, et al. The relationship between spontaneous rupture of membranes, labor, and microbial invasion of the amniotic cavity and amniotic fluid concentrations of prostaglandins and thromboxane B2 in term pregnancy. Am J Obstet Gynecol. 1993;168:1654–64. doi: 10.1016/0002-9378(93)90675-9. [DOI] [PubMed] [Google Scholar]
- 86.Baumann P, Romero R, Gonzelez R, Cotton DB, Mammen E. Evidence of topographic differences in amniotic fluid plasminogen activators/plasminogen activator inhibitor concentrations during spontaneous active labor at term. Am J Obstet Gynecol. 1994;170:270. [Google Scholar]
- 87.MacDonald PC, Casey ML. The accumulation of prostaglandins (PG) in amniotic fluid is an aftereffect of labor and not indicative of a role for PGE2 or PGF2 alpha in the initiation of human parturition. J Clin Endocrinol Metab. 1993;76:1332–9. doi: 10.1210/jcem.76.5.8496326. [DOI] [PubMed] [Google Scholar]
- 88.Romero R, Gonzalez R, Baumann P, et al. Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot Essent Fatty Acids. 1994;50:97–104. doi: 10.1016/0952-3278(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 89.Olah KS, Neilson JP, Johnson PM. Interleukin-6 in amniotic fluid obtained at forewater amniotomy compared with hindwater samples in women in spontaneous labour. Eur J Obstet Gynecol Reprod Biol. 1995;60:65–7. doi: 10.1016/0028-2243(95)02070-5. [DOI] [PubMed] [Google Scholar]
- 90.Slater DM, Berger LC, Newton R, Moore GE, Bennett PR. Expression of cyclooxygenase types 1 and 2 in human fetal membranes at term. Am J Obstet Gynecol. 1995;172:77–82. doi: 10.1016/0002-9378(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 91.Slater D, Dennes W, Sawdy R, Allport V, Bennett P. Expression of cyclooxygenase types-1 and -2 in human fetal membranes throughout pregnancy. J Mol Endocrinol. 1999;22:125–30. doi: 10.1677/jme.0.0220125. [DOI] [PubMed] [Google Scholar]
- 92.Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappaB activity which mediates cyclooxygenase-2 expression and is involved with the ’functional progester-one withdrawal’. Mol Hum Reprod. 2001;7:581–6. doi: 10.1093/molehr/7.6.581. [DOI] [PubMed] [Google Scholar]
- 93.Ackerman WE, Summerfield TL, Vandre DD, Robinson JM, Kniss DA. Nuclear factor-kappa B regulates inducible prostaglandin E synthase expression in human amnion mesenchymal cells. Biol Reprod. 2008;78:68–76. doi: 10.1095/biolreprod.107.061663. [DOI] [PubMed] [Google Scholar]
- 94.Paavola LG, Furth EE, Delgado V, et al. Striking changes in the structure and organization of rat fetal membranes precede parturition. Biol Reprod. 1995;53:321–38. doi: 10.1095/biolreprod53.2.321. [DOI] [PubMed] [Google Scholar]
- 95.Lei H, Kalluri R, Furth EE, Baker AH, Strauss JF., III Rat amnion type IV collagen composition and metabolism: implications for membrane breakdown. Biol Reprod. 1999;60:176–82. doi: 10.1095/biolreprod60.1.176. [DOI] [PubMed] [Google Scholar]
- 96.Than GN, Bohn H, Szabo DG. Advances in pregnancy-related protein research. Boca Raton: CRC Press; 1993. pp. 1–333. [Google Scholar]
- 97.Seppala M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002;23:401–30. doi: 10.1210/er.2001-0026. [DOI] [PubMed] [Google Scholar]
- 98.Seppala M. Advances in uterine protein research: reproduction and cancer. Int J Gynaecol Obstet. 2004;85:105–18. doi: 10.1016/j.ijgo.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 99.Park JK, Song M, Dominguez CE, et al. Glycodelin mediates the increase in vascular endothelial growth factor in response to oxidative stress in the endometrium. Am J Obstet Gynecol. 2006;195:1772–7. doi: 10.1016/j.ajog.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 100.Rachmilewitz J, Riely GJ, Tykocinski ML. Placental protein 14 functions as a direct T-cell inhibitor. Cell Immunol. 1999;191:26–33. doi: 10.1006/cimm.1998.1408. [DOI] [PubMed] [Google Scholar]
- 101.Julkunen M, Rutanen EM, Koskimies A, Ranta T, Bohn H, Seppala M. Distribution of placental protein 14 in tissues and body fluids during pregnancy. BJOG. 1985;92:1145–51. doi: 10.1111/j.1471-0528.1985.tb03027.x. [DOI] [PubMed] [Google Scholar]
