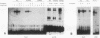Abstract
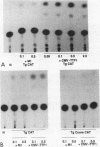
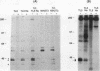
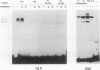
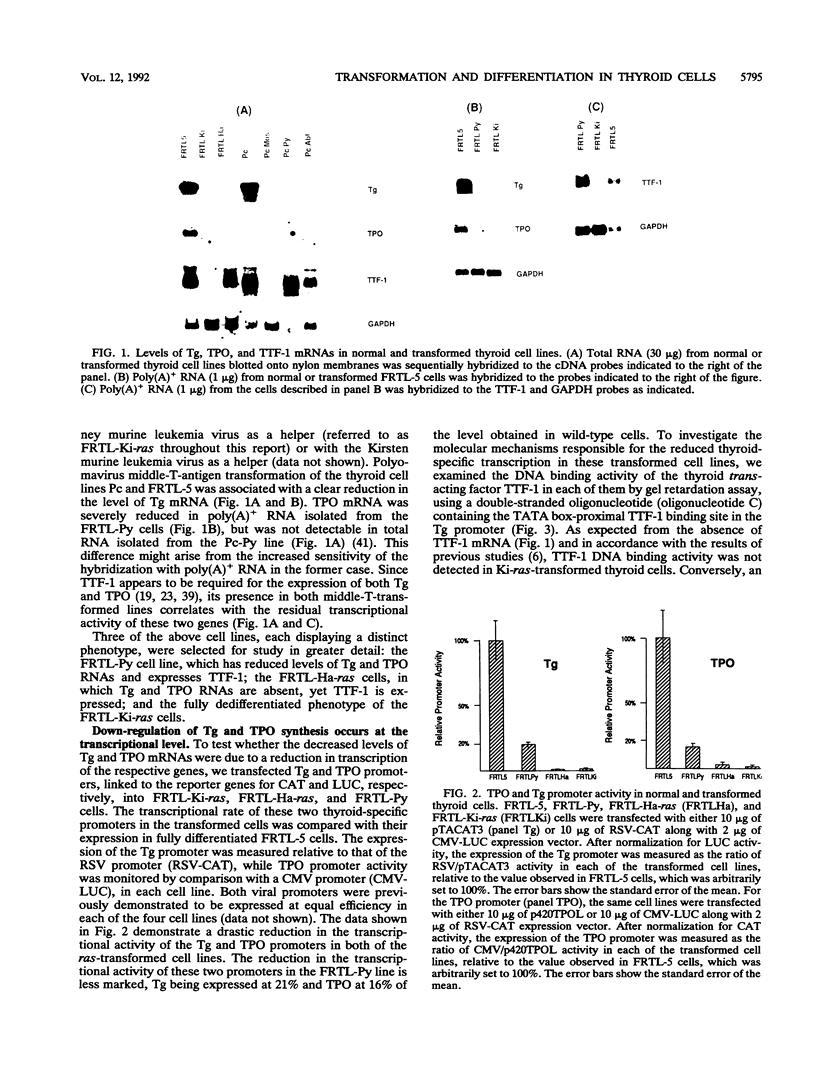
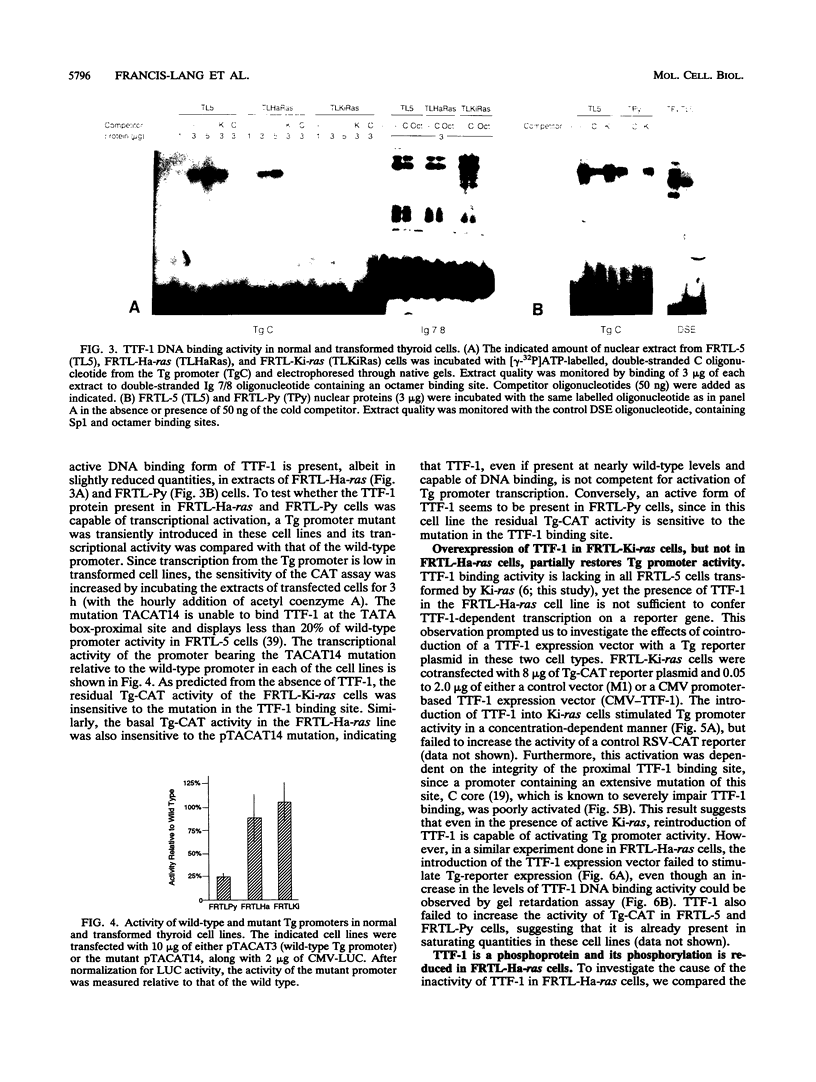
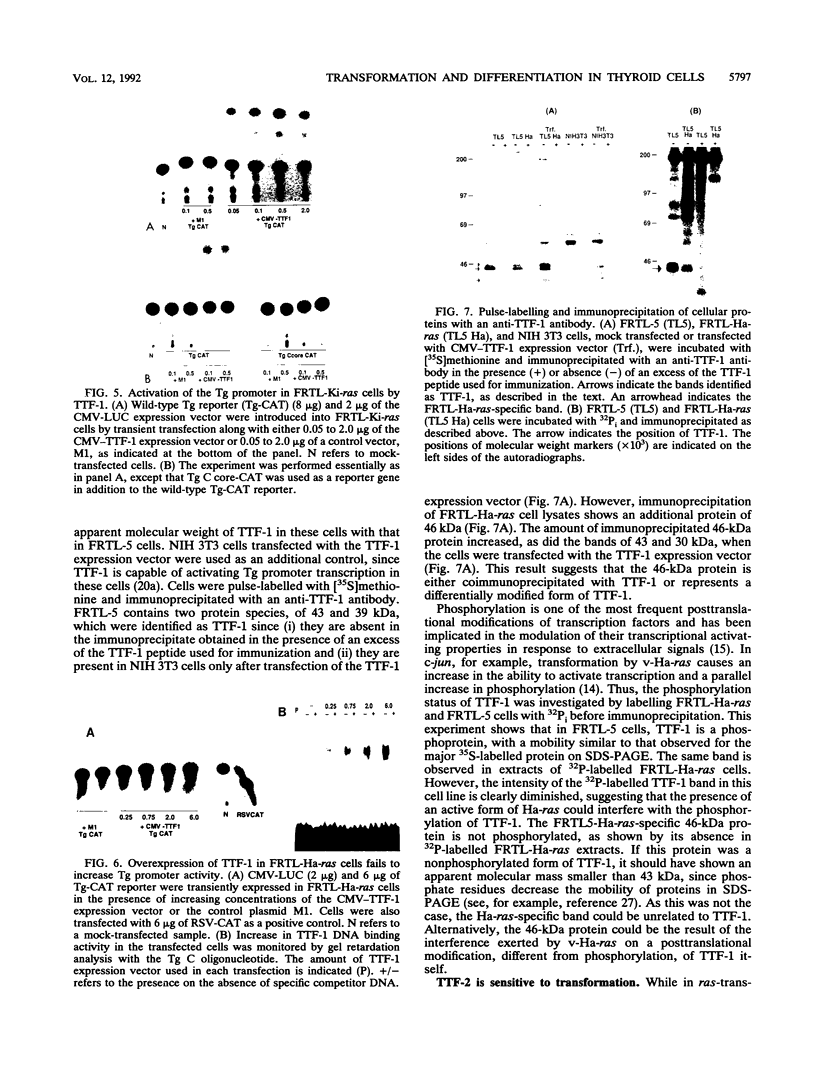
Transformation of the thyroid cell line FRTL-5 results in loss or reduction of differentiation as measured by the expression of thyroglobulin and thyroperoxidase, two proteins whose genes are exclusively expressed in thyroid follicular cells. The biochemical mechanisms leading to this phenomenon were investigated in three cell lines obtained by transformation of FRTL-5 cells with Ki-ras, Ha-ras, and polyomavirus middle-T oncogenes. With the ras oncogenes, transformation leads to undetectable expression of the thyroglobulin and thyroperoxidase genes. However, the mechanisms responsible for the extinction of the differentiated phenotype seem to be different for the two ras oncogenes. In Ki-ras-transformed cells, the mRNA encoding TTF-1, a transcription factor controlling thyroglobulin and thyroperoxidase gene expression, is severely reduced. On the contrary, nearly wild-type levels of TTF-1 mRNA are detected in Ha-ras-transformed cells. Furthermore, overexpression of TTF-1 can activate transcription of the thyroglobulin promoter in Ki-ras-transformed cells, whereas it has no effect on thyroglobulin transcription in the Ha-ras-transformed line. Expression of polyoma middle-T antigen in thyroid cells leads to only a reduction of differentiation and does not severely affect either the activity or the amount of TTF-1. Another thyroid cell-specific transcription factor, TTF-2, is more sensitive to transformation, since it disappears in all three transformed lines, and probably contributes to the reduced expression of the differentiated phenotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Coon H. G. Thyroid cells in culture. Int Rev Cytol Suppl. 1979;(10):163–172. doi: 10.1016/s0074-7696(08)60619-1. [DOI] [PubMed] [Google Scholar]
- Auersperg N., Roskelley C. Retroviral oncogenes: interrelationships between neoplastic transformation and cell differentiation. Crit Rev Oncog. 1991;2(2):125–160. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvedimento E. V., Obici S., Sanchez M., Gallo A., Musti A., Gottesman M. E. Reactivation of thyroglobulin gene expression in transformed thyroid cells by 5-azacytidine. Cell. 1989 Sep 22;58(6):1135–1142. doi: 10.1016/0092-8674(89)90511-4. [DOI] [PubMed] [Google Scholar]
