Abstract
The proliferation of eukaryotic cells is a highly regulated process that depends on the precise duplication of chromosomal DNA in each cell cycle. Regulation of the replication licensing system, which promotes the assembly of complexes of proteins termed Mcm2-7 onto replication origins, is responsible for preventing re-replication of DNA in a single cell cycle. Recent work has shown how the licensing system is directly controlled by cyclin-dependent kinases (CDKs). Repression of origin licensing is emerging as a ubiquitous route by which proliferative capacity is lowered, and Mcm2-7 proteins show promise as diagnostic markers of early cancer stages. These results have prompted us to propose a functional distinction between the proliferative state and the non-proliferative state (including G0) depending on whether origins are licensed.
Keywords: Replication licensing, DNA replication, Mcm2-7, cell proliferation, cell cycle, quiescence
If genetic stability is to be maintained, chromosomal DNA must be precisely duplicated in each cell cycle. In order to achieve this, the DNA polymerases and other DNA processing enzymes working at the replication fork must copy the template DNA with high accuracy. It is also crucial that the replication forks are assembled and disassembled correctly to ensure that all the genome is replicated and that no section of it is replicated more than once. This is a particular problem for eukaryotes, whose very large genomes mean that they must use thousands of replication origins (which each initiate a bi-directional pair of replication forks) to duplicate the entire genome in a reasonable period of time. The importance of strict cell cycle regulation of replication origins is outlined in Box 1. Here we review recent developments in our understanding of the replication licensing system which normally prevents replication origins from firing (initiating a bi-directional pair of forks) more than once in each cell cycle1-5. These recent results suggest that not only is the licensing system the key to ensuring precise chromosome duplication, but that it also plays an important role in determining the proliferative capacity of cells and has potentially important implications for cancer diagnostics.
Box 1.
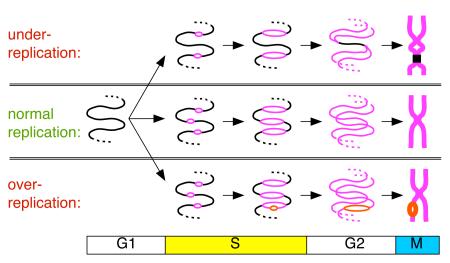
Ensuring precise chromosome replication. A small segment of chromosomal DNA, replicated from 3 origins is shown during the cell cycle. Middle panel: successful duplication. Top panel: under-replication due to the failure of one of the origins to fire. As sister chromatids are separated during anaphase, the chromosome is likely to be broken near the unreplicated section. Bottom panel: over-replication, due to one of the origins firing a second time in S phase. The local duplication of DNA in the vicinity of the over-firing origin is likley to represent an irreversible genetic change, and might be resolved to form a tandem duplication.
 unreplicated DNA;
unreplicated DNA;  replicated DNA;
replicated DNA;  re-replicated DNA The four stages of the cell cycle - G1, S (DNA synthesis), G2 and M (mitosis) - are shown below.
re-replicated DNA The four stages of the cell cycle - G1, S (DNA synthesis), G2 and M (mitosis) - are shown below.
The replication licensing system
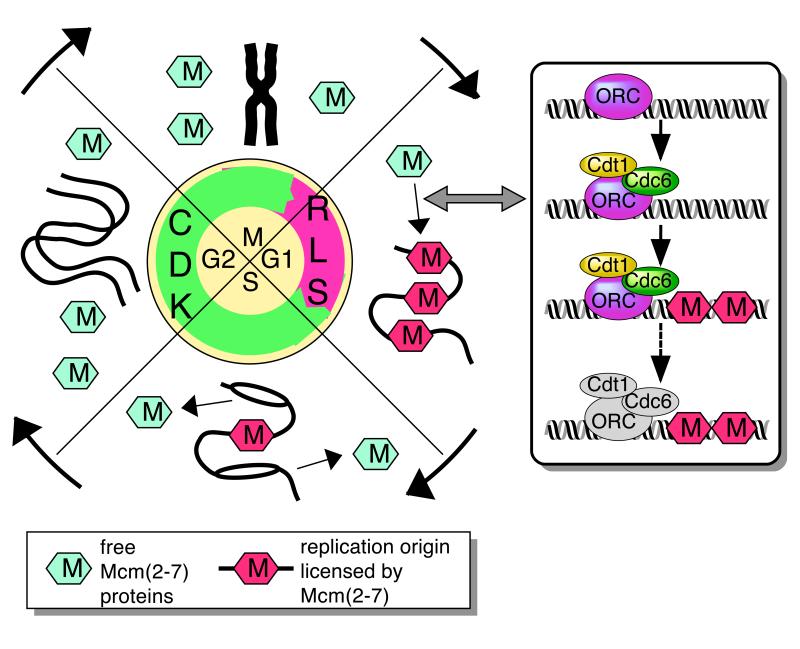
How does the cell know whether or not it has already replicated a section of DNA in S phase? What ensures the strict alternation of S phase and mitosis seen in most cells? Recent work has made it clear that the “licensing” of replication origins, achieved by their loading of mini-chromosome-maintenance 2-7 proteins (Mcm2, 3, 4, 5, 6 and 7), provides crucial information to the cell about whether the DNA has been replicated in the current cell cycle1-5. The Mcm2-7 polypeptides form a functional hexameric complex6 that probably comprises an important part of the “pre-Replicative Complex” of replication proteins found at replication origins during G1 phase. On exit from metaphase the replication licensing system becomes activated, so that each origin becomes loaded with Mcm2-7 (Fig 1). The replication licensing system (‘RLS’ in Fig 1) remains active throughout most of G1, but as cells approach S phase, it is inactivated. This means that in S phase, G2 and early mitosis, no further Mcm2-7 can be loaded onto origins. Only licensed origins containing Mcm2-7 can initiate a pair of replication forks, and as initiation occurs at an origin, the bound Mcm2-7 is displaced so that the origin cannot fire again (Fig 1). Mcm2-7 may function as the helicase that unwinds DNA ahead of each replication fork7, which would explain their displacement from origin DNA when forks are initiated. As a fail-safe mechanism, it is also envisaged that should an origin be passively replicated by a replication fork emanating from another origin, this would also displace the Mcm2-7 bound to it.
