Background: Eukaryotic circadian clocks require chromatin modifications and remodeling.
Results: SET1 is required for proper expression of the Neurospora clock gene frequency (frq). SET1 modifies chromatin at frq with the peak in H3K4me3 occurring after the peak in activation.
Conclusion: H3K4 methylation appears to mitigate White Collar complex (WCC)-mediated expression.
Significance: Chromatin is a key component underlying circadian oscillations in gene expression.
Keywords: Chromatin Immunoprecipitation (ChIP), Chromatin Modification, Circadian Rhythms, Gene Regulation, Neurospora
Abstract
The circadian oscillator controls time-of-day gene expression by a network of interconnected feedback loops and is reset by light. The requisite for chromatin regulation in eukaryotic transcription necessitates temporal regulation of histone-modifying and chromatin-remodeling enzymes for proper clock function. CHD1 is known to bind H3K4me3 in mammalian cells, and Neurospora CHD1 is required for proper regulation of the frequency (frq) gene. Based on this, we examined a strain lacking SET1 to determine the role of H3K4 methylation in clock- and light-mediated frq regulation. Expression of frq was altered in strains lacking set1 under both circadian- and light-regulated gene expression. There is a delay in the phasing of H3K4me3 relative to the peak in frq expression. White Collar 2 (WC-2) association with the frq promoter persists longer in Δset1, suggesting a more permissible chromatin state. Surprisingly, SET1 is required for DNA methylation in the frq promoter, indicating a dependence on H3K4me for DNA methylation. The data support a model where SET1 is needed for proper regulation by modulating chromatin at frq.
Introduction
The core mechanism underlying the circadian clock is coupled transcriptional/translational feedback loops that interact in a temporal manner to establish the proper period and phasing of clock gene expression (1–4). Predominant control comes from a negative feedback loop, although examples of rhythms occurring independent of transcription are known to occur (5, 6). The core feedback loop consists of a heterodimeric transcriptional activator complex that drives expression of inhibitors (7–9). This heterodimeric complex is composed of White Collar 1 (WC-1) and WC-2 in Neurospora (10), CLOCK, and CYCLE in Drosophila (11–13) and CLOCK and BMAL1 in mammals (14–17). These activate transcription of clock and clock-associated genes. Transcription and subsequent translation of negative elements eventually block further expression by inhibiting the positive elements. The negative elements are frequency (frq) and FRQ-interacting RNA helicase (frh) in Neurospora (18, 19), Period (Per) and Timeless (Tim) in Drosophila (20, 21), and Per and cryptochrome (Cry) in mammals (22–25). Both the positive and negative elements are post-translationally modified by a subset of kinases and phosphatases in tightly coupled reactions. These timed modifications ultimately lead to inactivation and turnover of the core clock proteins via the ubiquitin-proteasome pathway (26–37). Evidence suggests FRQ undergoes a conformational change induced by the kinase activity of casein kinase Ia that creates an accessible PEST1 domain needed for turnover (38). The molecular events occur such that circadian transcription oscillates in an ∼24-h period. Recent advances in our studies of the clock indicate that it controls chromatin structure, and proper chromatin structure is a requisite for normal clock gene oscillations, indicating higher order regulation in the activation and feedback mechanisms (39–43).
Eukaryotic chromatin packages DNA, and histone modifications help maintain the genome in a condensed or accessible state. The histones that compose the nucleosome have unstructured protruding amino-terminal tails that are modified individually or in tandem, and they serve as signaling platforms that can elicit a context-dependent outcome on gene expression (44, 45). Chromatin modifications and remodeling occur over the circadian cycle, and evidence indicates oscillations in facultative heterochromatic states (46). There are cycles in modifications considered permissive in the activation phases and repressive in the feedback inhibition phase. For example, rhythmic histone lysine acetylation has been observed in histone H3 in mPer1, mPer2, and Cry1 promoters with the peaks occurring during the transcriptionally active phase (47, 48). Affinity-purified CLOCK protein associates with the ubiquitous KAT3B, p300 (47), and has catalytic acetyltransferase activity (49), indicating the clock can direct chromatin modifications. Rhythms in acetylation have led to the identification of a growing list of histone deacetylases that include HDAC1 and HDAC2 (part of the Sin3B complex) (50) and the NAD-dependent histone deacetylase SIRT1 (51, 52). Moreover, deacetylation by the Sin3A complex and subsequent repressive modifications may be recruited by paraspeckle proteins NONO and PSF (41, 53).
Methylation and demethylation are also involved in circadian-regulated gene expression. During the repressive phase, there is di- and trimethylation of H3K27 at mPer1 and mPer2, which is dependent on the polycomb group protein EZH2 (KMT6) (54). Oscillating changes in H3K9 from acetylated to methylated states are observed at the albumin D-element binding protein (Dbp) gene, and HP1 is bound to H3K9me2 during the repressive phase (46). Methyl modifications considered activating also have a role in the clock. The mammalian KMT2, MLL1, associates with CLOCK-BMAL1 and directs H3K4 methylation at clock loci (46, 55). KMD5A (JARID1A) also associates with CLOCK-BMAL1 and is implicated in clock gene expression, but presumably, it does not demethylate H3K4 at clock loci. Instead it inhibits histone deacetylation (42). Additionally, the H3K36 lysine demethylase Jmj5B (KDM8) is rhythmically expressed in Arabidopsis and mammals, and it is needed to maintain WT period lengths (43).
Modifications to histones and DNA are recognized by cofactors and ATP-dependent chromatin-remodeling enzymes to elicit the proper transcriptional response. In Neurospora, two ATP-dependent chromatin-remodeling enzymes, Clockswitch (CSW-1/CRF10) (Saccharomyces cerevisiae Fun30, Schizosaccharomyces pombe Ftf3, Mus ETL1, and Homo sapiens SMARCAD1), and chromo-domain helicase DNA-binding protein 1 (CHD1), are needed for proper circadian regulation of frq. CSW-1 remodels chromatin at the frq c-box element to inhibit the association of WC-2 with the promoter, generating circadian-regulated accessible chromatin (40). CHD1 remodels chromatin at the frq antisense promoter and is needed to maintain the proper amplitude of clock gene expression (39). The CHD7 homologue Kismet in Drosophila is required for normal photoresponses and assists Cry-dependent transcriptional responses (57).
The combination of changing chromatin modifications and ATP-dependent remodeling at clock-associated loci suggests a complex mechanism of chromatin structural changes under the control of the clock that is needed for activation and feedback. Understanding how all this contributes to coordinate circadian-regulated gene expression, with respect to the timing and amplitude, is paramount to our basic knowledge of the biological clock and cellular homeostasis. In this study, we show that SET1 plays a role in circadian- and light-regulated gene expression. Specifically, H3K4 methylation appears to attenuate the expression of frq under circadian conditions. Moreover, loss of set1 results in elevated expression of a subset of light-activated genes. Under both circadian- and light-activated expression, the peak in H3K4 methylation at frq appears to lag the peak in gene expression, supporting the notion that in the absence of SET1 the chromatin at frq is more accessible.
EXPERIMENTAL PROCEDURES
Strains and Growth Conditions
All the strains utilized in this study are listed in Table 1. Neurospora conidia were suspended in 2% liquid culture medium (LCM) (1× Vogel's salts, 2% glucose, 0.17% arginine) and grown in 75-mm Petri dishes overnight at 30 °C to generate mycelia mats. Plugs were cut and used to inoculate flasks containing 50 ml of 2% LCM and grown at 25 °C for 2 days. For circadian time course experiments, strains were entrained with a standard light to dark transfer and harvested after a timed incubation in the dark (4, 8, 12, 16, 20, 24, 28, 32, 36, 40, and 44 h). Growth to examine the light response was done in a similar fashion except cultures were incubated in the light for 24 h, transferred to dark for 24 h, and then treated with saturating light for 15, 30, 60, 120, and 240 min. Tissue was harvested by filtration, frozen in liquid nitrogen, and then ground with a mortar and pestle in the presence of liquid nitrogen. Race tube medium consisted of 1× Vogel's salts, 0.1% glucose, 0.17% arginine, 50 mg/ml biotin, and 1.5% agar. Tubes were grown for 12–24 h in the light and transferred to the dark marking the growth front at 24-h intervals. All strains grown on race tubes contained the ras-1bd allele that facilitates observation of the rhythm in conidia formation (58), and all molecular analyses were done on deletion strains that were otherwise isogenic to FGSC2489. Luciferase experiments were performed as described using pVG110 (59), and data were acquired with μManager software and quantified with ImageJ (60).
TABLE 1.