- 102.Than GN, Tatra G, Szabo DG, Csaba IF, Bohn H. Beta lactoglobulin homologue placental protein 14 (PP14) in serum of patients with trophoblastic disease and non-trophoblastic gynecologic malignancy. Arch Gynecol Obstet. 1988;243:131–7. doi: 10.1007/BF00932079. [DOI] [PubMed] [Google Scholar]
- 103.Joshi SG, Smith RA, Stokes DK. A progestagen-dependent endometrial protein in human amniotic fluid. J Reprod Fertil. 1980;60:317–21. doi: 10.1530/jrf.0.0600317. [DOI] [PubMed] [Google Scholar]
- 104.Riittinen L, Julkunen M, Seppala M, Koistinen R, Huhtala ML. Purification and characterization of endometrial protein PP14 from midtrimester amniotic fluid. Clin Chim Acta. 1989;184:19–29. doi: 10.1016/0009-8981(89)90253-2. [DOI] [PubMed] [Google Scholar]
- 105.Chatzakis K, Wathen N, Campbell J, Iles R, Dawnay A, Chard T. Dramatic increase in levels of placental protein 14 in amniotic fluid at 10–15 weeks’ pregnancy. Early Hum Dev. 1994;36:113–6. doi: 10.1016/0378-3782(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 106.Julkunen M, Koistinen R, Sjoberg J, Rutanen EM, Wahlstrom T, Seppala M. Secretory endometrium synthesizes placental protein 14. Endocrinology. 1986;118:1782–6. doi: 10.1210/endo-118-5-1782. [DOI] [PubMed] [Google Scholar]
- 107.Jeschke U, Kunert-Keil C, Mylonas I, et al. Expression of glycodelin A in decidual tissue of preeclamptic, HELLP and intrauterine growth-restricted pregnancies. Virchows Arch. 2005;446:360–8. doi: 10.1007/s00428-004-1201-3. [DOI] [PubMed] [Google Scholar]
- 108.Connor JP, Brudney A, Ferrer K, Fazleabas AT. Glycodelin-A expression in the uterine cervix. Gynecol Oncol. 2000;79:216–9. doi: 10.1006/gyno.2000.5941. [DOI] [PubMed] [Google Scholar]
- 109.Toth B, Roth K, Kunert-Keil C, et al. Glycodelin protein and mRNA is downregulated in human first trimester abortion and partially up-regulated in mole pregnancy. J Histochem Cytochem. 2008;56:477–85. doi: 10.1369/jhc.2008.950600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foth D, Romer T. Glycodelin serum levels in women with ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;108:199–202. doi: 10.1016/s0301-2115(02)00437-2. [DOI] [PubMed] [Google Scholar]
- 111.Tee MK, Vigne JL, Yu J, Taylor RN. Natural and recombinant human glycodelin activate a proapoptotic gene cascade in monocyte cells. J Leukoc Biol. 2008;83:843–52. doi: 10.1189/jlb.0406291. [DOI] [PubMed] [Google Scholar]
- 112.Lam KK, Chiu PC, Chung MK, et al. Glycodelin-A as a modulator of trophoblast invasion. Hum Reprod. 2009;24:2093–103. doi: 10.1093/humrep/dep205. [DOI] [PubMed] [Google Scholar]
- 113.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abbaszade I, Liu RQ, Yang F, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274:23443–50. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- 115.Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999;274:25555–63. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- 116.Zhu H, Leung PC, MacCalman CD. Expression of ADAMTS-5/implantin in human decidual stromal cells: regulatory effects of cytokines. Hum Reprod. 2007;22:63–74. doi: 10.1093/humrep/del356. [DOI] [PubMed] [Google Scholar]
- 117.Hassan SS, Romero R, Haddad R, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–86. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 118.Meinert M, Malmstrom A, Tufvesson E, et al. Labour induces increased concentrations of biglycan and hyaluronan in human fetal membranes. Placenta. 2007;28:482–6. doi: 10.1016/j.placenta.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 119.Lappas M, Riley C, Rice GE, Permezel M. Increased expression of ac-FoxO1 protein in prelabor fetal membranes overlying the cervix: possible role in human fetal membrane rupture. Reprod Sci. 2009;16:635–41. doi: 10.1177/1933719109332831. [DOI] [PubMed] [Google Scholar]
- 120.Moore RM, Redline RW, Kumar D, et al. Differential expression of fibulin family proteins in the paracervical weak zone and other areas of human fetal membranes. Placenta. 2009;30:335–41. doi: 10.1016/j.placenta.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bollopragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200:104–11. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]