Figure 1.
Replication licensing and CDK activity through the cell cycle.
A small segment of chromosomal DNA is depicted at different cell cycle stages, with bound (red hexagons, M) or unbound (blue hexagons, M) Mcm2-7. The activity of the replication licensing system (RLS, purple) and cyclin-dependent kinases (CDK, green) at the different stages are show in the central circle. Inset, the sequence of events occurring as each origin binds Mcm2-7 and becomes licensed. The loading of ORC (purple), Cdt1 (yellow) and Cdc6 (green), and then Mcm2-7 (red, M) is shown. At some time after licensing is complete, ORC, Cdc6 and Cdt1 may become inactivated, as indicated by grey shading.
At least three other proteins are required for origins to load Mcm2-7 and become licensed (Fig 1, inset). The Origin Recognition Complex (ORC) first binds to each replication origin, and then recruits two other proteins, Cdc6 (called Cdc18 in Schizosaccharomyces pombe ) and Cdt1 (also known as RLF-B or double-parked)2-4,8,9. These proteins in turn load Mcm2-7 complexes and functionally license the origin. A crucial feature is that although ORC, Cdc6 and Cdt1 are all essential for Mcm2-7 loading, none of them are subsequently required to maintain the binding of Mcm2-7 to origins10-13. Furthermore it has been shown in Xenopus that once origin licensing is complete, ORC and Cdc6 (and probably Cdt1 as well) are no longer required for subsequent DNA replication11,12. As discussed below, an important consequence is that re-licensing of replicated DNA can be prevented by inhibition or removal of ORC, Cdc6 or Cdt1 once S phase has started, without displacing functional Mcm2-7 at licensed origins.
Overall control of origin licensing by cyclin-dependent kinases
Cyclin-dependent kinases (CDKs), master regulators of the cell cycle, are activated during late G1, where they eventually induce cells to progress through S phase, G2 and then mitosis; CDK activity is then abolished during late mitosis. Significantly, the licensing system is only active during late mitosis and G1 when CDK activity is low (Fig 1). Experiments performed in the fission yeast S. pombe first showed that if CDK activity were temporarily inhibited in cells at the G2 phase of the cell cycle, they would re-replicate their DNA14,15. This suggests that re-loading of Mcm(2-7) occurs in these CDK-deficient G2 cells. Subsequent work in a range of different organisms has supported this model by showing that CDKs can strongly inhibit the licensing system16-21. However, high CDK levels do not displace Mcm2-7 already bound to origins11,12, thus allowing the normal S phase programme to occur in the presence of S-phase-inducing CDKs (Fig 1). It is possible that different CDKs may differ in their ability to inhibit origin licensing, and CDKs normally active in G1 to prepare cells for S phase may be less repressive (see below).
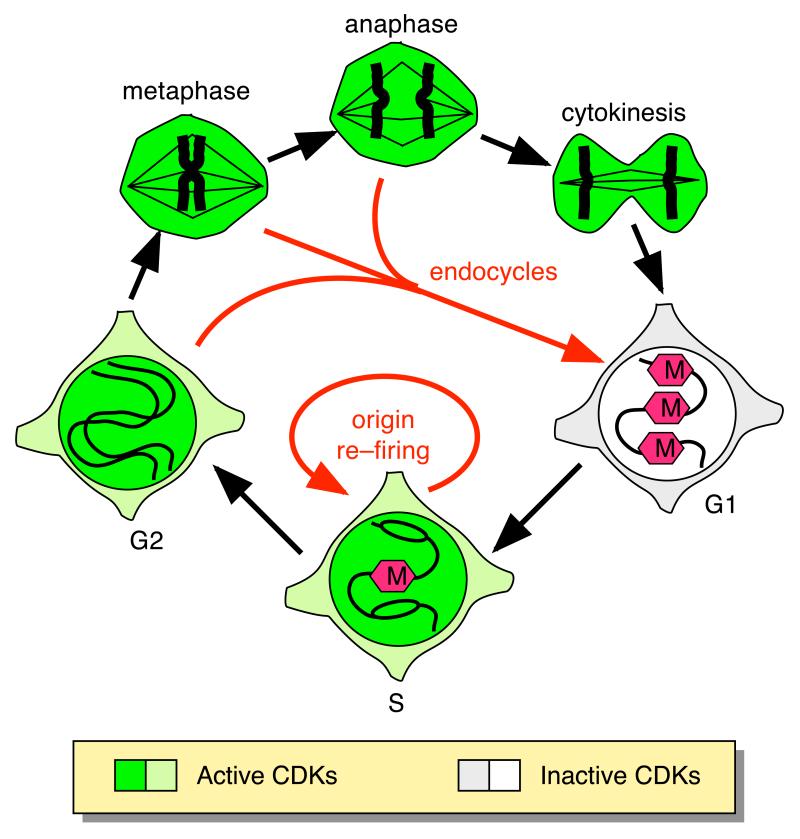
Sometimes cells undergo unusual cell cycles where the daughter cells acquire more DNA than their parents. This can occur as a consequence of developmental signals (such as in the increase in chromosome number seen during cardiac myocyte development), or as a consequence of some insult to the cell (such as chemically-induced polyploidy). It is useful to separate these unusual cell cycles into two distinct classes, shown schematically in Figure 2. The first class comprises endoreplication cell cycles (endocycles)22, where periods of alternating high and low CDK activity still occur. In endocycles, re-licensing of origins occurs when the CDK activity present in G2 or mitotic cells is abolished before cytokinesis has occurred. This G1 re-entry may occur from G2, metaphase or anaphase. The duplicated genomes are held in a single nucleus except in the case where nuclear division is completed and cytokinesis alone is suppressed, giving a binucleate cell. An example is the endoreplication cycles occurring during Drosophila development, where transient interruption of CDK levels after DNA replication leads to cycles of Mcm2-7 reloading and near-complete re-replication of the DNA when CDK levels are restored23-25. Because endocycles drive complete S phases, the resultant cells should have DNA contents that are powers of 2 (2, 4, 8 ,16 etc) larger than the DNA content in the starting cell. When mitotic CDK activation is abolished by mutation of the cdc13 (cyclin B) gene in S. pombe, multiple complete rounds of replication are seen and the cellular DNA content approximates this power series15.