Strains
| Strain | Genotype | Sourcea |
|---|---|---|
| FGSC2489 | OR74, A (WT) | FGSC |
| FGSC4200 | OR74, a (WT) | FGSC |
| FGSC15827 | ncu01206 (set-1)::hph, A | FGSC |
| FGSC15828 | ncu01206 (set-1)::hph, a | FGSC |
| FGSC16051 | ncu02104 (swd1)::hph, A | FGSC |
| FGSC13701 | ncu07885 (swd2)::hph, a | FGSC |
| FGSC11360 | ncu03244 (swd3)::hph, a | FGSC |
| FGSC14862 | ncu03037 (sdc1)::hph, a | FGSC |
| FGSC11914 | ncu06266 (dot1)::hph, a | FGSC |
| XB140-10 | set1::hph, A | This study |
| 94–40 | frq9, ras-1bd, A | 83 |
| XB77-1 | set1::hph, ras-1bd | This study |
| XB78-3 | swd1::hph, ras-1bd | This study |
| XB79-5 | swd2::hph, ras-1bd | This study |
| XB80-3 | swd3::hph, ras-1bd, a | This study |
| XB81-3 | sdc1::hph, ras-1bd, a | This study |
| XB98-3 | dim-2::hph, ras-1bd, a | 39 |
| XB136-6 | ras-1bd, A | This study |
| XB136-5 | ras-1bd, A | This study |
| 9014-VG3 | pVG110::his3, A | 59 |
| XB 157-25 | pVG110::his3, set1::hph | This study |
| XB 157-30 | pVG110::his3, set1::hph | This study |
| XB 157-31 | pVG110::his3, set1::hph | This study |
a FGSC: Fungal Genetics Stock Center.
RNA Extraction and Quantitative PCR
Total RNA was extracted using TRIzol (Invitrogen) following the manufacturer's guidelines. Briefly, 1 ml of TRIzol was added to an ∼100 μl volume of ground tissue from each sample and then vortexed vigorously. The mixture was subjected to centrifugation at 13,000 × g for 10 min to pellet cell debris. The organic TRIzol (800 μl) was transferred to a new tube, and the aqueous phase containing RNA was separated by the addition of 200 μl of chloroform followed by centrifugation at 13,000 × g for 10 min. The supernatant (250 μl) was transferred to a new tube, and the RNA was precipitated by the addition of isopropyl alcohol (250 μl) and centrifuged at 13,000 × g for 10 min. The RNA pellet was washed with 70% EtOH, then dried, and suspended in 50 μl of RNase-free 10 mm Tris-HCl, pH 7.5. Prior to the reverse transcription, DNA was removed by treatment with DNase I (Ambion TURBO DNA-free) following the manufacturer's guidelines. cDNA libraries were generated using a random hexamer and the High Capacity Reverse Transcriptase (Invitrogen). Quantitative PCR was performed using SYBR Green PCR Mastermix (Invitrogen) with oligonucleotides specific for frq, wc-1, vvd, dim-2, and al-2 all normalized relative to rac-1.
Immunoblot Analysis
Proteins were extracted from ground lysate in protein extraction buffer (PEB) (50 mm Hepes, pH 7.4, 137 mm KCl, 10% glycerol, and 1 mm EDTA) plus protease inhibitors (PI) (1 mm PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 mm DTT). 250 μl of ice-cold PEB + PI was mixed with a 100-μl volume of ground tissue and vortexed five times for 1 min with incubations on ice between vortexing and maintaining the lysates at ∼4 °C. Soluble protein was isolated by centrifugation at 13,000 × g for 10 min at 4 °C. The protein samples were normalized and diluted with 5× sample buffer (0.125 m Tris-HCl, pH 6.8, 25% glycerol, 4% SDS, 5% 2-mercaptoethanol, with bromphenol blue), to a final concentration of 2×. The protein was then heat-denatured, separated on 6.5% SDS-polyacrylamide gels, and then transferred to a PVDF membrane. Proteins were detected with primary polyclonal antibody specific for FRQ (31) and WC-1 (61). The blots were washed with PBST (phosphate-buffered saline with 0.3% Tween 20), incubated with secondary antibody, treated with chemiluminescent substrate, and then exposed to film.
Isolation of Nuclei
Nuclei were extracted from Neurospora tissue following an established protocol with modifications (62). 30 μl of conidia suspension was used to seed a 250-ml culture containing 2% LCM and grown under a standard time course procedure. Tissue was harvested and ground in the presence of 0.2 g of acid-washed glass beads (Sigma) and incubated in 4 ml of buffer A (1 m sorbitol, 7% Ficoll-PM70, 20% glycerol, 5 mm magnesium acetate, 3 mm calcium chloride, 50 mm Tris-HCl, pH 7.5, 3 mm DTT, 1 mm PMSF, 0.01 mg/ml pepstatin A, 0.01 mg/ml leupeptin) on ice. The lysate was filtered through cheesecloth, and 8 ml of buffer B (10% glycerol, 5 mm magnesium acetate, 25 mm Tris-HCl, pH 7.5, 1 mm PMSF, 1 μg/ml pepstatin A, 1 μg/ml leupeptin) was added dropwise to the filtered liquid with gentle stirring. The solution was spun at 3,000 × g for 7 min at 4 °C in an SW40 rotor (Beckman), and the supernatant was subjected to 6–10 strokes of homogenization using Wheaton Potter-Elvehjem Tissue Grinders (Fisher). Approximately 10 ml of homogenized supernatant was layered on a 2-ml step gradient consisting of 1 ml of buffer D (1 m sucrose, 10% glycerol, 5 mm magnesium acetate, 25 mm Tris-HCl, pH 7.5, 1 mm DTT, 1 mm PMSF, 0.01 mg/ml pepstatin A, 0.01 mg/ml leupeptin) layered on 1 ml of buffer D+ (1.2 m sucrose, 10% glycerol, 5 mm magnesium acetate, 25 mm Tris-HCl, pH 7.5, 1 mm DTT, 1 mm PMSF, 0.01 mg/ml pepstatin A, 0.01 mg/ml leupeptin) and centrifuged at 4,500 × g for 15 min at 4 °C. The resulting pellets were resuspended in 250 μl of buffer D, snap-frozen in liquid nitrogen, and stored at −80 °C. All nuclei were isolated under red light conditions to prevent photoactivation of WC-1.
Chromatin Immunoprecipitation
The ChIP assays followed the general outline described previously (63, 64) but modified and performed on isolated nuclei to increase efficiency in Neurospora (65). Isolated nuclei were suspended in 5 ml of buffer C (0.015 m Hepes, pH 7.4, 0.06 m KCl, 0.015 m NaCl, 0.01 m sodium bisulfite, 0.3 m sucrose, β-mercaptoethanol, 0.5 mm spermidine, 0.15 mm spermine), cross-linked in 1% formaldehyde, and then quenched with 0.1 m glycine for another 15-min incubation. The solution was centrifuged at 13,000 × g for 5 min to pellet the nuclei. Pellets were suspended in 5 ml of FA lysis buffer (0.05 m Hepes, pH 7.4, 0.15 m NaCl, 0.001 m EDTA, 1% Triton X-100, 0.1% SDS) containing protease inhibitors (0.002 mg/ml leupeptin, 0.002 mg/ml pepstatin A, 0.001 m PMSF) and sonicated four times at 50% power and then six times at 65% to an average size of 500 bp. Equal amounts of sheared chromatin was incubated with WC-2 (66) or H3K4me3 (Millipore) antibody plus protein A-conjugated magnetic beads (Dynabeads) overnight at 4 °C with constant mixing. The beads were washed five times with FA lysis buffer and then eluted twice with elution buffer (0.1 m sodium bicarbonate, 1.0% SDS) by incubating for 15 min at 42 °C. The cross-links were reversed by adding 2 μl of 5 m NaCl and incubated for a minimum of 4 h at 65 °C. Protein was removed by the addition of 4 μl of proteinase K (10 mg/ml), 4 μl of 1.0 m Tris-HCl, pH 6.5, 2 μl of 0.5 m EDTA, pH 8.0, and incubating at 42 °C for 1 h. DNA was purified using a Qiagen spin column or by a phenol/chloroform extraction. 2 μl of the purified DNA was used in a quantitative PCR with primers specific to the C-box, the PLRE, or the frq coding region (FCR)3 (40).
DNA Methylation and Southern Blot Analysis
Total Neurospora DNA was isolated from a 50-μl volume of ground tissue using the Gentra Puregene DNA isolation kit (Qiagen) following established guidelines (67). DNA methylation in the frq promoter was assayed by methylation-sensitive Southern blots using digoxigenin-labeled DNA probes (Roche Applied Science). Briefly, 10 μg of chromosomal DNA was digested with both BfuCI (methylation-sensitive) and DpnII (methylation-insensitive), then resolved on a 1.0% agarose gel, and transferred to Hybond N+ nitrocellulose membrane (GE Healthcare), and the region of the frq promoter was detected with digoxigenin-labeled PLREme probe. Complete digestion was determined by probing for a region of the mitochondrial genome.
