Abstract
The objective of this study was to examine the mechanism by which conjugated linoleic acid (CLA) reduces body fat. Young male mice were fed three combinations of fatty acids at three doses (0.06%, 0.2%, and 0.6%, w/w) incorporated into AIN76 diets for 7 weeks. The types of fatty acids were linoleic acid (control), an equal mixture of trans-10, cis-12 (10,12) CLA plus linoleic acid, and an equal isomer mixture of 10,12 plus cis-9, trans-11 (9,11) CLA. Mice receiving the 0.2% and 0.6% dose of 10,12 CLA plus linoleic acid or the CLA isomer mixture had decreased white adipose tissue (WAT) and brown adipose tissue (BAT) mass and increased incorporation of CLA isomers in epididymal WAT and liver. Notably, in mice receiving 0.2% of both CLA treatments, the mRNA levels of genes associated with browning, including uncoupling protein 1 (UCP1), UCP1 protein levels, and cytochrome c oxidase activity, were increased in epididymal WAT. CLA-induced browning in WAT was accompanied by increases in mRNA levels of markers of inflammation. Muscle cytochrome c oxidase activity and BAT UCP1 protein levels were not affected by CLA treatment. These data suggest a linkage between decreased adiposity, browning in WAT, and low-grade inflammation due to consumption of 10,12 CLA.
Keywords: obesity, weight loss supplement, glucose tolerance, uncoupling protein, steatosis
Obesity is recognized as the most widespread nutritional disease in the United States (1). Excess body fat increases chronic disease risk and mortality (2). Many Americans are consuming dietary supplements to lose weight or prevent weight gain, including supplements containing equal mixtures of cis-9, trans-11 (9,11) conjugated linoleic acid (CLA) and trans-10, cis-12 (10,12) CLA. Indeed, consuming a mixture of these CLA isomers, or 10,12 CLA alone, reduced body fat in many animal (reviewed in Ref. 3) and human studies (4). Rodents that consumed higher amounts of the CLA than humans (e.g., 0.5–1.5% CLA in the diet or 600–1,800 mg/kg body weight) lost body fat more rapidly but concurrently developed side effects, including chronic inflammation, insulin resistance, and lipoatrophy (5). Notably, intermediate levels of mixed CLA isomers (i.e., 0.1–0.3%, w/w) reduced adiposity in mice without adversely affecting liver weight or lipid content (6). However, individual isomers were not fed, the level of reduction in adiposity in the 0.1% group was marginal, and anti-obesity mechanisms were not identified (6). In contrast, clinical trials routinely use lower doses of CLA (e.g., 3–6 g/day or 35–70 mg/kg body weight), although the relative decrease in adiposity is not as rapid or great as in the higher doses used in rodent studies.
It has been reported that only the 10,12 CLA isomer reduces adiposity or delipidates adipocytes; however, at relatively high doses it also causes adverse side effects in mice (5) and some humans (7, 8). In contrast, the 9,11 CLA isomer has anti-inflammatory and adipogenic properties (9), and it improves insulin sensitivity in mice (10). Controversy still exists concerning the dose- and isomer-specific effects of CLA on adiposity, anti-obesity mechanism(s) of action, and adverse metabolic consequences. Continued existence of this controversy represents an important problem because, until it is resolved, realizing the potential health benefits of taking CLA to decrease or prevent obesity and its adverse side effects will likely remain largely beyond reach.
Proposed anti-obesity mechanisms of 10,12 CLA include i) increased energy metabolism and expenditure, ii) decreased adipogenesis, iii) decreased lipogenesis and increased lipolysis, iv) inflammation, and v) adipocyte apoptosis (reviewed in Ref. 3). One possible linkage of these potential mechanisms to body fat loss, especially inflammation and energy expenditure, is browning of white adipose tissue (WAT). This would have important medical implications, because an increased brown fat-like signature is associated with lower risk of developing obesity in mice (11) and humans (12). We have shown that 10,12 CLA increases cyclooxygenase (COX)-2 expression and prostaglandin (PG) production in human adipocytes (13), which have been associated with induction of brown fat-like adipocytes in WAT (14, 15), suggesting a link between inflammation and browning. Consistent with this hypothesis, relatively high levels of an equal mixture of 10,12 and 9,11 CLA [1.5% (16)] or 10,12 CLA alone [0.5% (17); 1% (18)] increase markers of brown fat-like adipocytes, including uncoupling protein 1 (UCP1) in WAT in mice. However, these high levels of 10,12 CLA alone or mixed CLA isomers also cause adverse side effects as stated above.
Based on these data, we wanted to determine in mice i) the extent to which 10,12 CLA or a CLA isomer mixture given at low (i.e., an effective dose used in clinical trials), intermediate (i.e., 3.3-fold the effective clinical dose), and high doses (i.e., 10-fold the effective clinical dose) shifted free fatty acids (FFA) away from adipocyte storage and toward oxidation versus ectopic deposition; and ii) the role of WAT browning and inflammation in mediating the anti-obesity properties of CLA. In this study, we demonstrated that an intermediate dose of 10,12 CLA (i.e., 0.1% 10,12 CLA plus 0.1% linoleic acid) or the CLA isomer mixture (i.e., 0.1% 10,12 CLA plus 0.1% 9,11 CLA) i) incorporated into WAT and liver, ii) decreased total WAT depot weight, iii) increased the mRNA levels of markers of browning, browning activators, and low-grade inflammation, and iv) increased the protein levels of UCP1, carnitine palmitoyltransferase (CPT)-1b, and COX-2 and the activity of cytochrome c oxidase in epididymal (EPI) WAT without decreasing food intake or causing steatosis or insulin resistance.