Figure 2.
Different routes by which cellular DNA content may increase.
A small segment of chromosomal DNA is depicted at different cell cycle stages. Red hexagons show Mcm2-7 bound to origins in G1 and S. Outer ring (black arrows): the normal sequence of cell cycle events. Red arrows show routes by which cellular DNA content is increased (endocycles and origin re-firing). CDK activity in S, G2 and M is denoted by green colouring.
The second way that DNA content might increase is shown in the lower part of Figure 2. In this case, the cell re-licenses and re-initiates forks at one or more replication origins before S phase has been completed (origin re-firing). The S phase CDK levels do not drop, but re-licensing occurs because the licensing components (ORC, Cdc6, Cdt1 or Mcm2-7) cannot respond to the inhibitory CDK signal. Since the re-licensing and re-replication of one particular origin would not be co-ordinated with any other origin, the resultant cellular DNA content would not represent any simple multiple of the original DNA content. If certain origins are more prone to re-firing, there would be localised amplification of the DNA surrounding them (much as is shown in the bottom panel of Box 1). One of the best studied examples of this phenomenon in a physiological system occurs within ovarian follicle cells during Drosophila oogenesis, where certain genes are amplified to ensure sufficient production of chorion proteins that make up the eggshell. This occurs by the repeated initiation of replication origins close to the amplified genes in the presence of constant CDK levels22,26.
CDK targets for preventing re-licensing of origins
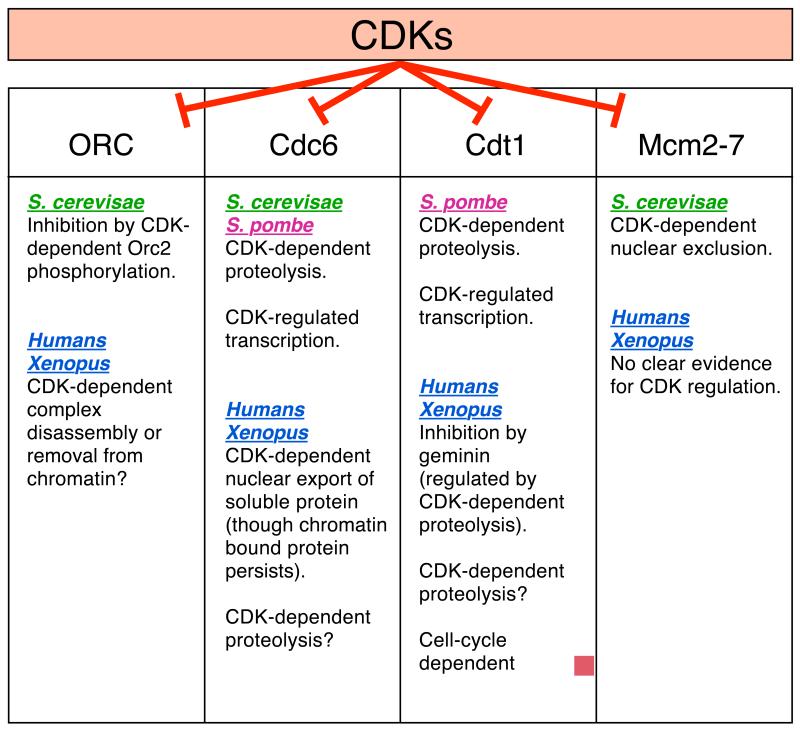
Significant progress has recently been made in understanding the mechanisms by which CDKs inhibit replication licensing. Mounting evidence suggests that CDKs are likely to block licensing by a number of partially redundant mechanisms, which is perhaps not surprising given the importance of preventing over-replication of genomic DNA. Indeed, it appears that all four proteins known to be involved in origin licensing (ORC, Cdc6, Cdt1 and Mcm2-7) may each be independently downregulated as a consequence of CDK activity. The redundancy of CDK control over licensing is dramatically demonstrated in a recent paper by Nguyen et al5. When CDK inhibition of ORC, Cdc6 and Mcm2-7 was specifically abrogated in the yeast Saccharomyces cerevisiae, partial re-replication of the genome occurred. This strongly supports the idea that the role of CDKs in preventing re-replication of DNA is primarily mediated by preventing re-licensing of DNA. Abrogation of CDK regulation on individual pairs of these proteins (ORC+Cdc6, ORC+Mcm2-7 or Cdc6+Mcm2-7) failed to induce significant re-replication of DNA, thus demonstrating the redundancy of the CDK control mechanisms. Since re-replication was only partial when all three of these CDK controls were abolished5, there may be further pathways preventing re-replication that were still active in these cells. It is also consistent with re-replication being due to “origin re-firing” (Fig 2) rather than by CDK oscillation.