RESULTS
In Neurospora, CSW-1 and CHD1 remodel chromatin and are required for proper regulation of frq (39, 40). However, it is unclear how these enzymes associate with chromatin. In mammals, CHD1 is known to bind to H3K4me3 via the double chromodomain, whereas binding of yeast CHD1 to chromatin is still unresolved (68–70). It seems likely that modifications to histones, possibly methylation, might serve as the recognition motif for either or both enzymes. Therefore, we took a systematic approach and began examining lysine methyltransferase deletion strains from the Neurospora knock-out collection for clock phenotypes. A strain lacking the KMT2 enzyme, SET1 (FGSC15827), was backcrossed to a strain carrying the ras-1bd allele, and rhythms in conidia formation were analyzed on race tubes. Comparison of the Δset1, ras-1bd strain to an isogenic clock wild type (WT) on race tubes indicated an apparent defect in clock function as indicated by a lack of robust oscillations in banding (Fig. 1A). SET1 is part of a large holoenzymatic complex (also known as COMPASS for complex associated with SET1) that contains several additional subunits (71). To examine the requirement of the other SET1/COMPASS subunits in clock function, deletion strains corresponding to SET1/COMPASS subunits were examined in a similar manner. Strains lacking two of the subunits (SWD1 and SWD3) appeared identical to Δset1 and are thus also required for clock-dependent SET1 activity. Surprisingly, some subunits (SWD2 and SDC1) appeared to be dispensable for rhythms in conidia formation, with the banding pattern being indistinguishable from WT. Most notably, a strain lacking SWD2, which is the only essential subunit of SET1/COMPASS in Saccharomyces, is viable in Neurospora and appeared identical to the clock-WT. This may suggest that certain subunits of SET1/COMPASS could function differently at the Neurospora clock gene than in lower eukaryotes.
FIGURE 1.
SET1 is required for normal circadian rhythms. A, Δset1 (XB77-1) and other subunits found in the COMPASS complex were grown on race tubes alongside WT (XB136-6) to determine the circadian phenotype. Data are presented for seven circadian cycles after a light to dark transfer. An apparent arrhythmic conidiation phenotype is observed for Δset1. Additional subunits of the Set1/COMPASS are shown, and the data indicate that swd2 and sdc1 are dispensable for circadian-regulated gene expression. B, WT (FGSC 2489) and Δset1 (XB140-10) were entrained with a standard light to dark transfer and harvested after incubation in the dark (DD) for the indicated time. The corresponding circadian time (CT) is shown. qRT-PCR data indicate an apparent clock defect in circadian-regulated frq expression. A cDNA library was generated using random hexamers to compare Δset1 to WT (FGSC 2489) over a 2-day circadian cycle, and levels of frq were determined with oligonucleotides specific for the frq transcript. Error bars represent the mean ± S.D., and the asterisk marks a statistically significant difference. C, immunoblot analysis of the FRQ protein confirms the clock defect observed in the qRT-PCR data. LLC, constant light. D, strand-specific qRT-PCR was performed on WT (FGSC 2498) and Δset1 (XB140-10) probing for the antisense frq transcript, ASfrq. Sub, subjective day and subjective night.
The apparent arrhythmic phenotype of SET1/COMPASS subunits indicated that H3K4 methylation is required for rhythmic expression of frq, or there was phenotypic masking of an underlying rhythm. The defect in clock function was further examined using quantitative RT-PCR looking at the rhythm in frq mRNA. The results shown in Fig. 1B indicate that Δset1 has an altered rhythm in expression compared with WT. In particular, there seems to be a defect in negative feedback inhibition as indicated by the high levels of expression in Δset1 relative to the WT at the DD24 time point (Fig. 1B, WT CT 12–13, marked with an asterisk). To further define the defect in frq expression, rhythmic levels of the FRQ protein were examined by immunoblot analysis using antibodies specific to FRQ. There appears to a defect in FRQ expression that is consistent with the qRT-PCR results and race tubes (Fig. 1C) with newly synthesized FRQ replacing hyperphosphorylated forms that are degraded. This is in comparison with the rhythm observed in WT. These data suggest that H3K4 methylation is required for efficient feedback inhibition of the central clock gene. This result contrasts recent findings in the mammalian clock, where the KMT2, MLL1, is required for activation and rhythmic expression of mPer2 (55). Moreover, these data are consistent with the initial characterization of the set1 mutant in Saccharomyces indicating that it was involved in rDNA silencing and silencing of genes near telomeres (72, 73), but it conflicts with the notion that H3K4 methylation is solely a mark for activation.
It is possible that the defect observed in frq expression may be due to misregulation of the antisense frq transcript (ASfrq). To explore the possibility that SET1-dependent methylation was affecting ASfrq regulation, we examined ASfrq transcript levels by strand-specific qRT-PCR but were unable to detect any notable defects (Fig. 1D). However, it is often difficult to measure differences in ASfrq because it is expressed at a very low level. Regardless of the mechanism, it is clear that frq is misregulated in strains lacking SET1. Moreover, based on the race tube phenotype, other clock-controlled genes are also likely affected, but it is unclear if this is direct or indirect.
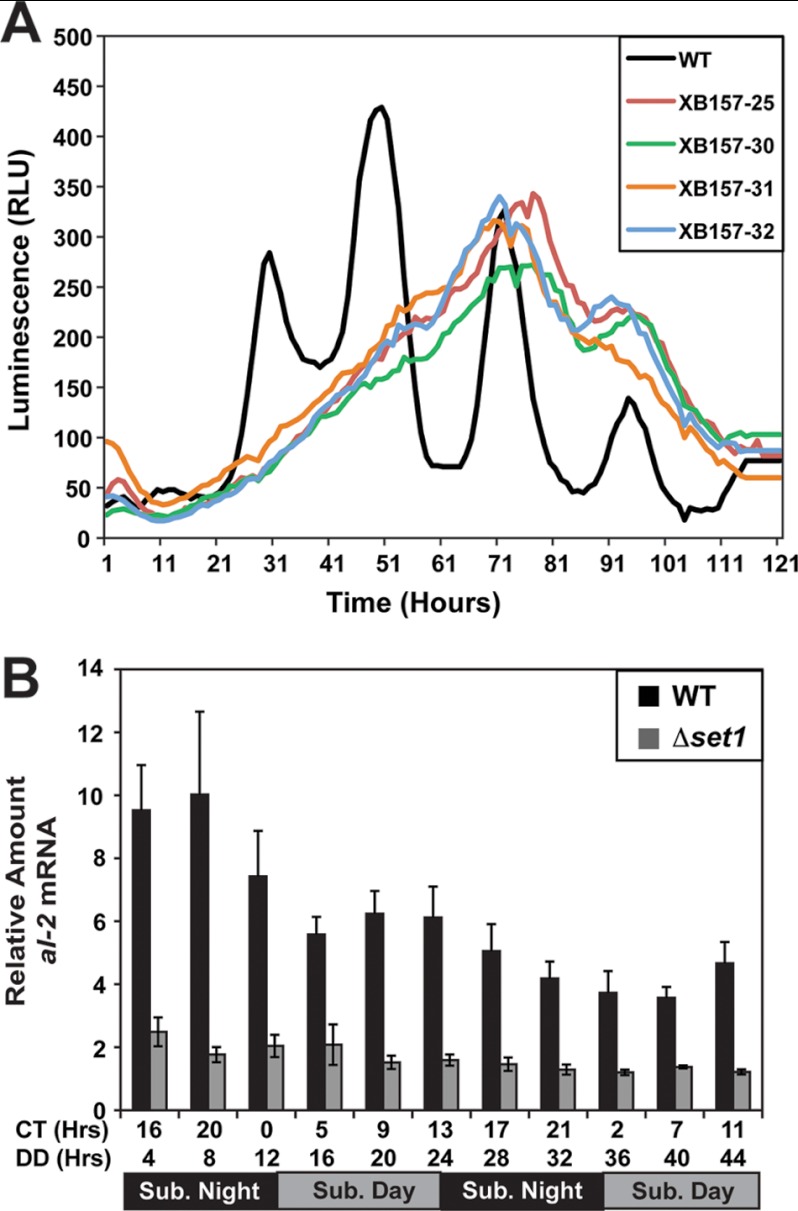
To further define the defect in clock function, we used a frq-luciferase reporter construct consisting of the frq promoter fused to a codon-optimized luciferase (59). Luminescence was examined over 5 days sampling every hour. We were unable to detect any discernable rhythm in any spores examined (Fig. 2A), although we cannot rule out an underlining rhythm that may be masked due to defects in luciferase protein turnover or possible alterations in ATP levels. There also appeared to be a delay in activation relative to WT, indicating SET1 may function as an activating mark when the frq promoter is fused to luciferase and integrated at a separate genomic locus (i.e. frq-luciferase may behave as a ccg).