METHODS
Experimental design and diets
129Sv male mice (n = 90) were obtained from Jackson Laboratories (Bar Harbor, ME) at 5–6 weeks of age and housed in pairs in a 12 h light/12 h dark cycle, temperature-controlled room. Ethical treatment of animals was assured by the UNCG Institutional Animal Care and Use Committee. After 1 week of acclimation to a standard rodent semipurified diet, mice were randomly assigned to one of nine dietary treatments (n = 10 mice per treatment; supplementary Table I) for 7 weeks in this 3 × 3 factorial design (i.e., three levels of fatty acids and three types of fatty acids). The types of fatty acids used were linoleic acid (#1024, control FA indicated as “L” in the figures and tables); 10,12 CLA (#1249); and 9,11 CLA (#1245) purchased from Matreya LLC (Pleasant Gap, PA) (supplementary Table II). The three doses of fatty acids in the diet (w/w) were 0.06% (low), 0.2% (intermediate), and 0.6% (high), equivalent to 70, 240, and 700 mg/kg body weight, respectively, assuming a 25 g mouse consumes 3 g of food per day. The low-dose (0.06%) diets were supplemented with i) 0.06% linoleic acid, ii) 0.03% 10,12 CLA plus 0.03% linoleic acid, or iii) 0.03% 10,12 CLA plus 0.03% 9,11 CLA. The intermediate-dose (0.2%) and high-dose (0.6%) diets were formulated in a similar manner. This design ensured that each of the two types of 10,12 CLA-containing diets (i.e., 10,12 CLA plus linoleic acid and 10,12 CLA plus 9,11 CLA) within a given dose had the same amount of 10,12 CLA, allowing for determining additive or synergistic effects of 9,11 CLA when combined with 10,12 CLA, as is found in commercial CLA preparations. The low dose of mixed CLA isomers was chosen based on a clinical trial by Raff et al. (19) using one of the higher doses of mixed CLA (i.e., 6.4 g of 40/40% 9,11 CLA plus 10,12 CLA) in healthy subjects (i.e., postmenopausal women with BMI < 35.0) weighing on average 71 kg; equivalent to 77 mg of total CLA isomers or 62 mg 9,11 plus 10,12 CLA isomers/kg body weight. Total body fat mass was decreased in this mixed CLA group compared with controls after 16 weeks (19).
Treatments were added to a standard, semipurified rodent diet (D12450B; AIN76 containing 10% kcal from fat and 35% from sucrose) by Research Diets Inc., (New Brunswick, NJ). Diets were pelleted and packed under inert gas in individual 2.5 kg foil bags and stored at −20°C until use. The actual fatty acid profile of these diets is shown in supplementary Table III. Fresh diet was provided twice per week to minimize oxidization. Mice had ad libitum access to both food and water. Food intake and body weight were measured weekly. Mice were euthanized by CO2 narcosis followed by cervical dislocation and exsanguination. Trunk blood was collected and serum was harvested for measuring markers of inflammation and lipoatrophy. WAT depots were harvested, weighed, frozen in liquid N2, and stored at −80°C until analysis.
FFA profile of the diets and tissues
Lipid extraction and fatty acid methylation.
The dietary samples were ground into a powder with a coffee grinder (Black and Decker, Towson, MD). Total lipids from 1–2 g of the ground samples in triplicate were extracted with 50 ml petroleum ether continuously for 4 h using a Glodfisch extraction apparatus (Labconco, Kansas City, MO). The liver and muscle tissue samples (100 mg) were homogenized in 0.9 ml water with a Sonic Dismembrator (550, Fisher Scientific, Pittsburgh, PA) for 30 s, and total lipids were subsequently extracted (20). The EPI WAT samples (30–50 mg) were used with no further processing. An internal standard containing 0.5 mg of heptadecanoic acid was then added to each lipid extraction and the EPI WAT. The fatty acids in the extracted lipids and the adipose tissues were methylated following alkali treatment. The fatty acids methyl esters were dissolved in 0.1–0.5 ml of hexane and analyzed using GC-MS.
GC-MS analysis.
Fatty acid methyl esters were separated on a DB-23 capillary column (122-2332), 30 m × 0.25 mm, film thickness 0.25 μm (Agilent Technologies, Wilmington, DE) (21). Mass spectrometric analysis was conducted using a 6890 N model gas chromatograph (Agilent Technologies) equipped with an Agilent Technologies 5973N mass spectrometric detector. The oven temperature was programmed from 50°C to 100°C at 10°C/min, then to 200°C at 4°C/min, held for 2 min, and finally to 220°C at 4°C/min, held for 12 min. The average helium velocity was 36 cm/sec, and the split ratio was 100:1. The temperatures of the MSD electron ionization source and quadropoles were 230°C and 150°C, respectively. One microliter of methyl ester was manually injected, and the total fatty acid amounts were determined by the areas of the total ions for each fatty acid (Tables 1 and 2, supplementary Table IV).
Intraperitoneal glucose tolerance tests
Glucose tolerance tests (GTT) were performed during week 6 on nonanesthetized mice. Mice were deprived of food for 8 h and given an intraperitoneal glucose injection (Sigma-Aldrich, St. Louis, MO) at a dose of 1 g/kg body weight. Blood was obtained from the tail vein, and glucose levels were determined at 0, 5, 15, 30, 60, and 120 min following glucose administration using Contour blood glucose monitoring system (Bayer Diabetes Care, Tarrytown, NY). Total GTT area under the curve (AUC) was calculated as described (22).
Analysis of serum cytokine, chemokine, triglyceride, FFA, and insulin levels
Serum tumor necrosis factor (TNF)α, interleukin (IL)-6, IL-1β, and monocyte chemoattractant protein (MCP)-1 levels were determined using the Bio-Plex magnetic bead-multiplex immunoassay on the Bio-Plex 200 system, according to the manufacturer's instructions (Bio-Rad, Hercules, CA). Serum triglyceride (TG) and FFA levels were determined using commercial assays from Thermo Scientific (Infinity TG assay #TR22421 and TG standards #TR22923, Norcross, GA) and Wako Diagnostics (#NEFA-HR-2, Richmond, VA), respectively. Serum insulin levels were detected using an ultrasensitive mouse insulin kit (Crystal Chem Inc., Downers Grove, IL). The homeostasis model assessment method (HOMA) for insulin resistance (IR) used the following formula: [fasting insulin concentration (ng/ml) × 24 × fasting glucose concentration (mg/dl)] / 405 (23).