Precisely how do CDKs block origin licensing? As discussed in more detail below, each of the four origin proteins (ORC, Cdc6, Cdt1 and Mcm2-7) may be independently regulated by CDKs. Figure 3 summarises these different mechanisms. This is not a complete list, and further inhibitory pathways may well be discovered. One striking feature shown in Figure 3 is that although the four origin proteins are highly conserved throughout the eukaryotic kingdom, the way that they are regulated by CDKs differ significantly. The regulation of these four different proteins by CDKs will now be discussed in turn.
Figure 3.
Pathways for CDK inhibition of origin licensing.
The ways that CDKs inhibit the activity of ORC, Cdc6, Cdt1 and Mcm2-7 in S. cerevisiae, S. pombe, Xenopus and humans.
CDK regulation of ORC
In S. cerevisiae, the Orc2 subunit of ORC is phosphorylated by CDKs in late G1, S phase, G2 and mitosis5. When these phosphorylation sites were genetically removed from the ORC2 gene, cells could undergo partial re-replication under conditions where Cdc6 and Mcm2-7 regulation by CDKs had been abrogated. The phosphorylation of Orc2 during the later stages of the cell cycle appears to be highly conserved throughout eukaryotic evolution, and has also been reported in S. pombe and Xenopus (frog). Phosphorylation of the S. pombe Orc2 homologue (Orp2) by CDK has recently been shown to regulate activity of the ORC complex, as deletion of CDK phosphorylation sites enhances the re-replication seen when the S. pombe Cdc6 homologue (Cdc18) is over-expressed27. The situation in metazoans appears somewhat more complex. In Xenopus, exposure of chromatin to high CDK levels (such as occur during mitosis) can release ORC from chromatin11,12, and this appears to play a small but significant role in preventing origin re-licensing8. Although ORC appears to bind DNA with increased affinity in early G1 when CDK activity is low, binding then weakens, not due to increases in CDK activity, but as a consequence of origin licensing (“licensing-dependent origin inactivation”), a process which may help to prevent re-licensing of origins later in the cell cycle12. In mammalian cells there is also evidence that the Orc1 subunit of ORC can be released from DNA during S phase and mitosis, leaving Orc2 still bound28,29. This effect is likely to be due to the high CDK levels present at this stage.
CDK regulation of Cdc6
In yeasts, Cdc6 abundance is tightly controlled during the cell division cycle. CDC6 transcription peaks during late mitosis and G130-32, with transcription being under the control of the CDK-dependent transcription factor SWI4/Cdc1031,33. Protein levels are also controlled by cell-cycle specific degradation, probably involving two or more different pathways34,35, but again controlled by CDK activity34-36. To abolish both regulatory mechanisms and to permit DNA re-replication in S. cerevisiae, Nguyen et al5 therefore expressed from a constitutively active promoter a truncated form of Cdc6 that is resistant to cell-cycle specific degradation.
In vertebrate cells, the regulation of Cdc6 is less well understood. Cdc6 appears to be present throughout the vertebrate cell cycle. Although cell cycle-dependent degradation of Cdc6 protein is seen, significant amounts of chromatin-bound Cdc6 are observed during G1, S and G2 phases37,38. Much of the soluble Cdc6 protein, however, is translocated from the nucleus to the cytoplasm when CDKs are activated in late G1, thus preventing it from further interaction with replication origins39-41. One possible explanation for these apparently contradictory results is that the chromatin-bound form of Cdc6 seen in S and G2 is not competent to support origin licensing, but may play another role in cell cycle regulation.
CDK regulation of Cdt1
First identified in S. pombe as a gene induced by the CDK-dependent transcription factor Cdc10, Cdt1 mRNA and protein levels peak in late mitosis and early G142-44. In HeLa cells, levels of Cdt1 also peak during G143,45. Since a Cdt1 homologue has not yet been identified in S. cerevisiae, it is possible that the reason why re-replication was only partial in S. cerevisiae cells containing unregulated ORC, Cdc6 and Mcm2-7 was due to Cdt1 activity being inhibited by CDKs5.
Vertebrate cells are also able to control Cdt1 activity through a specific inhibitor called geminin8,45,46; no geminin homologue has yet been identified in yeast. In metaphase-arrested Xenopus eggs, geminin inhibition of Cdt1 is the major pathway for prevention of origin licensing8. The abundance of geminin, however, has been shown to be indirectly regulated by CDKs. Geminin is specifically degraded during late mitosis by the Anaphase Promoting Complex (APC/C) that is also responsible for degrading cyclins and which itself is regulated by CDKs46. In mammalian cells, geminin levels remain low throughout G1 (when the APC/C is active) and levels rise again during S and G2, when the APC/C becomes inactivated and CDK levels rise45,46. APC/C regulation has been suggested as a means by which the specialized endocycles of Drosophila, mentioned above, are controlled22.
CDK regulation of Mcm2-7
In S. cerevisiae, Mcm2-7 are present in the nucleoplasm only during late mitosis and early G1. Soluble Mcm2-7 are excluded from nuclei at other stages of the cell cycle, though origin-bound Mcm2-7 is still seen in S phase nuclei47-49. The nuclear exclusion of Mcm2-7 is dependent on the presence of CDK activity. Exclusion can be mediated by the S. cerevisiae G1 cyclins (Clns) as well as the B-type cyclins (Clbs)48, though the B-type cyclins may be more effective49. This nuclear exclusion, which formed a major part of the original licensing factor model1 is an effective way of preventing re-replication of DNA as it separates the licence (Mcm2-7) from its substrate (DNA). However, there is little evidence that Mcm2-7 activity is regulated by subcellular localisation in other organisms. Mcm2-7 has been reported to be constitutively nuclear in S. pombe, Drosophila, Xenopus and a range of mammalian cells. Indeed, there is little evidence that Mcm2-7 are negatively regulated late in the cell cycle of other organisms (e.g. ref. 19). However, the possibility of undiscovered modes of Mcm2-7 regulation (such as controlling its assembly into an active hexamer6) is still open.