FIGURE 2.
Characterization of expression defects in Δset1. A, clock rhythms were examined using the frq promoter fused to codon-optimized firefly luciferase. Strains FGSC 9014 with pVG110 and four independent isolates containing both Δset1 and pVG110 (XB157) were grown on luciferin race tubes, and luminescence was monitored for 120 h with sampling each hour. RLU, relative light units. B, quantitative RT-PCR comparing the steady-state levels of the al-2 transcript in WT (FGSC 2489) compared with Δset1 (XB140-10) at the indicated times. Sub, subjective day and subjective night.
To determine whether the apparent defect in repression is unique to frq, we proceeded to examine expression of the albino-2 (al-2) gene in the set1 deletion (Fig. 2B). We observed a lower level of al-2 expression in Δset1 compared with WT that is consistent with the canonical role of SET1 in gene activation at all time points examined.
H3K4 Methylation Occurs at frq
To further define the role of H3K4 methylation in circadian-regulated frq expression, we performed a chromatin immunoprecipitation (ChIP) using antibodies that recognize H3K4me3. The ChIP data indicate that H3K4me3 is present at the frq locus throughout the entire circadian cycle when compared with the levels observed in Δset1 and the IgG negative control (Fig. 3). As expected, H3K4me3 is predominantly present in the frq-coding region (Fig. 3B), and this is consistent with reports noting that H3K4 methylation is normally localized to coding regions and the transcriptional start site of genes (74, 75). There is also a low level of H3K4me3 in the proximity of the C-box element (Fig. 3A). Moreover, there appears to be a low amplitude rhythm in H3K4me3 with peaks occurring during the transcriptional repressive phase (CT9 to CT13). The peak in H3K4me3 corresponds to the repressive phase of frq transcription, whereas the trough in methylation corresponds to the activation phase. These data, and the frq expression defect observed in Δset1, support the notion that H3K4me3 modulates frq and that it is likely a direct effect.
FIGURE 3.
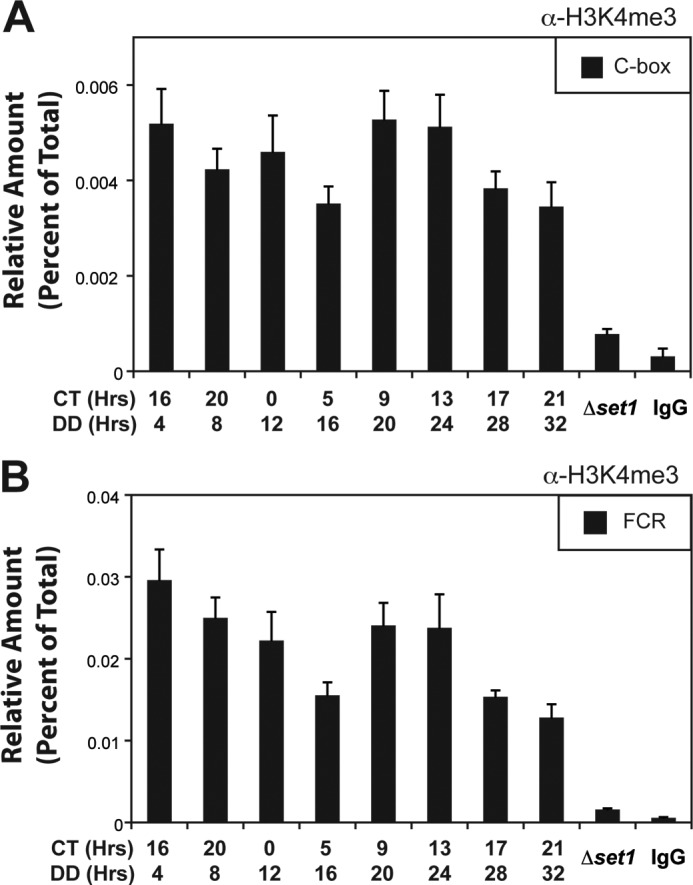
H3K4 methylation at frq under circadian conditions. ChIP analysis of H3K4me3 occurring at the frq locus. Cross-linked, sonicated lysates enriched for nuclei were isolated from WT (FGSC 2489) under circadian-entrained conditions and immunoprecipitated with an antibody that recognizes H3K4me3. The amount of DNA associated with the modification was determined by quantitative PCR and examined at the C-box (A) and within the FCR (B). A strain lacking SET1 (XB140-10) and a nonspecific IgG were used as negative controls. Values are the average of four independent ChIP experiments, and the error bars indicate the mean ± S.D. (p < 0.05 comparing peak, CT 13–16, and trough, CT 5). DD, constant dark; CT, circadian time.
WC-2 Binding Is Altered without SET1
The defects in frq expression observed in Δset1 led us to reason that binding of WC-2 to the frq promoter might also be affected in this strain. Therefore, we examined WC-2 binding to the frq C-box promoter element over one and a half circadian cycles by ChIP using polyclonal antibodies specific for WC-2 (66). Shown in Fig. 4 are the average results of four ChIP experiments for each strain. The results demonstrate a defect in WC-2 binding to the C-box element in Δset1 relative to WT. We observe a rhythm in WC-2 binding in WT with a peak occurring between CT0 and CT5 (DD12 to DD16) (Fig. 4A). In contrast, there was a delay of ∼4 h in the peak of WC-2 binding in Δset1 (Fig. 4B), which would imply a delay in transcription or a defect in negative feedback inhibition of the frq gene consistent with the rhythm in frq mRNA.
FIGURE 4.
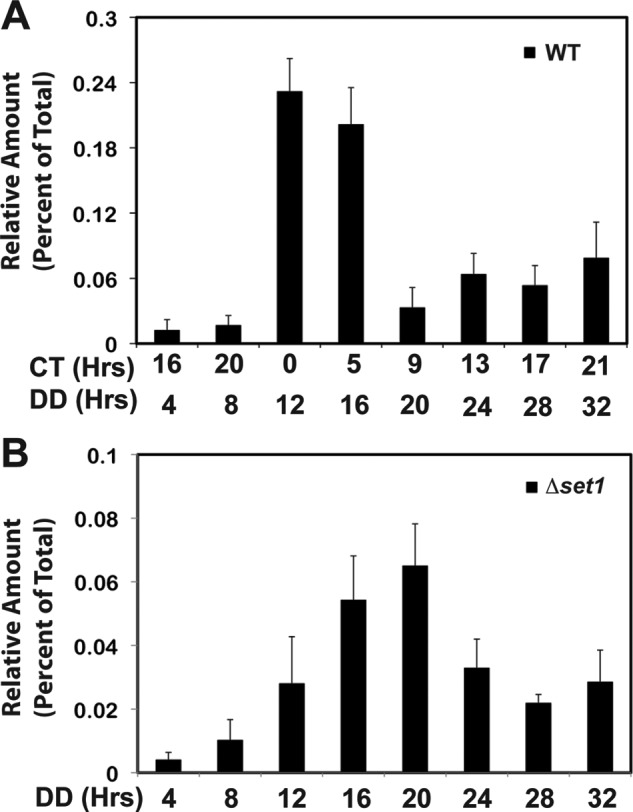
WC-2 binding to the C-box in WT and Δset1. ChIP analysis of WC-2 binding to the C-box was performed on nuclei isolated from WT (FGSC 2489) (A) and Δset1 (XB140-10) (B). Values are the average of four ChIP experiments normalized to nonspecific background, and error bars indicate the mean ± S.D. DD, dark; CT, circadian time.