- Avvedimento V. E., Musti A. M., Ueffing M., Obici S., Gallo A., Sanchez M., DeBrasi D., Gottesman M. E. Reversible inhibition of a thyroid-specific trans-acting factor by Ras. Genes Dev. 1991 Jan;5(1):22–28. doi: 10.1101/gad.5.1.22. [DOI] [PubMed] [Google Scholar]
- Avvedimento V. E., Musti A., Fusco A., Bonapace M. J., Di Lauro R. Neoplastic transformation inactivates specific trans-acting factor(s) required for the expression of the thyroglobulin gene. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1744–1748. doi: 10.1073/pnas.85.6.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal V. R., Park A., Tjian R. v-Src and EJ Ras alleviate repression of c-Jun by a cell-specific inhibitor. Nature. 1991 Jul 11;352(6331):165–168. doi: 10.1038/352165a0. [DOI] [PubMed] [Google Scholar]
- Bengal E., Ransone L., Scharfmann R., Dwarki V. J., Tapscott S. J., Weintraub H., Verma I. M. Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell. 1992 Feb 7;68(3):507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- Berlingieri M. T., Akamizu T., Fusco A., Grieco M., Colletta G., Cirafici A. M., Ikuyama S., Kohn L. D., Vecchio G. Thyrotropin receptor gene expression in oncogene-transfected rat thyroid cells: correlation between transformation, loss of thyrotropin-dependent growth, and loss of thyrotropin receptor gene expression. Biochem Biophys Res Commun. 1990 Nov 30;173(1):172–178. doi: 10.1016/s0006-291x(05)81037-x. [DOI] [PubMed] [Google Scholar]
- Berlingieri M. T., Portella G., Grieco M., Santoro M., Fusco A. Cooperation between the polyomavirus middle-T-antigen gene and the human c-myc oncogene in a rat thyroid epithelial differentiated cell line: model of in vitro progression. Mol Cell Biol. 1988 May;8(5):2261–2266. doi: 10.1128/mcb.8.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Bohmann D. Transcription factor phosphorylation: a link between signal transduction and the regulation of gene expression. Cancer Cells. 1990 Nov;2(11):337–344. [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Carbone A., Gusella G. L., Radzioch D., Varesio L. Human Harvey-ras is biochemically different from Kirsten- or N-ras. Oncogene. 1991 May;6(5):731–737. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitareale D., Lonigro R., Sinclair A. J., Di Lauro R. A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. EMBO J. 1989 Sep;8(9):2537–2542. doi: 10.1002/j.1460-2075.1989.tb08391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletta G., Pinto A., Di Fiore P. P., Fusco A., Ferrentino M., Avvedimento V. E., Tsuchida N., Vecchio G. Dissociation between transformed and differentiated phenotype in rat thyroid epithelial cells after transformation with a temperature-sensitive mutant of the Kirsten murine sarcoma virus. Mol Cell Biol. 1983 Nov;3(11):2099–2109. doi: 10.1128/mcb.3.11.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-Lang H., Price M., Polycarpou-Schwarz M., Di Lauro R. Cell-type-specific expression of the rat thyroperoxidase promoter indicates common mechanisms for thyroid-specific gene expression. Mol Cell Biol. 1992 Feb;12(2):576–588. doi: 10.1128/mcb.12.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco A., Berlingieri M. T., Di Fiore P. P., Portella G., Grieco M., Vecchio G. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987 Sep;7(9):3365–3370. doi: 10.1128/mcb.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco A., Pinto A., Tramontano D., Tajana G., Vecchio G., Tsuchida N. Block in the expression of differentiation markers of rat thyroid epithelial cells by transformation with Kirsten murine sarcoma virus. Cancer Res. 1982 Feb;42(2):618–626. [PubMed] [Google Scholar]
- Georges E., Mushynski W. E. Chemical modification of charged amino acid moieties alters the electrophoretic mobilities of neurofilament subunits on SDS/polyacrylamide gels. Eur J Biochem. 1987 Jun 1;165(2):281–287. doi: 10.1111/j.1432-1033.1987.tb11439.x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guazzi S., Price M., De Felice M., Damante G., Mattei M. G., Di Lauro R. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990 Nov;9(11):3631–3639. doi: 10.1002/j.1460-2075.1990.tb07574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isozaki O., Kohn L. D., Kozak C. A., Kimura S. Thyroid peroxidase: rat cDNA sequence, chromosomal localization in mouse, and regulation of gene expression by comparison to thyroglobulin in rat FRTL-5 cells. Mol Endocrinol. 1989 Nov;3(11):1681–1692. doi: 10.1210/mend-3-11-1681. [DOI] [PubMed] [Google Scholar]
- Konieczny S. F., Drobes B. L., Menke S. L., Taparowsky E. J. Inhibition of myogenic differentiation by the H-ras oncogene is associated with the down regulation of the MyoD1 gene. Oncogene. 1989 Apr;4(4):473–481. [PubMed] [Google Scholar]