Licensing and the definition of the proliferative state
Only a small proportion of the cells that make up a multicellular organism are likely to be actively engaged in the cell division cycle at any one time. Some non-proliferating cells will have permanently withdrawn from the cell cycle as a result of terminal differentiation or senescence. Others can be easily stimulated to divide again by appropriate environmental signals such as growth factors; these cells are said to be ‘quiescent’, or in the G0 phase of the cell cycle. Evidence from a range of different organisms and cell types suggest that both G0 and permanently arrested cells have lost Mcm2-7 proteins and are functionally unlicensed50-56. Not only is Mcm2-7 removed from DNA as cells pass into G0, but the unbound protein is also lost from the cells. A similar reduction in Cdc6 protein is seen in G0 and permanently arrested cells53,54,56-58. ORC levels, however, seem to remain high on progression into quiescence52,53. The persistence of ORC in non-proliferating cells is consistent with it having a function in these cells that is independent of DNA replication, such as transcriptional silencing59.
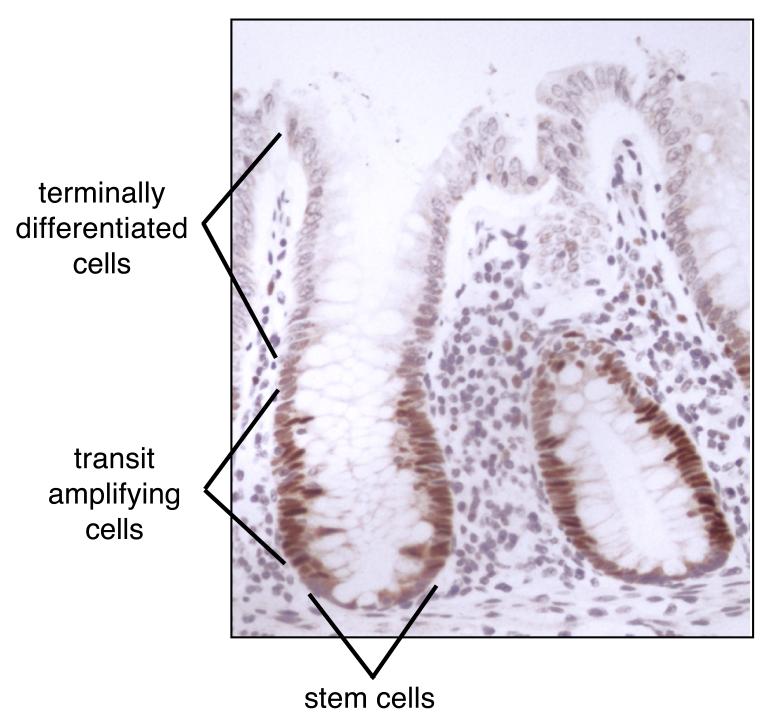
A recent study of Mcm2-7 proteins in a variety of human tissues by Stoeber et al56 consolidates these results, and suggests that the removal of the replication licence is a common pathway by which proliferation is restrained. An example is shown in Fig 4, where a section of human colon has been immunostained for Mcm2. The terminally differentiated (non-proliferating) cells at the top of the crypt contain virtually no Mcm2. At the base of the crypts are the stem cells, which although having a high capacity for self-renewal, divide relatively infrequently. These stem cells contain intermediate levels of Mcm2, consistent with them being at different points through the G1 - G0 transition. Between the stem cells and the terminally differentiated cells are the transit amplifying cells which are actively proliferating. These proliferating cells stain the most strongly for Mcm2.
Figure 4.
Mcm2 protein expression in differentiating colonic epithelial cells.
Indirect immunoperoxidase staining of colon with an anti-Mcm2 antibody. Anatomical regions containing stem cells, rapidly proliferating cells and terminally differentiated cells are indicated. Reproduced from ref. 56 with permission.
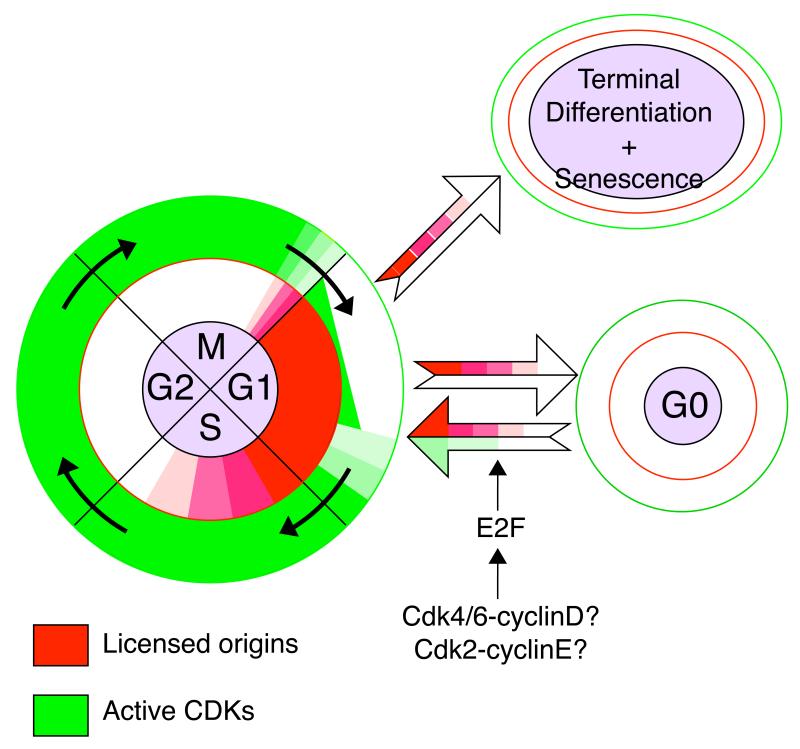
The correlation between entry into G0 and terminal differentiation with loss of Mcm2-7 is shown schematically in Figure 5. During late mitosis and early G1, replication origins become licensed, and they stay licensed throughout the G1 period. From G1, cells have the choice to either enter S phase or to withdraw from the cell cycle. Entry into S phase is driven by rising CDK activity, which leads to the initiation of DNA replication and subsequent removal of Mcm2-7. Withdrawal from the cell cycle is accompanied by the gradual loss of Mcm2-7 in the absence of CDK activity or DNA replication. On re-entry into the cell cycle from G0, origins are re-licensed and only then can CDK activity induce entry into S phase53. The licensing pathway that is followed during the G0/G1 transition may well be regulated differently from the licensing pathway that occurs on exit from mitosis. In particular, many of the pre-RC genes involved in licensing appear to have binding sites for the E2F transcription factor which is active during late G1 or on exit from G09,51,57,60-65. Full E2F activity is dependent on Cdk4/6-cyclin D and Cdk2-cyclin E, suggesting that at the levels necessary to activate E2F, these particular CDKs may not inhibit licensing (Fig 5).