Role of SET1 in Light-activated Gene Expression
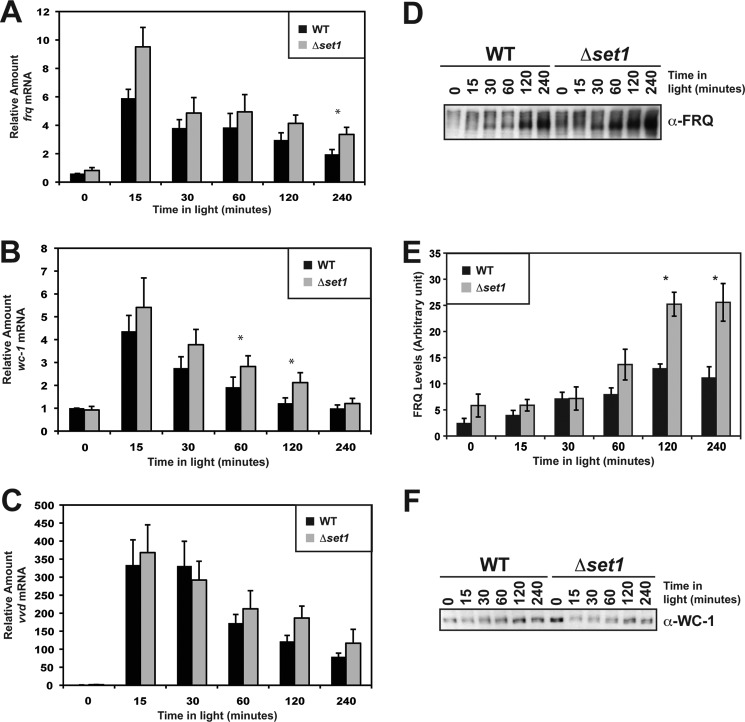
H3K4me3 is correlated with transcriptionally active chromatin and is thus thought to facilitate expression even though it was originally implicated in silencing (72, 73). The apparent defect in frq repression from the qRT-PCR may result from a defect in down-regulation. In conjunction, the phase delay in the H3K4me3 rhythm at frq led us to wonder if SET1 is involved in activation or in modulating down-regulation of frq. To accomplish this, we examined the role of SET1 in the Neurospora light response to see if there was any difference in expression under these conditions. We performed qRT-PCR in Δset1 compared with WT, examining frq, wc-1, and vvd (Fig. 5, A–C). Interestingly, the general trend was that Δset1 appeared to have a slightly elevated level of light-induced frq and wc-1 but not vvd at a subset of time points tested, although not all time points had a 95% confidence level. The lack of high confidence differences presumably stemmed from minor variability in the cDNA library construction, efficiency between experiments, and in the case with light-activated genes, it may be difficult to observe increases greater than maximum expression. Support for this comes from photoadaptation experiments where Neurospora is unresponsive to a higher intensity light pulse during the adaptation phase. Regardless of the cause, it is clear that there are consistently higher levels of frq and wc-1 in response to light.
FIGURE 5.
Δset1 has slightly higher frq and wc-1 expression under light-inducing conditions. Light-induced gene expression was examined in Δset1 (XB140-10) compared with WT (FGSC 2489), and relative levels of frq (A), wc-1 (B), and vvd (C) were determined by qRT-PCR. D, FRQ protein levels were also examined and appeared to be higher at later time points in Δset1 relative to WT. E, quantification of FRQ protein levels in response to light. Values are the normalized average of four independent experiments. F, level of WC-1 is generally lower in Δset1 compared with WT. All error bars represent the S.E.
To further define frq expression in Δset1, we investigated FRQ protein levels in response to light. Immunoblot analysis of FRQ indicates that FRQ expression is elevated in Δset1 versus WT (Fig. 5, D and E). In contrast, WC-1 protein levels in Δset1 (Fig. 5F) did not vary substantially comparing WT and Δset1, even though there are minor increases in wc-1 transcript at some time points (Fig. 5B). This may be due to turnover of WC-1 protein by the proteasome. This is a common mode of regulation found in numerous transcription factors (76), including both CLOCK an BMAL1 (77), and WC-1 has been shown to be degraded in response to light (78).
H3K4me3 in Response to Light
The observation that Δset1 has an increase in the expression of light-responsive genes, combined with phase-delayed peaks in H3K4me3 under circadian conditions, led us to examine the kinetics of H3K4 methylation in response to light. We performed ChIP on cross-linked and sonicated lysates from enriched nuclei using H3K4me3 antibodies probing regions within the frq locus. There appear to be no significant change in H3K4me3 after exposure to light for 15 min at the C-box, the PLRE, and within the FCR (Fig. 6), even though this corresponds to the peak in frq expression (Fig. 5A). However, at 30 min, a time corresponding to the start of VIVID (VVD)-dependent light repression, the levels of H3K4me3 rose and remained elevated with only a slow and steady decrease over the course of the next 2 h. After 4 h in the light, the amount of H3K4me3 returned to the levels observed in the dark (data not shown). The kinetics of H3K4 methylation is consistent with the notion that SET1 plays a role in modulating the amplitude of the light response. However, we cannot rule out the possibility that the low levels of H3K4 methylation seen after 15 min of light are the result of a reduction of nucleosome occupancy within the frq locus.
FIGURE 6.
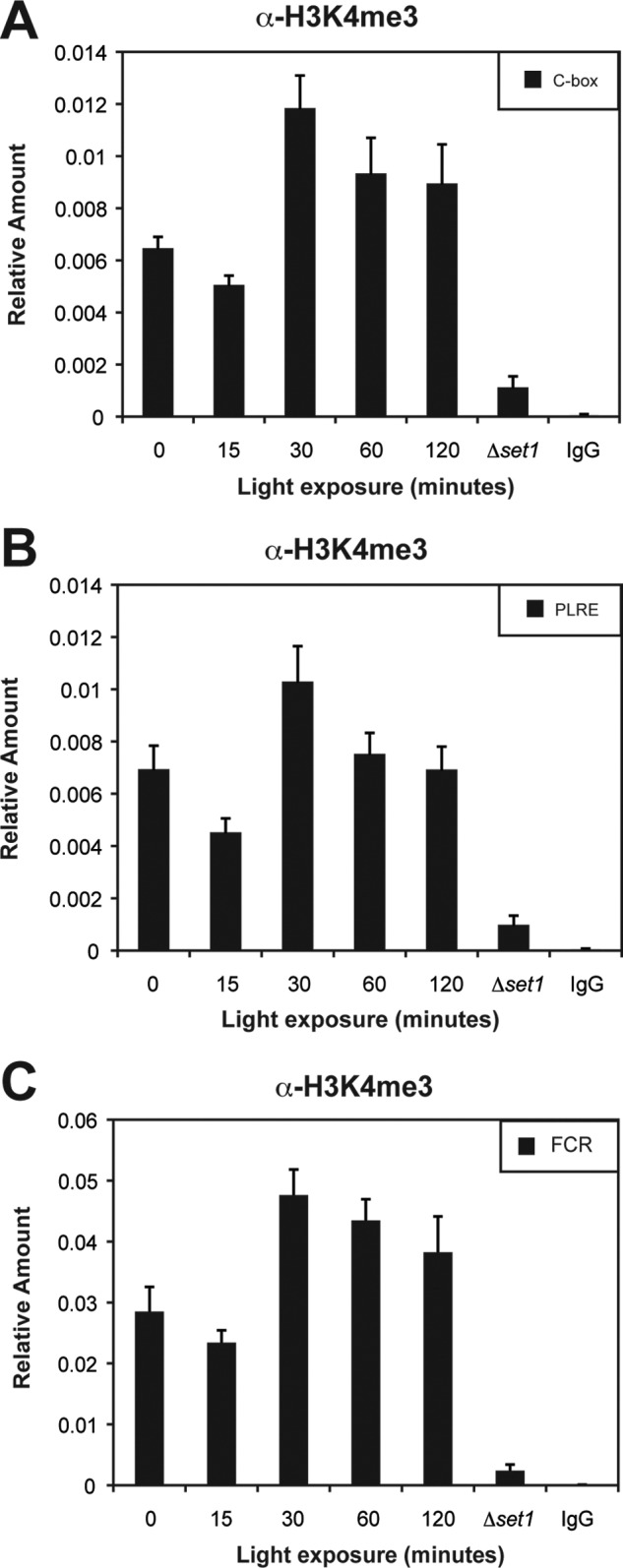
H3K4me3 under light-induced conditions. H3K4me3 within the frq locus was examined by ChIP after light induction at the C-box (A), PLRE (B), and within FCR (C). The amount of DNA associated with H3K4me3 was determined by quantitative PCR at the indicated times in the light. Sheared chromatin from Δset1 and a nonspecific IgG were used as controls. Average values are from four independent experiments. The errors bars represent the S.E.
DNA Methylation at frq Requires SET1
To further define the role of SET1 in regulating the chromatin state at frq, we examined the levels of DNA methylation in the frq promoter. The impetus for this experiment arose from the observation that there is typically a strict anti-correlation between H3K4 methylation and DNA methylation. Normally when H3K4 methylation is high, there is an absence of DNA methylation, and when DNA methylation is high, genes are typically devoid of H3K4 methylation. In fact, it has been shown that the mammalian SET1/COMPASS contains a CXXC protein, Cfp1, which is targeted to unmethylated CpG islands (79). The observation that H3K4me3 is present at all times examined in the ChIP experiments, combined with the observation that DNA sequences within the frq promoter are methylated under circadian entrainment conditions, led us to examine this relationship. We proceeded to examine if there were defects in normal DNA methylation at frq in Δset1 by performing a methyl-sensitive Southern blot. We compared total genomic DNA digested with the methylation-sensitive restriction endonuclease BfuCI and its methylation-insensitive isoschizomer, DpnII, followed by Southern blot analysis using a probe specific to the frq promoter (80). We probed DNA isolated from two specific time points (DD12 and DD24, which correspond to WT CT0 and CT12, respectively) from WT and Δset1 using the clock-defective frq9 (hypomethylated) and Δdim-2 (no methylation) as controls (Fig. 7A). To ensure that the lack of BfuCI digestion was due to DNA methylation, we stripped and probed the blots again and then examined a region in the mitochondrial genome (Fig. 7B). Mitochondrial DNA, which is never methylated, but is at much higher concentrations relative to nuclear DNA, was completely digested confirming that the partially digested DNA at frq was due to DNA methylation.