Liver TG content and staining
Liver TG levels were measured by extracting lipids in 2:1 CHCl3/methanol in glass tubes at room temperature overnight. After centrifugation and reextraction, the pooled lipid extract was dried down under N2 gas at 55°C and redissolved in a measured volume of 2:1 CHCl3/methanol. Dilute H2SO4 was added to the sample, which was then vortexed and centrifuged to split the phases. The aqueous upper phase was aspirated and discarded, and an aliquot of the bottom phase was removed and dried down; 1% triton-X100 in CHCl3 was then added, and the solvent was evaporated. Deionized water was added to each tube and vortexed until the solution was clear. Lipids were then quantified using the TG/GB kit (Roche). Next, the delipidated liver was dried down at 60°C for 1–2 h. Then, 1N NaOH was added and incubated at 60°C, with vortexing every 30 min to ensure complete tissue dissolution. Lowry protein assay was performed to determine liver protein concentration. For liver histological analyses, formalin-fixed, paraffin-embedded liver tissues were sliced and stained with hematoxylin and eosin.
Tissue RNA analysis and real-time quantitative PCR
Epididymal, inguinal (ING), and retroperitoneal (RET) WAT were harvested, and total RNA was extracted using RNeasy Lipid Tissue Kit (Qiagen, Valencia, CA) combined with RNase-Free DNase Set (Qiagen). EPI WAT was isolated from the bilateral intra-abdominal visceral depots attached to the epididymis. ING WAT was isolated from the bilateral superficial subcutaneous WAT depots between the skin and muscle fascia just anterior to the lower segment of the hind limbs. RET WAT was isolated from the bilateral visceral depots in the abdominal cavity behind the peritoneum on the dorsal side of the kidneys. Mesenteric WAT was isolated from a glue-like visceral net located in the mesentery of the intestines. RNA integrity was assessed using Agilent RNA 6000 Nano Kit on an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). For real-time qPCR, 1 μg total RNA from mouse tissues was converted into first-strand cDNA by using a high-capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The qPCR was performed in a 7500 FAST real-time PCR system by using Taqman gene expression assays (Applied Biosystems). Fold differences in gene expression were calculated as 2−ΔΔCt using the endogenous reference gene TATA-binding protein (TBP).
Cytochrome c oxidase
Cytochrome c oxidase, the final protein complex in the mitochondrial electron transport chain, was used as an indicator of mitochondria content in tissue. Approximately 50 mg of the gastrocnemius-plantaris muscle group was homogenized on ice in a Potter Elvehjem homogenizer in 19 vol of 50 mM KH2PO4, 0.1 mM EDTA, and 0.1% Triton X-100 (pH 7.4), and then centrifuged at 10,000 g for 5 min at 4°C. Fat from the EPI depot was homogenized in 4 vol of 50 mM KH2PO4, 0.1 mM EDTA, and 0.25% Triton X-100 (pH 7.4). Enzyme activity in the muscle supernatant and fat homogenate was determined polarographically at 37°C with a Clark-type oxygen electrode as previously described (24).
Immunoblotting
Cellular protein was harvested in a phosphate buffered saline (pH = 7.5) lysis buffer containing 1% NP40, 0.5% SDS, 30 μl/ml aprotinin, 1 mM phenylmethylsulfonlyl fluoride, and 1 mM sodium orthovanadate. The samples were homogenized on ice and centrifuged at 15,000 g for 15 min at 4°C; then the protein concentration was determined in the supernatant using the bicinchoninic acid assay. Equal amounts of protein were separated using 4–12% NuPage mini precast gels (Invitrogen Inc.), and transferred to PVDF membranes (Bio-Rad Inc.) as previously described (13). Membranes were then blocked with 5% milk in TBST for 30 min and washed with TBST 5 min three times. Overnight incubation of membranes at 4°C with primary antibodies for UCP1 (catalog no. ab23841, Abcam, Cambridge, MA); CPT-1b (catalog no. sc-20670, Santa Cruz Biotechnology Inc., Santa Cruz, CA); COX-2 (catalog no. sc-1745, Santa Cruz Biotechnology); and β actin (catalog no. sc-1616, Santa Cruz Biotechnology) at 1:800, 1:400, 1:800, and 1:2,000, dilutions, respectively, followed by 1 h exposure at room temperature to horseradish peroxidase-conjugated secondary antibodies at 1:5000 dilutions unless otherwise indicated. Blots were exposed to chemiluminescence reagent for 1 min, and X-ray films were developed using a SRX-101A Konica Minolta film developer. Densitometry was performed on blots using the Kodak 4400 CF Image Station with the Kodak Molecular Imaging software.
Statistics
Statistical analyses were performed using a two-way ANOVA testing the main effects of fatty acid dose (0.06. 0.2, 0.6%) and fatty acid type (linoleic acid, 10,12 CLA, CLA isomer mixture) and their full-factorial interaction (dose × type) using the JMP version 8.0 program (SAS, Cary, NC), unless otherwise indicated. The significance levels of the main effects and interactions are shown in supplementary Tables V and VI. Tukey's multicomparison test was conducted to detect significant treatment differences among the interactions (P < 0.05). Data for cytochrome c oxidase were analyzed using a one-way ANOVA and Tukey's HSD test to compute individual pairwise comparisons of means (P < 0.05). Data are expressed as means ± SEM.
RESULTS
CLA isomers incorporate into WAT and liver, but not muscle
To determine the extent to which CLA isomers incorporate into WAT, liver, and muscle fatty acids, we measured the fatty acid profile of these tissues. Indeed, 10,12 CLA and 9,11 CLA incorporated into EPI WAT (Table 1) and liver (Table 2) in a dose-dependent manner. Notably, mice supplemented with 10, 12 CLA or the CLA mixture had 5- to 20-fold higher concentrations of CLA isomers in EPI WAT compared with liver, demonstrating the accumulation in WAT and potential impact on cells comprising WAT. In contrast, CLA supplementation did not increase the CLA isomer content of muscle, with the exception of 9,11 CLA in the 0.2% dose of mixed CLA isomers (supplementary Table IV). There were no remarkable changes in the profiles of saturated, monounsaturated, or polyunsaturated fatty acid in WAT or muscle. In liver, however, the high dose of 10,12 CLA and the CLA mixture increased 5-fold the abundance of saturated and monounsaturated fatty acids.