Figure 5.
Entry and exit of cycling cells into quiescence and terminal differentiation.
On the left is a cartoon of cells passing through four phases of the cell division cycle. The presence of licensed origins is shown in red, and the presence of active CDKs is shown in green. When cells pass from G1 into G0 or terminal differentiation, origins become unlicensed. When G0 cells are stimulated to re-enter G1 their origins become re-licensed. It is currently unclear whether CDK reactivation of the E2F transcription system is required for this re-licensing to occur.
Detection of Mcm2-7 is therefore a powerful way of assessing the proliferative potential of cells. Since there is currently no formal way of distinguishing G1 from G0 cells, we would like to propose that the description of cells being in G1 should be limited only to cells with licensed origins. The G0 state can then be defined as: a reversible withdrawal from the cell cycle characterised by unlicensed origins and the absence of CDK activity. We believe that this definition would be useful as it has clear functional significance in distinguishing G0 or differentiated cells that are not proliferating from G1 cells engaged in the cell division cycle. This distinction, between cells containing Mcm2-7 and cells lacking it, has potential as a diagnostic marker since cancers with a high proportion of proliferating cells (high growth fraction) should stain strongly for Mcm2-754,56,66,67. This powerful new approach has already given promising results in the diagnosis of cervical54, urothelial66 and bronchial cancers67.
In their survey of Mcm2-7 levels in different human tissues, Stoeber et al56 reported that the glandular epithelial cells of the breast showed an interesting exception to the correlation between Mcm2-7 presence and active proliferation. In breast cells from non-pregnant and non-lactating women, a large percentage of glandular epithelial cells (47-65%) expressed Mcm2-7 while only a small percentage (~6%) showed signs of proliferation. During pregnancy, when these cells proliferate rapidly, almost of all them contained Mcm2-7, but expression plunged to <3% during lactation when the glandular epithelial cells undergo differentiation to the secretory state. The persistence of Mcm2-7 in non-proliferating breast may be an evolutionary relic from times when women spent most of their fertile years either pregnant or lactating, and when it was therefore unnecessary for these cells to be able to withdraw from the G1 state. An important question raised by this finding is whether licensed but slowly proliferating cells such as these have a higher risk of undergoing malignant transformation. Since the lack of origin licensing may be an important mechanism restraining the proliferation of G0 cells, it is possible that failure to down-regulate the licensing system (as in these breast cells) may make transition to uncontrolled proliferation significantly easier to achieve. These new observations may therefore have important implications for the pathogenesis of breast cancer, and urgently demand further study.
Concluding Remarks
The results discussed in this review show that the replication licensing system not only plays a central role in ensuring precise chromosome duplication in each cell cycle, but it also plays an important role in down-regulating the proliferative capacity of cells when they withdraw from the cell cycle. This conclusion appears to have important practical implications for cancer diagnosis, which we expect will be consolidated by future work.
Acknowledgements
Thanks to Inke Näthke and Margret Michalski-Blow for comments on the manuscript. JJB is supported by Cancer Research Campaign [CRC] grant SP2385/0101. BJH is supported by a UBI studentship.
Glossary
- CDK
cyclin dependent kinase, consisting of a small kinase subunit complexed with an activating cyclin subunit. Key activator of cell cycle transitions.
- Licensing
the loading of functional Mcm2-7 hexamers onto replication origins to enable them to support a single round of replication.
- Mcm2-7
6 related minichromosome maintenance proteins (Mcm2, 3, 4, 5, 6 and 7) found in a hexameric complex.
- ORC
the origin recognition complex, comprising 6 polypeptides: Orc1, 2, 3, 4, 5 and 6.
- pre-RC
the pre-replicative complex of proteins bound to replication origins during G1 but not G2 of the cell cycle.
- Replication licensing system
the set of proteins required to license replication origins.
- Replication origin
a site on chromosomal DNA where a bi-directional pair of replication forks initiate.