FIGURE 7.
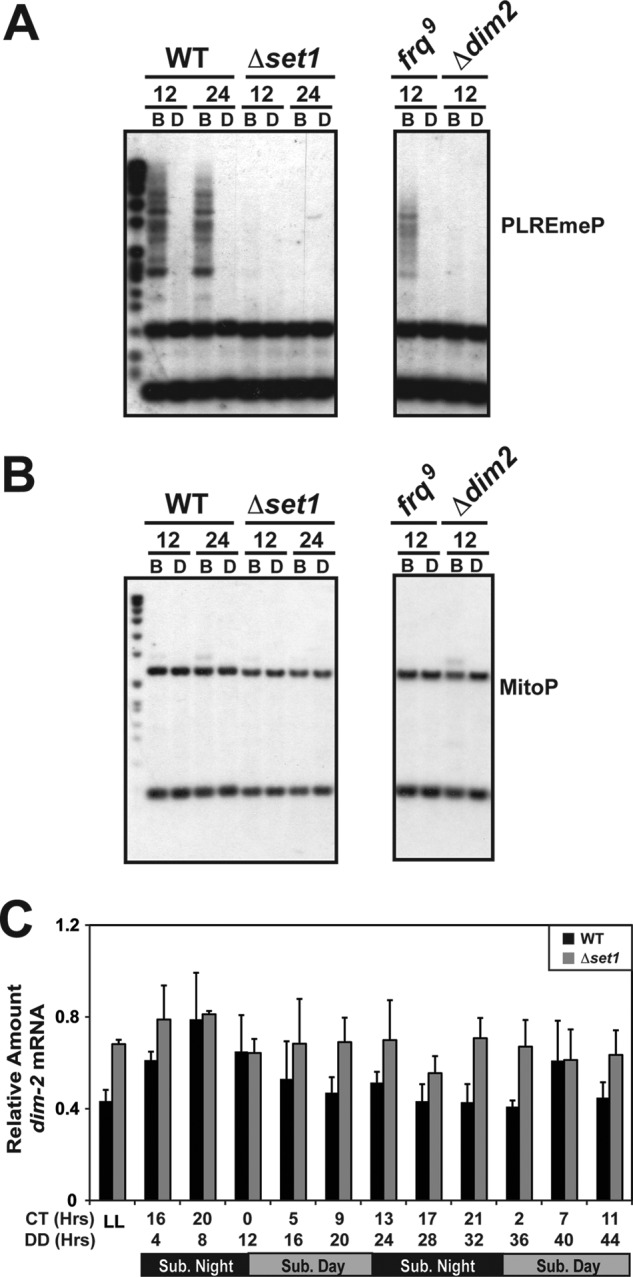
DNA methylation at frq requires SET1. A, methylation-sensitive Southern blot was performed on WT compared with Δset1 from DNA isolated from 12- and 24-h time points. DNA was digested with BfuCI (B) and DpnII (D) and resolved on a 1% agarose gel, and DNA methylation was determined by probing a region of the frq promoter that is methylated. A strain with the frq9 allele (hypomethylated) and a strain lacking the DNA methyltransferase dim-2 were added as controls. All the samples shown are contained on the same blot but separated to remove unrelated information. B, blot was stripped and probed a second time for a region within the mitochondrial genome. MitoP, mitochondrial probe. C, expression of the dim-2 transcript was analyzed by qRT-PCR in WT compared with Δset1.
The complete dependence of DNA methylation on H3K4 methylation observed in Δset1 was indistinguishable from the Δdim-2 mutant. This is a significant observation because of the anti-correlation between DNA methylation and H3K4 methylation stated above. A possible explanation for this observation may be the lack of expression of dim-2 in Δset1. However, this was ruled out because dim-2 expression is largely unaffected in strains lacking SET1 (Fig. 7C). The molecular mechanism behind DNA methylation at frq is still unresolved, but it is clear in this case that DNA methylation is dependent on H3K4 methylation. However, we cannot rule out the possibility that expression of other genes needed for DNA methylation are low (or not expressed) in the absence of SET1.
DISCUSSION
Both CSW-1 and CHD1 remodel chromatin at frq and are needed for the maintenance of circadian rhythms in Neurospora and either may associate with chromatin via histone methylation. With that in mind, we set out to determine whether methyl modifications to chromatin are involved in circadian rhythms and whether they share similar phenotypes to either Δcsw-1 or Δchd1. We began by examining a subset of histone methyltransferases and identified SET1/COMPASS. Strains lacking set1 or a subset of other SET1/COMPASS subunits have an apparent arrhythmic clock phenotype on race tubes. Further analysis of molecular rhythms either by qRT-PCR, Western blot, or using a frq-luciferase reporter construct confirms the defect in frq expression in Δset1. There is also a delay in H3K4me3 following the peak in frq expression under both circadian- and light-induced gene expression suggesting that H3K4me3 may play a role in the down-regulation of frq. We cannot rule out the possibility that activation is delayed under circadian conditions due to the loss of set1 or the excess abundance of light-expressed FRQ, which would lengthen the period and give it an apparent defect in inhibition. Moreover, it is possible that splicing of the frq transcript is delayed or less efficient in the absence of set1, and this would be consistent with reports showing that H3K4 methylation serves as modification linking CHD1 with components of the spliceosome (69). This is unlikely to be the case here because Neurospora CHD1 is more similar to yeast CHD1 and likely does not associate with H3K4me3 (68), and the phenotypic differences between Δchd1 and Δset1 indicate they are unlikely to be in a parallel pathway. Also, it is not entirely consistent with light activation experiments, where we routinely observed higher levels of frq, suggesting that SET1 may serve to repress expression at this locus. This is certainly unexpected because of the strong correlation between H3K4me3 and transcriptional activation and raises the following question. How prevalent is this throughout the genome? When we examined expression of al-2, we found that it behaved as one might predict for an activating modification, and the al-2 transcript was much lower than in WT. Microarray studies in Saccharomyces indicates that genes close to telomeres have an increase in expression in Δset1, but this is likely due to its role as an antisilencing factor and would be limited to telomeric regions and/or heterochromatic regions (81). Might frq be regulated in a similar fashion to genes near telomeres? Only time will tell, but many of the chromatin-modifying enzymes implicated in clock function also function at telomeric regions, most notably SIRT2 (51, 52). In addition, the seemingly contradictory observation that SET1 plays a role in silencing at frq fits nicely with the notion that the clock is regulated by facultative heterochromatin and may indicate a spreading of facultative heterochromatin to an adjacent loci in Δset1 leading to elevated levels of expression.
Consistent with the observed phenotypes and defects in molecular rhythms, we also find defects in rhythmic binding of the transcription factor WC-2. WC-2 normally has a robust rhythm in binding to the C-box sequence. In the absence of SET1, binding is different from what is typically observed in WT. Combined with the detectable rhythms in H3K4me3 observed at frq, it seems likely that SET1 functions as a direct regulator of frq expression. Assuming for a moment that the clock is regulated by facultative heterochromatin, loss of set1 may cause a spreading in heterochromatin that leads to defects in silencing (as is the case with telomeric regions), and this would then generate more accessible chromatin that would allow WC-2 to bind longer and lead to longer periods with elevated expression.
The notion that SET1 is involved in modulating inhibition of frq in Neurospora is based on the compilation of the data. First, we always see elevated levels of frq transcript and FRQ protein in response to light in Δset1. Second, we observed elevated levels of frq mRNA expression at the DD24 time point (WT CT12), a time when frq is normally inhibited. Third, Δset1 is phenotypically and molecularly identical to Δcsw-1, and this strain also has an apparent defect in negative feedback inhibition (40). Whether CSW-1 binds to the H3K4 methyl modification will certainly need to be tested in later studies. These data on Δset1 appear to contrast an elegant report on the role of MLL1 in the mammalian circadian system (55). However, the observation that WDR5 is associated with both PER1 and PER2 indicates that the process in the mammalian system is more complicated than current literature indicates (82).