TABLE 1.
Fatty acid content of epididymal WAT
| 0.06% Diet |
0.2% Diet |
|||||
| Tissue (mg/g) | L | 10 | M | L | 10 | M |
| C10:0 | 0.22 ± 0.03a | 0.18 ± 0.02a | 0.22 ± 0.02a | 0.21 ± 0.03a | 0.06 ± 0.01b | 0.06 ± 0.00b |
| C12:0 | 1.3 ± 0.1ab | 1.5 ± 0.2a | 1.8 ± 0.1a | 1.5 ± 0.2a | 0.8 ± 0.1b | 0.8 ± 0.0b |
| C14:0 | 17.2 ± 0.6bc | 21.3 ± 0.4a | 19.7 ± 0.8ab | 17.1 ± 1.3bc | 15.0 ± 0.8c | 14.2 ± 0.4c |
| C14:1 | 2.6 ± 0.2ab | 3.1 ± 0.1a | 2.8 ± 0.2ab | 2.4 ± 0.2b | 1.0 ± 0.1c | 1.1 ± 0.1c |
| C15:0 | 1.5 ± 0.1 | 1.5 ± 0.0 | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.0 |
| C16:0 | 141 ± 5 | 154 ± 2 | 144 ± 3 | 149 ± 9 | 153 ± 3 | 154 ± 3 |
| C16:1 | 108 ± 4b | 139 ± 3a | 120 ± 5ab | 107 ± 7b | 82 ± 4c | 81 ± 4c |
| C17:1 | 2.4 ± 0.1bc | 3.0 ± 0.0a | 2.8 ± 0.1ab | 2.4 ± 0.1c | 2.4 ± 0.1c | 2.6 ± 0.1bc |
| C18:0 | 7.2 ± 0.4 | 5.7 ± 0.2 | 6.0 ± 0.3 | 7.0 ± 0.5 | 5.1 ± 0.1 | 5.2 ± 0.1 |
| C18:1cis | 200 ± 6 | 244 ± 3 | 227 ± 5 | 202 ± 10 | 243 ± 6 | 244 ± 3 |
| C18:2cis | 168 ± 4b | 195 ± 7a | 184 ± 6ab | 177 ± 8ab | 173 ± 6ab | 160 ± 4b |
| C18:2trans | 1.00 ± 0.17 | 1.00 ± 0.06 | 0.91 ± 0.03 | 0.96 ± 0.11 | 0.91 ± 0.07 | 0.97 ± 0.05 |
| 9,11 CLA | 1.14 ± 0.05c | 1.25 ± 0.04c | 3.14 ± 0.30b | 1.10 ± 0.05c | 1.06 ± 0.12c | 7.52 ± 0.14a |
| 10,12 CLA | 0.22 ± 0.06c | 0.92 ± 0.04b | 1.00 ± 0.07b | 0.27 ± 0.03c | 2.32 ± 0.21a | 2.39 ± 0.06a |
| C18:3n3 | 16.7 ± 0.7a | 16.3 ± 0.6a | 14.8 ± 0.8a | 16.1 ± 0.9a | 8.8 ± 0.5b | 8.6 ± 0.3b |
| C18:3n6 | 1.03 ± 0.08 | 0.98 ± 0.05 | 0.92 ± 0.07 | 0.88 ± 0.07 | 1.07 ± 0.08 | 1.02 ± 0.06 |
| C20:0 | 0.25 ± 0.02 | 0.21 ± 0.01 | 0.24 ± 0.01 | 0.20 ± 0.02 | 0.17 ± 0.02 | 0.20 ± 0.01 |
| C20:1 | 2.31 ± 0.11 | 2.25 ± 0.1 | 2.35 ± 0.13 | 2.42 ± 0.2 | 2.38 ± 0.1 | 2.47 ± 0.08 |
| C20:2 | 1.35 ± 0.11 | 0.81 ± 0.02 | 0.82 ± 0.04 | 1.29 ± 0.09 | 0.59 ± 0.08 | 0.67 ± 0.01 |
| C20:3n6 | 0.48 ± 0.04 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.46 ± 0.04 | 0.20 ± 0.01 | 0.19 ± 0.01 |
| C20:4n6 | 0.85 ± 0.08 | 0.52 ± 0.02 | 0.54 ± 0.04 | 0.92 ± 0.05 | 0.74 ± 0.04 | 0.71 ± 0.03 |
| SAF | 169 ± 6 | 179 ± 7 | 182 ± 6 | 178 ± 11 | 176 ± 5 | 174 ± 3 |
| MUFA | 315 ± 9 | 377 ± 15 | 355 ± 10 | 316 ± 17 | 330 ± 8 | 332 ± 6 |
| PUFA | 191 ± 5ab | 217 ± 8a | 206 ± 7ab | 198 ± 8ab | 189 ± 6ab | 182 ± 4b |
Means ± SEM, not sharing a common letter within the same row differ (P < 0.05). Data not available for 0.6% diet analysis due to insufficient amounts of epididymal WAT. L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA. Bolded data represent detected amounts of 9,11 CLA and 10,12 CLA.
TABLE 2.