References
- 1.Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- 2.Diffley JFX. Building the perfect switch. Current Biol. 2001;11:R367–370. doi: 10.1016/s0960-9822(01)00196-8. [DOI] [PubMed] [Google Scholar]
- 3.Lei M, Tye BK. Initiating DNA synthesis: from recruiting to activating the MCM complex. J. Cell Sci. 2001;114:1447–1454. doi: 10.1242/jcs.114.8.1447. [DOI] [PubMed] [Google Scholar]
- 4.Blow JJ. Control of chromosomal DNA replication in the early Xenopus embryo. EMBO J. 2001;20:3293–3297. doi: 10.1093/emboj/20.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen VQ, et al. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature. 2001;411:1068–1073. doi: 10.1038/35082600. [DOI] [PubMed] [Google Scholar]
- 6.Prokhorova TA, Blow JJ. Sequential MCM/P1 subcomplex assembly is required to form a heterohexamer with replication licensing activity. J. Biol. Chem. 2000;275:2491–2498. doi: 10.1074/jbc.275.4.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labib K, Diffley JF. Is the MCM2-7 complex the eukaryotic DNA replication fork helicase? Curr. Opin. Genet. Dev. 2001;11:64–70. doi: 10.1016/s0959-437x(00)00158-1. [DOI] [PubMed] [Google Scholar]
- 8.Tada S, et al. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat. Cell Biol. 2001;3:107–113. doi: 10.1038/35055000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker AJ, et al. Drosophila Double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 2000;14:1765–1776. [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan S, et al. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5611–5616. doi: 10.1073/pnas.94.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua XH, Newport J. Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J. Cell Biol. 1998;140:271–281. doi: 10.1083/jcb.140.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowles A, et al. Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J. Cell Sci. 1999;112:2011–2018. doi: 10.1242/jcs.112.12.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiorano D, et al. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- 14.Broek D, et al. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- 15.Hayles J, et al. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 16.Dahmann C, et al. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Current Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- 17.Hua XH, et al. A role for Cdk2 kinase in negatively regulating DNA replication during S phase of the cell cycle. J. Cell Biol. 1997;137:183–192. doi: 10.1083/jcb.137.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itzhaki JE, et al. Construction by gene targeting in human cells of a ‘conditional’ CDC2 mutant that rereplicates its DNA. Nature Genet. 1997;15:258–265. doi: 10.1038/ng0397-258. [DOI] [PubMed] [Google Scholar]
- 19.Mahbubani HM, et al. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J. Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates S, et al. Cell cycle arrest and DNA endoreduplication following p21(Waf1/Cip1) expression. Oncogene. 1998;17:1691–1703. doi: 10.1038/sj.onc.1202104. [DOI] [PubMed] [Google Scholar]
- 21.Noton E, Diffley JFX. CDK inactivation is the only essential function of the APC/C and the mitotic exit network proteins for origin resetting during mitosis. Mol. Cell. 2000;5:85–95. doi: 10.1016/s1097-2765(00)80405-0. [DOI] [PubMed] [Google Scholar]
- 22.Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: More for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 23.Follette PJ, et al. Fluctuations in Cyclin E levels are required for multiple rounds of endocycle S phase in Drosophila. Current Biol. 1998;8:235–238. doi: 10.1016/s0960-9822(98)70089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su TT, O’Farrell PH. Chromosome association of minichromosome maintenance proteins in Drosophila endoreplication cycles. J. Cell Biol. 1998;140:451–460. doi: 10.1083/jcb.140.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss A, et al. Continuous Cyclin E expression inhibits progression through endoreduplication cycles in Drosophila. Current Biol. 1998;8:239–242. doi: 10.1016/s0960-9822(98)70090-9. [DOI] [PubMed] [Google Scholar]
- 26.Calvi BR, et al. Cell cycle control of chorion gene amplification. Genes Dev. 1998;12:734–744. doi: 10.1101/gad.12.5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vas A, et al. Control of DNA rereplication via Cdc2 phosphorylation sites in the origin recognitiion complex. Mol. Cell. Biol. 2001;21:5767–5777. doi: 10.1128/MCB.21.17.5767-5777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natale DA, et al. Selective instability of Orc1 protein accounts for the absence of functional origin recognition complexes during the M-G(1) transition in mammals. EMBO J. 2000;19:2728–2738. doi: 10.1093/emboj/19.11.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreitz S, et al. The human origin recognition complex protein 1 dissociates from chromatin during S phase in HeLa cells. J. Biol. Chem. 2001;276:6337–6342. doi: 10.1074/jbc.M009473200. [DOI] [PubMed] [Google Scholar]
- 30.Zwerschke W, et al. The Saccharomyces cerevisiae Cdc6 gene is transcribed at late mitosis and encodes a ATP/GTPase controlling S-phase initiation. J. Biol. Chem. 1994;269:23351–23356. [PubMed] [Google Scholar]
- 31.McInerny CJ, et al. A novel Mcm1-dependent element in the SWI4, CLN3, CDC6, and CDC47 promoters activates M/G(1)-specific transcription. Genes Dev. 1997;11:1277–1288. doi: 10.1101/gad.11.10.1277. [DOI] [PubMed] [Google Scholar]
- 32.Baum B, et al. Cdc18 transcription and proteolysis couple S phase to passage through mitosis. EMBO J. 1998;17:5689–5698. doi: 10.1093/emboj/17.19.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly TJ, et al. The fission yeast Cdc18+ gene-product couples S-phase to Start and mitosis. Cell. 1993;74:371–382. doi: 10.1016/0092-8674(93)90427-r. [DOI] [PubMed] [Google Scholar]