- Lassar A. B., Thayer M. J., Overell R. W., Weintraub H. Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1. Cell. 1989 Aug 25;58(4):659–667. doi: 10.1016/0092-8674(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Lazzaro D., Price M., de Felice M., Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991 Dec;113(4):1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Lee N. T., Kamikubo K., Chai K. J., Kao L. R., Sinclair A. J., Nayfeh S. N., Chae C. B. The deoxyribonucleic acid regions involved in the hormonal regulation of thyroglobulin gene expression. Endocrinology. 1991 Jan;128(1):111–118. doi: 10.1210/endo-128-1-111. [DOI] [PubMed] [Google Scholar]
- Lewin B. Oncogenic conversion by regulatory changes in transcription factors. Cell. 1991 Jan 25;64(2):303–312. doi: 10.1016/0092-8674(91)90640-k. [DOI] [PubMed] [Google Scholar]
- Musti A. M., Ursini V. M., Avvedimento E. V., Zimarino V., Di Lauro R. A cell type specific factor recognizes the rat thyroglobulin promoter. Nucleic Acids Res. 1987 Oct 26;15(20):8149–8166. doi: 10.1093/nar/15.20.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisteban P., Acebrón A., Polycarpou-Schwarz M., Di Lauro R. Insulin and insulin-like growth factor I regulate a thyroid-specific nuclear protein that binds to the thyroglobulin promoter. Mol Endocrinol. 1992 Aug;6(8):1310–1317. doi: 10.1210/mend.6.8.1406708. [DOI] [PubMed] [Google Scholar]
- Santisteban P., Kohn L. D., Di Lauro R. Thyroglobulin gene expression is regulated by insulin and insulin-like growth factor I, as well as thyrotropin, in FRTL-5 thyroid cells. J Biol Chem. 1987 Mar 25;262(9):4048–4052. [PubMed] [Google Scholar]
- Sinclair A. J., Lonigro R., Civitareale D., Ghibelli L., Di Lauro R. The tissue-specific expression of the thyroglobulin gene requires interaction between thyroid-specific and ubiquitous factors. Eur J Biochem. 1990 Oct 24;193(2):311–318. doi: 10.1111/j.1432-1033.1990.tb19339.x. [DOI] [PubMed] [Google Scholar]
- Zannini M., Francis-Lang H., Plachov D., Di Lauro R. Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol. 1992 Sep;12(9):4230–4241. doi: 10.1128/mcb.12.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrilli R., Formisano S., Di Jeso B. Hormonal regulation of thyroid peroxidase in normal and transformed rat thyroid cells. Mol Endocrinol. 1990 Jan;4(1):39–45. doi: 10.1210/mend-4-1-39. [DOI] [PubMed] [Google Scholar]
- Zimarino V., Wu C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. 1987 Jun 25-Jul 1Nature. 327(6124):727–730. doi: 10.1038/327727a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]