Fatty acid content of liver
| 0.06% Diet |
0.2% Diet |
0.6% Diet |
|||||||
| Tissue (mg/g) | L | 10 | M | L | 10 | M | L | 10 | M |
| C12:0 | 0.02 ± 0.00c | 0.02 ± 0.00c | 0.02 ± 0.00c | 0.04 ± 0.01bc | 0.05 ± 0.01bc | 0.05 ± 0.02bc | 0.02 ± 0.00c | 0.07 ± 0.01ab | 0.10 ± 0.02a |
| C14:0 | 0.50 ± 0.06bc | 0.66 ± 0.13bc | 0.49 ± 0.08bc | 0.83 ± 0.18bc | 1.16 ± 0.12bc | 1.23 ± 0.28b | 0.42 ± 0.07c | 2.19 ± 0.22a | 2.62 ± 0.31a |
| C14:1 | 0.05 ± 0.01d | 0.08 ± 0.01bcd | 0.06 ± 0.01cd | 0.09 ± 0.02bcd | 0.12 ± 0.02abcd | 0.15 ± 0.05abc | 0.05 ± 0.01d | 0.16 ± 0.02ab | 0.2 ± 0.03a |
| C15:0 | 0.07 ± 0.01d | 0.07 ± 0.01d | 0.06 ± 0.01d | 0.09 ± 0.01cd | 0.15 ± 0.01b | 0.14 ± 0.02bc | 0.06 ± 0.01d | 0.22 ± 0.02a | 0.25 ± 0.02a |
| C16:0 | 15 ± 1bc | 16 ± 2bc | 13 ± 1c | 20 ± 3bc | 30 ± 2b | 25 ± 4bc | 12 ± 2c | 65 ± 5a | 77 ± 7a |
| C16:1 | 3.5 ± 0.4b | 4.7 ± 0.8b | 3.5 ± 0.5b | 5.6 ± 1.1b | 8.1 ± 0.9b | 8.6 ± 2.0b | 3.0 ± 0.5b | 15.6 ± 2.2a | 18.8 ± 3.1a |
| C17:1 | 0.10 ± 0.01d | 0.13 ± 0.02cd | 0.10 ± 0.01d | 0.16 ± 0.03bcd | 0.30 ± 0.03b | 0.26 ± 0.05bc | 0.09 ± 0.02d | 0.57 ± 0.05a | 0.66 ± 0.05a |
| C18:0 | 2.6 ± 0.1bc | 1.8 ± 0.2d | 2.2 ± 0.1cd | 2.6 ± 0.1bc | 3.0 ± 0.1b | 2.0 ± 0.1cd | 2.2 ± 0.2cd | 4.1 ± 0.2a | 3.8 ± 0.3a |
| C18:1cis | 18 ± 2bc | 20 ± 3bc | 15 ± 1c | 26 ± 4bc | 35 ± 3b | 30 ± 5bc | 15 ± 2c | 79 ± 6a | 95 ± 9a |
| C18:2cis | 9 ± 1 | 10 ± 1 | 9 ± 1 | 12 ± 2 | 17 ± 1 | 14 ± 2 | 8 ± 1 | 13 ± 1 | 13 ± 1 |
| C18:2trans | 0.04 ± 0.01b | 0.04 ± 0.01b | 0.02 ± 0.01b | 0.06 ± 0.02b | 0.08 ± 0.01b | 0.06 ± 0.02b | 0.02 ± 0.01b | 0.19 ± 0.02a | 0.26 ± 0.03a |
| 9,11 CLA | 0.07 ± 0.01c | 0.09 ± 0.01c | 0.15 ± 0.02c | 0.09 ± 0.01c | 0.14 ± 0.01c | 0.51 ± 0.08b | 0.07 ± 0.01c | 0.11 ± 0.01c | 1.63 ± 0.09a |
| 10,12 CLA | 0.04 ± 0.00c | 0.06 ± 0.01c | 0.06 ± 0.01c | 0.04 ± 0.00c | 0.17 ± 0.01b | 0.13 ± 0.02b | 0.04 ± 0.00c | 0.35 ± 0.03a | 0.41 ± 0.02a |
| C18:3n3 | 0.43 ± 0.06 | 0.49 ± 0.09 | 0.41 ± 0.04 | 0.55 ± 0.11 | 0.74 ± 0.05 | 0.63 ± 0.12 | 0.29 ± 0.05 | 0.27 ± 0.03 | 0.3 ± 0.02 |
| C18:3n6 | 0.17 ± 0.02 | 0.18 ± 0.03 | 0.15 ± 0.02 | 0.24 ± 0.06 | 0.33 ± 0.03 | 0.22 ± 0.05 | 0.14 ± 0.03 | 0.29 ± 0.02 | 0.29 ± 0.02 |
| C20:0 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.09 ± 0.02 | 0.15 ± 0.02 | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.12 ± 0.01 | 0.09 ± 0.01 |
| C20:1 | 0.3 ± 0.04cd | 0.33 ± 0.06cd | 0.23 ± 0.02d | 0.35 ± 0.05cd | 0.53 ± 0.03bc | 0.33 ± 0.05cd | 0.26 ± 0.05d | 0.76 ± 0.07ab | 0.97 ± 0.13a |
| C20:2 | 0.26 ± 0.03bc | 0.24 ± 0.03c | 0.22 ± 0.02c | 0.28 ± 0.03abc | 0.38 ± 0.03ab | 0.26 ± 0.03bc | 0.23 ± 0.04c | 0.29 ± 0.03abc | 0.40 ± 0.03a |
| C20:3n3 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 |
| C20:3n6 | 0.38 ± 0.03abc | 0.28 ± 0.02cd | 0.36 ± 0.02bcd | 0.41 ± 0.03ab | 0.49 ± 0.01a | 0.34 ± 0.02bcd | 0.32 ± 0.05bcd | 0.30 ± 0.02bcd | 0.24 ± 0.02d |
| C20:4n6 | 4.0 ± 0.2bcd | 2.8 ± 0.4d | 4.0 ± 0.2bcd | 4.4 ± 0.3abc | 5.2 ± 0.1ab | 4.1 ± 0.2bcd | 3.6 ± 0.5cd | 5.4 ± 0.2a | 4.2 ± 0.3abc |