- 34.Elsasser S, et al. Phosphorylation controls timing of Cdc6p destruction: A biochemical analysis. Mol. Biol. Cell. 1999;10:3263–3277. doi: 10.1091/mbc.10.10.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drury LS, et al. The cyclin-dependent kinase Cdc28p regulates distinct modes of Cdc6p proteolysis during the budding yeast cell cycle. Current Biol. 2000;10:231–240. doi: 10.1016/s0960-9822(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 36.Jallepalli PV, et al. Regulation of the replication initiator protein p65(cdc18) by CDK phosphorylation. Genes Dev. 1997;11:2767–2779. doi: 10.1101/gad.11.21.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coverley D, et al. Chromatin-bound Cdc6 persists in S and G(2) phases in human cells, while soluble Cdc6 is destroyed in a cyclin A-cdk2 dependent process. J. Cell Sci. 2000;113:1929–1938. doi: 10.1242/jcs.113.11.1929. [DOI] [PubMed] [Google Scholar]
- 38.Mendez J, Stillman B. Chromatin association of human origin recognition complex, Cdc6, and minichromosome maintenance proteins during the cell cycle: Assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha P, et al. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol. Cell. Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen BO, et al. Phosphorylation of mammalian CDC6 by Cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delmolino LM, et al. Multiple mechanisms regulate subcellular localization of human CDC6. J. Biol. Chem. 2001;276:26947–26954. doi: 10.1074/jbc.M101870200. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann JF, Beach D. cdt1 is an essential target of the Cdc10/Sct1 transcription factor: requirement for DNA replication and inhibition of mitosis. EMBO J. 1994;13:425–434. doi: 10.1002/j.1460-2075.1994.tb06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishitani H, et al. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature. 2000;404:625–628. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- 44.Yanow SK, et al. Expression of Cdc18/Cdc6 and Cdt1 during G2 phase induces initiation of DNA replication. EMBO J. 2001;20:4648–4656. doi: 10.1093/emboj/20.17.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wohlschlegel JA, et al. Inhibition of eukaryotic replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 46.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 47.Hennessy KM, et al. Subcellular localization of yeast CDC46 varies with the cell cycle. Genes Dev. 1990;4:2252–2263. doi: 10.1101/gad.4.12b.2252. [DOI] [PubMed] [Google Scholar]
- 48.Labib K, et al. G1-phase and B-type cyclins exclude the DNA-replication factor Mcm4 from the nucleus. Nat. Cell Biol. 1999;1:415–422. doi: 10.1038/15649. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen VQ, et al. Clb/Cdc28 kinases promote nuclear export of the replication initiator proteins Mcm2-7. Current Biol. 2000;10:195–205. doi: 10.1016/s0960-9822(00)00337-7. [DOI] [PubMed] [Google Scholar]
- 50.Su TT, O’Farrell PH. Chromosome association of minichromosome maintenance proteins in Drosophila mitotic cycles. J Cell Biol. 1997;139:13–21. doi: 10.1083/jcb.139.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuruga H, et al. Expression, nuclear localization and interactions of human MCM/P1 proteins. Biochem. Biophys. Res. Commun. 1997;236:118–125. doi: 10.1006/bbrc.1997.6865. [DOI] [PubMed] [Google Scholar]
- 52.Musahl C, et al. Stability of the replicative Mcm3 protein in proliferating and differentiating human cells. Exp. Cell Res. 1998;241:260–264. doi: 10.1006/excr.1998.4041. [DOI] [PubMed] [Google Scholar]
- 53.Stoeber K, et al. Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. EMBO J. 1998;17:7219–7229. doi: 10.1093/emboj/17.24.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams GH, et al. Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14932–14937. doi: 10.1073/pnas.95.25.14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun W, et al. The replication capacity of intact mammalian nuclei in Xenopus egg extracts declines with quiescence, but the residual DNA synthesis is independent of Xenopus MCM proteins. J. Cell Sci. 2000;113:683–695. doi: 10.1242/jcs.113.4.683. [DOI] [PubMed] [Google Scholar]
- 56.Stoeber K, et al. DNA replication licensing and human cell proliferation. J. Cell Sci. 2001;114:2027–2041. doi: 10.1242/jcs.114.11.2027. [DOI] [PubMed] [Google Scholar]
- 57.Yan Z, et al. Cdc6 is regulated by E2F and is essential for DNA replication in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3603–3608. doi: 10.1073/pnas.95.7.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams RS, et al. A human protein related to yeast Cdc6p. Proc. Natl. Acad. Sci. U.S.A. 1997;94:142–147. doi: 10.1073/pnas.94.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox CA, et al. The origin recognition complex has essential functions in transcriptional silencing and chromosomal replication. Genes Dev. 1995;9:911–924. doi: 10.1101/gad.9.8.911. [DOI] [PubMed] [Google Scholar]
- 60.Asano M, Wharton RP. E2F mediates developmental and cell cycle regulation of ORC1 in Drosophila. EMBO J. 1999;18:2435–2448. doi: 10.1093/emboj/18.9.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leone G, et al. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev. 1998;12:2120–2130. doi: 10.1101/gad.12.14.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohtani K, et al. Regulation of cell growth-dependent expression of mammalian CDC6 gene by the cell cycle transcription factor E2F. Oncogene. 1998;17:1777–1785. doi: 10.1038/sj.onc.1202105. [DOI] [PubMed] [Google Scholar]
- 63.Ohtani K, et al. Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F. Oncogene. 1999;18:2299–2309. doi: 10.1038/sj.onc.1202544. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki S, et al. Cloning and characterization of human MCM7 promoter. Gene. 1998;216:85–91. doi: 10.1016/s0378-1119(98)00323-0. [DOI] [PubMed] [Google Scholar]
- 65.Tsuruga H, et al. HsMCM6: A new member of the human MCM/P1 family encodes a protein homologous to fission yeast Mis5. Genes Cells. 1997;2:381–399. doi: 10.1046/j.1365-2443.1997.1290327.x. [DOI] [PubMed] [Google Scholar]
- 66.Stoeber K, et al. Immunoassay for urothelial cancers that detects DNA replication protein Mcm5 in urine. Lancet. 1999;354:1524–1525. doi: 10.1016/S0140-6736(99)04265-8. [DOI] [PubMed] [Google Scholar]
- 67.Tan D-F, et al. MCM2 - a promising marker for premalignant lesions of the lung: a cohort study. BioMed Central Cancer. 2001;1:6. doi: 10.1186/1471-2407-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]