One of the more intriguing aspects of this report is the idea that DNA methylation is dependent on H3K4 methylation, albeit possibly indirectly. How DNA methylation at frq is established is still unknown, but it seems likely that it is a complex cascade of oscillating chromatin modifications and may involve alternating transcription of the sense and antisense transcripts producing Dicer-independent small interfering RNAs that direct the DNA methylation (56). SET1 is just one component in this process, and if H3K4 is missing, subsequent modifications that are direct may, by default, be absent. In other words, loss of H3H4me2/3 may cause a defect in a subsequent modification (i.e. H3K9me3) that is needed for DNA methylation. The exact molecular mechanisms leading to DNA methylation at frq will certainly take time to determine, but no longer is there a hard and fast rule that if H3K4 methylation is absent, then DNA is likely methylated. Regardless of the details that will certainly be worked out in the future, it is clear that in all eukaryotic clocks studied to date, clock regulation of chromatin structure is a vital component of both the activation phase and repressive phase of the transcriptional negative feedback cycle.
Acknowledgments
We thank Dr. Peter Kahn and Dr. Tom Kusch for helpful comments on this manuscript and Dr. Luis Larrondo for luciferase stains and assistance with the data analysis. We are grateful to Dr. Kevin McCluskey at the Fungal Genetics Stock Center who provided deletion strains generated by the Neurospora Knock-out Consortium (supported by National Institutes of Health Grant P01GM068087).
This work was supported, in whole or in part, by National Institutes of Health Grant R01GM101378 (to W. J. B.). This work was also supported by the School of Environmental and Biological Science at Rutgers University and the Charles and Joanna Busch Memorial Fund.
- FCR
- frq-coding region
- qRT
- quantitative RT
- PLRE
- proximal light-regulated element.
REFERENCES
- 1. Dunlap J. C. (1999) Molecular bases for circadian clocks. Cell 96, 271–290 [DOI] [PubMed] [Google Scholar]
- 2. Young M. W., Kay S. A. (2001) Time zones: a comparative genetics of circadian clocks. Nat. Rev. Genet. 2, 702–715 [DOI] [PubMed] [Google Scholar]
- 3. Panda S., Hogenesch J. B., Kay S. A. (2002) Circadian rhythms from flies to human. Nature 417, 329–335 [DOI] [PubMed] [Google Scholar]
- 4. Bell-Pedersen D., Cassone V. M., Earnest D. J., Golden S. S., Hardin P. E., Thomas T. L., Zoran M. J. (2005) Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 6, 544–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Neill J. S., Reddy A. B. (2011) Circadian clocks in human red blood cells. Nature 469, 498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomita J., Nakajima M., Kondo T., Iwasaki H. (2005) No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science 307, 251–254 [DOI] [PubMed] [Google Scholar]
- 7. Reppert S. M., Weaver D. R. (2002) Coordination of circadian timing in mammals. Nature 418, 935–941 [DOI] [PubMed] [Google Scholar]
- 8. Schibler U., Sassone-Corsi P. (2002) A web of circadian pacemakers. Cell 111, 919–922 [DOI] [PubMed] [Google Scholar]
- 9. Heintzen C., Liu Y. (2007) The Neurospora crassa circadian clock. Adv. Genet. 58, 25–66 [DOI] [PubMed] [Google Scholar]
- 10. Crosthwaite S. K., Dunlap J. C., Loros J. J. (1997) Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science 276, 763–769 [DOI] [PubMed] [Google Scholar]
- 11. Darlington T. K., Wager-Smith K., Ceriani M. F., Staknis D., Gekakis N., Steeves T. D., Weitz C. J., Takahashi J. S., Kay S. A. (1998) Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 280, 1599–1603 [DOI] [PubMed] [Google Scholar]
- 12. Rutila J. E., Suri V., Le M., So W. V., Rosbash M., Hall J. C. (1998) CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93, 805–814 [DOI] [PubMed] [Google Scholar]
- 13. Allada R., White N. E., So W. V., Hall J. C., Rosbash M. (1998) A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93, 791–804 [DOI] [PubMed] [Google Scholar]
- 14. Antoch M. P., Song E. J., Chang A. M., Vitaterna M. H., Zhao Y., Wilsbacher L. D., Sangoram A. M., King D. P., Pinto L. H., Takahashi J. S. (1997) Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell 89, 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gekakis N., Staknis D., Nguyen H. B., Davis F. C., Wilsbacher L. D., King D. P., Takahashi J. S., Weitz C. J. (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 [DOI] [PubMed] [Google Scholar]
- 16. Hogenesch J. B., Gu Y. Z., Jain S., Bradfield C. A. (1998) The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. U.S.A. 95, 5474–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bunger M. K., Wilsbacher L. D., Moran S. M., Clendenin C., Radcliffe L. A., Hogenesch J. B., Simon M. C., Takahashi J. S., Bradfield C. A. (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aronson B. D., Johnson K. A., Dunlap J. C. (1994) Circadian clock locus frequency: protein encoded by a single open reading frame defines period length and temperature compensation. Proc. Natl. Acad. Sci. U.S.A. 91, 7683–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng P., He Q., He Q., Wang L., Liu Y. (2005) Regulation of the Neurospora circadian clock by an RNA helicase. Genes Dev. 19, 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardin P. E., Hall J. C., Rosbash M. (1990) Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536–540 [DOI] [PubMed] [Google Scholar]
- 21. Sehgal A., Rothenfluh-Hilfiker A., Hunter-Ensor M., Chen Y., Myers M. P., Young M. W. (1995) Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science 270, 808–810 [DOI] [PubMed] [Google Scholar]
- 22. Shearman L. P., Zylka M. J., Weaver D. R., Kolakowski L. F., Jr., Reppert S. M. (1997) Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19, 1261–1269 [DOI] [PubMed] [Google Scholar]
- 23. Sun Z. S., Albrecht U., Zhuchenko O., Bailey J., Eichele G., Lee C. C. (1997) RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 24. Tei H., Okamura H., Shigeyoshi Y., Fukuhara C., Ozawa R., Hirose M., Sakaki Y. (1997) Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389, 512–516 [DOI] [PubMed] [Google Scholar]
- 25. Kume K., Zylka M. J., Sriram S., Shearman L. P., Weaver D. R., Jin X., Maywood E. S., Hastings M. H., Reppert S. M. (1999) mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205 [DOI] [PubMed] [Google Scholar]
- 26. Kloss B., Price J. L., Saez L., Blau J., Rothenfluh A., Wesley C. S., Young M. W. (1998) The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iϵ. Cell 94, 97–107 [DOI] [PubMed] [Google Scholar]
- 27. Lin J. M., Kilman V. L., Keegan K., Paddock B., Emery-Le M., Rosbash M., Allada R. (2002) A role for casein kinase 2α in the Drosophila circadian clock. Nature 420, 816–820 [DOI] [PubMed] [Google Scholar]
- 28. Martinek S., Inonog S., Manoukian A. S., Young M. W. (2001) A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105, 769–779 [DOI] [PubMed] [Google Scholar]
- 29. Liu Y., Loros J., Dunlap J. C. (2000) Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc. Natl. Acad. Sci. U.S.A. 97, 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lowrey P. L., Shimomura K., Antoch M. P., Yamazaki S., Zemenides P. D., Ralph M. R., Menaker M., Takahashi J. S. (2000) Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garceau N. Y., Liu Y., Loros J. J., Dunlap J. C. (1997) Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell 89, 469–476 [DOI] [PubMed] [Google Scholar]
- 32. Chiu J. C., Vanselow J. T., Kramer A., Edery I. (2008) The phospho-occupancy of an atypical SLIMB-binding site on PERIOD that is phosphorylated by DOUBLETIME controls the pace of the clock. Genes Dev. 22, 1758–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. He Q., Cha J., He Q., Lee H. C., Yang Y., Liu Y. (2006) CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 20, 2552–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee C., Etchegaray J. P., Cagampang F. R., Loudon A. S., Reppert S. M. (2001) Post-translational mechanisms regulate the mammalian circadian clock. Cell 107, 855–867 [DOI] [PubMed] [Google Scholar]
- 35. Ko H. W., Jiang J., Edery I. (2002) Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature 420, 673–678 [DOI] [PubMed] [Google Scholar]
- 36. He Q., Cheng P., He Q., Liu Y. (2005) The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev. 19, 1518–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He Q., Cheng P., Yang Y., He Q., Yu H., Liu Y. (2003) FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 22, 4421–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Querfurth C., Diernfellner A. C., Gin E., Malzahn E., Höfer T., Brunner M. (2011) Circadian conformational change of the Neurospora clock protein FREQUENCY triggered by clustered hyperphosphorylation of a basic domain. Mol. Cell 43, 713–722 [DOI] [PubMed] [Google Scholar]
- 39. Belden W. J., Lewis Z. A., Selker E. U., Loros J. J., Dunlap J. C. (2011) CHD1 remodels chromatin and influences transient DNA methylation at the clock gene frequency. PLoS Genet. 7, e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belden W. J., Loros J. J., Dunlap J. C. (2007) Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol. Cell 25, 587–600 [DOI] [PubMed] [Google Scholar]