| C20:5n5 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.01 |
| C22:0 | 0.02 ± 0.00b | 0.00 ± 0.00b | 0.01 ± 0.00b | 0.01 ± 0.01b | 0.02 ± 0.01b | 0.05 ± 0.01b | 0.01 ± 0.00b | 0.03 ± 0.01b | 0.18 ± 0.05a |
| C22:1 | 0.07 ± 0.01a | 0.06 ± 0.01ab | 0.08 ± 0.00a | 0.10 ± 0.01a | 0.10 ± 0.01a | 0.07 ± 0.01a | 0.07 ± 0.01a | 0.05 ± 0.02ab | 0.02 ± 0.01b |
| C22:5 | 0.31 ± 0.03ab | 0.24 ± 0.02c | 0.31 ± 0.03ab | 0.37 ± 0.06ab | 0.45 ± 0.02ab | 0.31 ± 0.05ab | 0.34 ± 0.05ab | 0.65 ± 0.05a | 0.41 ± 0.05ab |
| C22:6n3 | 2.3 ± 0.2bc | 2.1 ± 0.4bc | 2.6 ± 0.2abc | 2.5 ± 0.2abc | 3.4 ± 0.1a | 2.4 ± 0.1abc | 2.0 ± 0.3c | 3.0 ± 0.2ab | 2.3 ± 0.2bc |
| C24:0 | 0.08 ± 0.01b | 0.07 ± 0.01b | 0.09 ± 0.01b | 0.09 ± 0.02b | 0.14 ± 0.01a | 0.08 ± 0.01b | 0.05 ± 0.01b | 0.08 ± 0.01b | 0.05 ± 0.01b |
| SAT | 18 ± 2bc | 19 ± 3bc | 16 ± 1c | 24 ± 3bc | 34 ± 2b | 29 ± 4bc | 15 ± 2c | 72 ± 5a | 84 ± 8a |
| MUFA | 22 ± 2bc | 26 ± 4bc | 19 ± 2c | 33 ± 5bc | 44 ± 4b | 39 ± 7bc | 18 ± 3c | 96 ± 8a | 115 ± 11a |
| PUFA | 18 ± 1bc | 17 ± 2c | 17 ± 1bc | 21 ± 2abc | 28 ± 1a | 23 ± 2abc | 15 ± 2c | 24 ± 1ab | 23 ± 1abc |
Means ± SEM, not sharing a common letter within the same row differ (P < 0.05). L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA. Bolded data represent detected amounts of 9,11 CLA and 10,12 CLA.
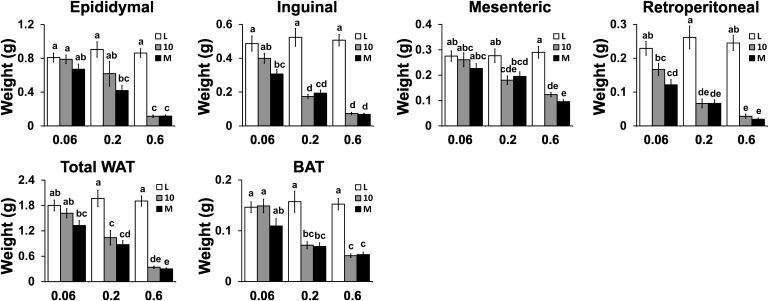
Intermediate and high doses of CLA treatments decrease adiposity
The intermediate and high doses of both CLA treatments decreased total body weight gain (supplementary Table V) and total WAT depot weights (e.g., adiposity index; Fig. 1) compared with the linoleic acid (L) controls. Both CLA treatments decreased body and WAT depot weights to the same degree, with the EPI and ING depots losing the most grams of WAT. The abundance of several fatty acids in the EPI depot was decreased by the intermediate dose of both CLA treatments, including C10:0, C14:1, C16:1, and C18:3 n3 (Table 1). The weight of subscapular brown adipose tissue (BAT) was also decreased by intermediate and high doses of both CLA treatments (Fig. 1). Although food intake was not significantly (P > 0.05) reduced by CLA treatments, it was 4–7% lower in mice fed 10,12 CLA compared with mice fed linoleic acid alone. The high dose of both CLA treatments impaired the efficiency of food conversion to body weight gain compared with the linoleic acid controls (supplementary Table V). Collectively, these data show that the reduction in adiposity by CLA is dose- and WAT depot-dependent and that the inclusion of 9,11 CLA with 10,12 CLA in the diets of mice does not further reduce adiposity.
Fig. 1.
Intermediate and high doses of CLA treatments decrease adiposity. Young, male 129SV mice (N = 10 per treatment group) were fed for 7 weeks a standard, purified mouse diet containing 0.06, 0.2, or 0.6% linoleic acid control (L), 10,12 CLA plus linoleic acid (10), or 10,12 CLA plus 9,11 CLA (M). Epididymal, inguinal, retroperitoneal, mesenteric, and BAT depots were excised and weighed. Means ± SEM without a common letter differ (P < 0.05).
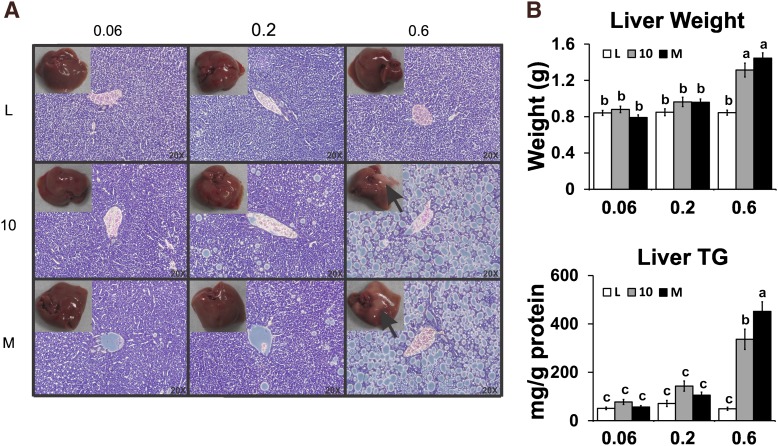
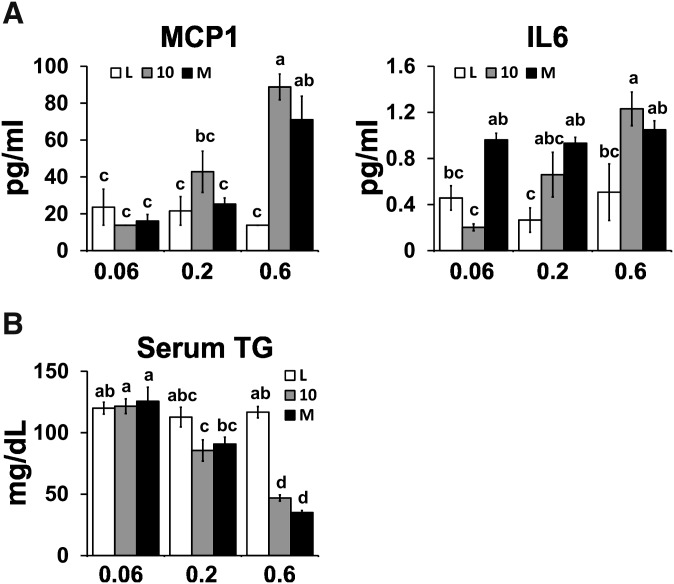
High dose of CLA treatments cause hepatic steatosis, hepatomegaly, and elevated serum levels of MCP-1
Given the reported adverse side effects of high doses of CLA in rodents (reviewed in Refs. 3, 5), we examined the effects of three doses of CLA on these outcomes. The high dose, but not the low or intermediate doses, of both CLA treatments caused hepatic steatosis (Fig. 2), increased FA levels (data not shown), hepatomegaly (Fig. 2B), and elevated serum levels of MCP-1 (Fig. 3A), a proinflammatory chemokine that recruits macrophages to WAT. Serum levels of IL-6 were elevated in the intermediate dose of the CLA isomer mixture (Fig. 3A). Notably, none of the CLA treatments increased serum levels of IL-1β, TNFα, or FFA (data not shown), nor increased TG levels (Fig. 3B), nor impaired fasting glucose, insulin, or glucose tolerance (supplementary Table V). These data suggest that there is a threshold dose (i.e., 0.1% 10,12 CLA plus 0.1% linoleic acid or 0.2% mixed CLA isomers) for both CLA treatments in mice that reduce adiposity without causing steatosis.
Fig. 2.
High dose of CLA treatments cause hepatic steatosis and hepatomegaly. A: Livers were excised and weighed, and then a section of liver was fixed in formalin, sliced, and stained with hematoxylin and eosin. B: Liver weights were recorded, liver TG and protein contents were measured, and the ratio of TG per milligram of protein was used as an indicator of steatosis. Means ± SEM without a common letter differ (P < 0.05). L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA.
Fig. 3.
High dose of CLA treatments increases serum MCP-1 levels. A: Serum levels of MCP-1 and IL-6. B: Serum TG levels. Means ± SEM without a common letter differ (P < 0.05). L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA.
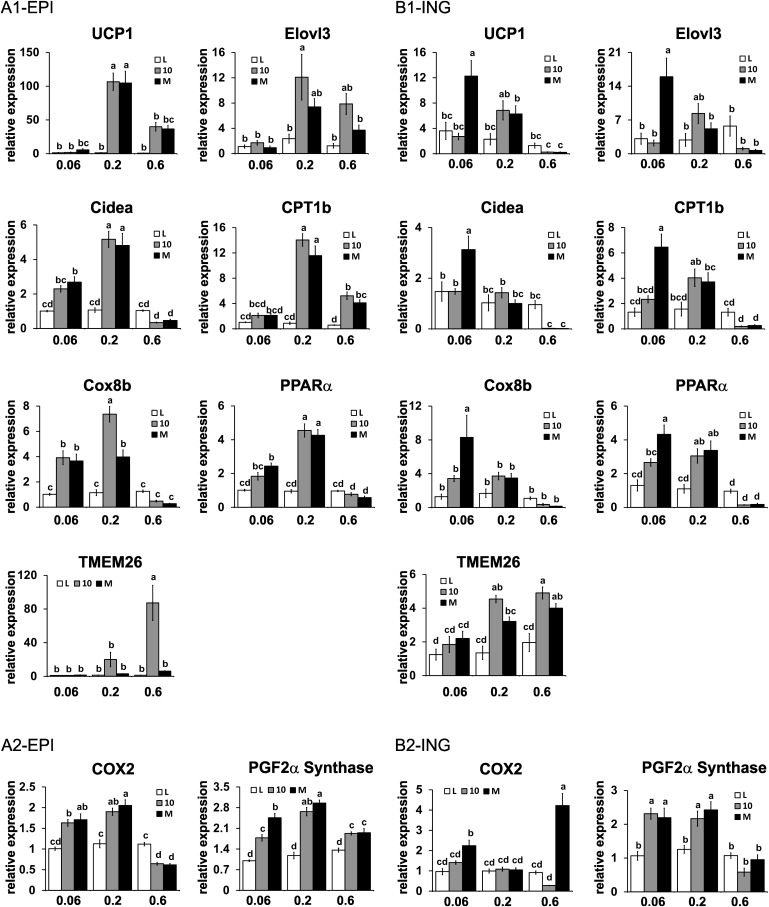
Intermediate or low doses of 10,12 CLA or mixed CLA isomers increase mRNA markers or activators of browning in a WAT depot-specific manner
To better understand how CLA reduces adiposity, we measured the mRNA levels of genes associated with browning and fatty acid oxidation in visceral (i.e., EPI, RET) and subcutaneous (i.e., ING) WAT. CLA treatments impacted multiple markers of browning in WAT in a depot-specific manner (Fig. 4, supplementary Fig. I). For example, UCP1 (uncouples respiration from ATP synthesis), elongation of very long chain fatty acids 3 protein (Evol3; elongates fatty acids), cell death-induced DNA fragmentation factor-a-like effector A (Cidea; regulates lipid droplet formation), CPT-1b (facilitates fatty acid transport into mitochondria for oxidation), cytochrome c oxidase VIII b (Cox8b; promotes electron transfer during mitochondrial respiration), and peroxisome proliferator-activated receptor α (PPARα induces genes associated with fatty acid oxidation) were increased approximately 1- to 100-fold by the intermediate dose of 10,12 CLA or the CLA isomer mixture in EPI WAT (Fig. 4A1). In RET WAT, the intermediate dose of both CLA treatments increased the expression of CPT-1b, Cox8b, and PPARα (supplementary Fig. IA). In contrast, only the low dose of the CLA isomer mixture increased these markers of browning in ING WAT (Fig. 4B1). Interestingly, the mRNA levels of transmembrane protein (TMEM) 26, a gene expressed in 129Sv mice and human beige adipocytes, which are distinct from white or brown adipocytes and inducible with cAMP analogs or cold exposure (25), were increased by the high dose or the intermediate and high dose of 10,12 CLA in the EPI and ING depots, respectively. Unexpectedly, the high doses of both CLA treatments did not increase markers of browning in EPI, RET, or ING WAT, with the exception of CPT-1b in EPI WAT, even though these CLA-treated mice had markedly decreased adiposity and impaired food conversion efficiencies, a hallmark of increased thermogenesis.