- 41. Duong H. A., Robles M. S., Knutti D., Weitz C. J. (2011) A molecular mechanism for circadian clock negative feedback. Science 332, 1436–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DiTacchio L., Le H. D., Vollmers C., Hatori M., Witcher M., Secombe J., Panda S. (2011) Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science 333, 1881–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones M. A., Covington M. F., DiTacchio L., Vollmers C., Panda S., Harmer S. L. (2010) Jumonji domain protein JMJD5 functions in both the plant and human circadian systems. Proc. Natl. Acad. Sci. U.S.A. 107, 21623–21628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J. (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 [DOI] [PubMed] [Google Scholar]
- 45. Smith E., Shilatifard A. (2010) The chromatin signaling pathway: diverse mechanisms of recruitment of histone-modifying enzymes and varied biological outcomes. Mol. Cell 40, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ripperger J. A., Schibler U. (2006) Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat. Genet. 38, 369–374 [DOI] [PubMed] [Google Scholar]
- 47. Etchegaray J. P., Lee C., Wade P. A., Reppert S. M. (2003) Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421, 177–182 [DOI] [PubMed] [Google Scholar]
- 48. Curtis A. M., Seo S. B., Westgate E. J., Rudic R. D., Smyth E. M., Chakravarti D., FitzGerald G. A., McNamara P. (2004) Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J. Biol. Chem. 279, 7091–7097 [DOI] [PubMed] [Google Scholar]
- 49. Doi M., Hirayama J., Sassone-Corsi P. (2006) Circadian regulator CLOCK is a histone acetyltransferase. Cell 125, 497–508 [DOI] [PubMed] [Google Scholar]
- 50. Naruse Y., Oh-hashi K., Iijima N., Naruse M., Yoshioka H., Tanaka M. (2004) Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol. Cell. Biol. 24, 6278–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., Guarente L. P., Sassone-Corsi P. (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Asher G., Gatfield D., Stratmann M., Reinke H., Dibner C., Kreppel F., Mostoslavsky R., Alt F. W., Schibler U. (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–328 [DOI] [PubMed] [Google Scholar]
- 53. Guillaumond F., Boyer B., Becquet D., Guillen S., Kuhn L., Garin J., Belghazi M., Bosler O., Franc J. L., François-Bellan A. M. (2011) Chromatin remodeling as a mechanism for circadian prolactin transcription: rhythmic NONO and SFPQ recruitment to HLTF. FASEB J. 25, 2740–2756 [DOI] [PubMed] [Google Scholar]
- 54. Etchegaray J. P., Yang X., DeBruyne J. P., Peters A. H., Weaver D. R., Jenuwein T., Reppert S. M. (2006) The polycomb group protein EZH2 is required for mammalian circadian clock function. J. Biol. Chem. 281, 21209–21215 [DOI] [PubMed] [Google Scholar]
- 55. Katada S., Sassone-Corsi P. (2010) The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat. Struct. Mol. Biol. 17, 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee H. C., Li L., Gu W., Xue Z., Crosthwaite S. K., Pertsemlidis A., Lewis Z. A., Freitag M., Selker E. U., Mello C. C., Liu Y. (2010) Diverse pathways generate microRNA-like RNAs and Dicer-independent small interfering RNAs in fungi. Mol. Cell 38, 803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dubruille R., Murad A., Rosbash M., Emery P. (2009) A constant light-genetic screen identifies KISMET as a regulator of circadian photoresponses. PLoS Genet. 5, e1000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Belden W. J., Larrondo L. F., Froehlich A. C., Shi M., Chen C. H., Loros J. J., Dunlap J. C. (2007) The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 21, 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gooch V. D., Mehra A., Larrondo L. F., Fox J., Touroutoutoudis M., Loros J. J., Dunlap J. C. (2008) Fully codon-optimized luciferase uncovers novel temperature characteristics of the Neurospora clock. Eukaryot. Cell 7, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Edelstein A., Amodaj N., Hoover K., Vale R., Stuurman N. (2010) Computer control of microscopes using μManager. Curr. Protoc. Mol. Biol. Chapter 14, Unit 14.20.1–14.20.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee K., Loros J. J., Dunlap J. C. (2000) Interconnected feedback loops in the Neurospora circadian system. Science 289, 107–110 [DOI] [PubMed] [Google Scholar]
- 62. Luo C., Loros J. J., Dunlap J. C. (1998) Nuclear localization is required for function of the essential clock protein FRQ. EMBO J. 17, 1228–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Komarnitsky P., Cho E. J., Buratowski S. (2000) Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14, 2452–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kuras L., Struhl K. (1999) Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399, 609–613 [DOI] [PubMed] [Google Scholar]
- 65. Smith K. M., Sancar G., Dekhang R., Sullivan C. M., Li S., Tag A. G., Sancar C., Bredeweg E. L., Priest H. D., McCormick R. F., Thomas T. L., Carrington J. C., Stajich J. E., Bell-Pedersen D., Brunner M., Freitag M. (2010) Transcription factors in light and circadian clock signaling networks revealed by genome-wide mapping of direct targets for Neurospora white collar complex. Eukaryot. Cell 9, 1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Denault D. L., Loros J. J., Dunlap J. C. (2001) WC-2 mediates WC-1-FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J. 20, 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M., Litvinkova L., Weiss R. L., Borkovich K. A., Dunlap J. C. (2006) A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U.S.A. 103, 10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sims R. J., 3rd, Chen C. F., Santos-Rosa H., Kouzarides T., Patel S. S., Reinberg D. (2005) Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J. Biol. Chem. 280, 41789–41792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sims R. J., 3rd, Millhouse S., Chen C. F., Lewis B. A., Erdjument-Bromage H., Tempst P., Manley J. L., Reinberg D. (2007) Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol. Cell 28, 665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Flanagan J. F., Blus B. J., Kim D., Clines K. L., Rastinejad F., Khorasanizadeh S. (2007) Molecular implications of evolutionary differences in CHD double chromodomains. J. Mol. Biol. 369, 334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miller T., Krogan N. J., Dover J., Erdjument-Bromage H., Tempst P., Johnston M., Greenblatt J. F., Shilatifard A. (2001) COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proc. Natl. Acad. Sci. U.S.A. 98, 12902–12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Briggs S. D., Bryk M., Strahl B. D., Cheung W. L., Davie J. K., Dent S. Y., Winston F., Allis C. D. (2001) Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 15, 3286–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Krogan N. J., Dover J., Khorrami S., Greenblatt J. F., Schneider J., Johnston M., Shilatifard A. (2002) COMPASS, a histone H3 (lysine 4) methyltransferase required for telomeric silencing of gene expression. J. Biol. Chem. 277, 10753–10755 [DOI] [PubMed] [Google Scholar]
- 74. Liu C. L., Kaplan T., Kim M., Buratowski S., Schreiber S. L., Friedman N., Rando O. J. (2005) Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol. 3, e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Barski A., Cuddapah S., Cui K., Roh T. Y., Schones D. E., Wang Z., Wei G., Chepelev I., Zhao K. (2007) High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 [DOI] [PubMed] [Google Scholar]
- 76. Collins G. A., Tansey W. P. (2006) The proteasome: a utility tool for transcription? Curr. Opin. Genet. Dev. 16, 197–202 [DOI] [PubMed] [Google Scholar]
- 77. Stratmann M., Suter D. M., Molina N., Naef F., Schibler U. (2012) Circadian Dbp transcription relies on highly dynamic BMAL1-CLOCK interaction with E boxes and requires the proteasome. Mol. Cell 48, 277–287 [DOI] [PubMed] [Google Scholar]
- 78. Talora C., Franchi L., Linden H., Ballario P., Macino G. (1999) Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J. 18, 4961–4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Thomson J. P., Skene P. J., Selfridge J., Clouaire T., Guy J., Webb S., Kerr A. R., Deaton A., Andrews R., James K. D., Turner D. J., Illingworth R., Bird A. (2010) CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature 464, 1082–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tamaru H., Selker E. U. (2001) A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414, 277–283 [DOI] [PubMed] [Google Scholar]
- 81. Venkatasubrahmanyam S., Hwang W. W., Meneghini M. D., Tong A. H., Madhani H. D. (2007) Genome-wide, as opposed to local, antisilencing is mediated redundantly by the euchromatic factors Set1 and H2A.Z. Proc. Natl. Acad. Sci. U.S.A. 104, 16609–16614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brown S. A., Ripperger J., Kadener S., Fleury-Olela F., Vilbois F., Rosbash M., Schibler U. (2005) PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science 308, 693–696 [DOI] [PubMed] [Google Scholar]
- 83. Loros J. J., Richman A., Feldman J. F. (1986) A recessive circadian clock mutation at the frq locus of Neurospora crassa. Genetics 114, 1095–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]