Fig. 4.
Intermediate dose of CLA treatments increases markers of browning in epididymal (EPI) WAT. mRNA levels of markers of brown fat-like adipocytes were measured in EPI (A) or inguinal (ING) (B) WAT by real-time qPCR. (A1) and (B1) are direct markers of browning, and (A2) and (B2) are markers of prostaglandin production that are associated with the activation of browning. Means ± SEM without a common letter differ (P < 0.05). L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA.
We have previously shown that 10,12 CLA increases the expression of COX-2, a nuclear factor kappa B (NF-κB)-inducible gene, and PG production in human adipocytes (13), which has been linked to induction of brown fat-like adipocytes in WAT (14, 15). Consistent with our hypothesis, the low and intermediate doses of CLA treatments increased the expression of COX-2 and PGF2α synthase in EPI WAT (Fig. 4A2), the depot where CLA had its greatest effects on markers of browning. In RET (supplementary Fig. IB) and ING WAT (Fig. 4B2), the low dose of the CLA isomer mixture increased COX-2 and PGF2α synthase expression, which is consistent with the induction of browning in these depots. The intermediate dose of both CLA treatments increased PGF2α synthase expression in all three WAT depots.
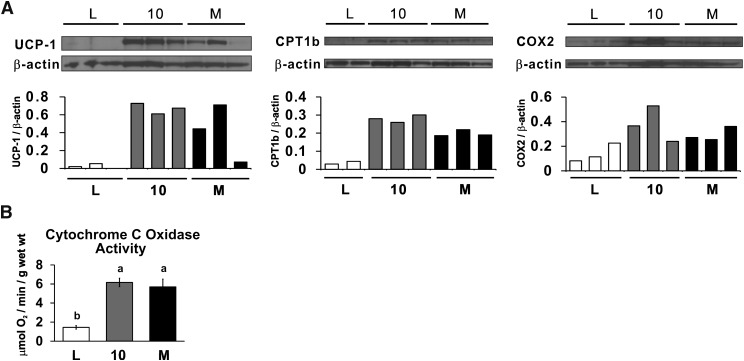
Intermediate dose of CLA treatments increase the protein or activity levels of browning markers in WAT
Consistent with the mRNA data above, 0.1% 10,12 CLA plus 0.1% linoleic acid (10) and 0.2% CLA isomer mixture (M) increased the protein levels of UCP1, CPT-1b, and COX-2 and the activity of cytochrome c oxidase in EPI WAT compared with the linoleic acid (L) controls (Fig. 5). In contrast, UCP1 protein levels in BAT and cytochrome c oxidase activity in muscle were not affected by CLA treatment (data not shown). Taken together, these data suggest that the CLA-mediated reduction in adiposity is more likely due to increased mitochondria activity in WAT and not in BAT or muscle.
Fig. 5.
Intermediate dose (0.2%) of CLA treatments increases the protein or activity levels of browning markers in epididymal (EPI) WAT. A: Protein levels of UCP1, CPT-1b, COX-2, and β actin (load control) were measured in homogenates of EPI WAT. B: Activity of cytochrome c oxidase was measured in homogenates of EPI WAT. Means ± SEM without a common letter differ (P < 0.05). L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA.
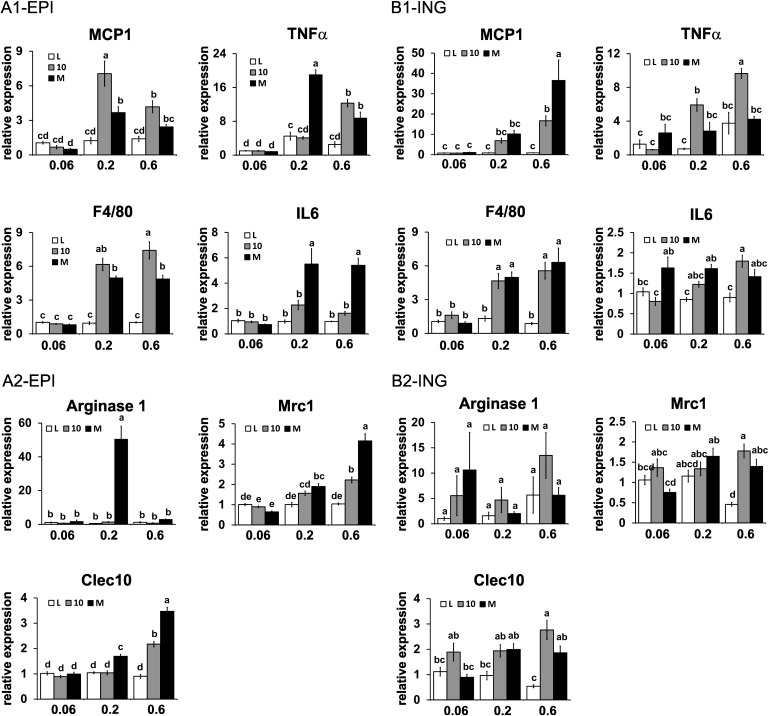
CLA treatments increase mRNA markers of low-grade inflammation in WAT
Increased NF-κB-driven inflammation has recently been shown to prevent obesity by increasing energy expenditure (26, 27). Furthermore, low-grade inflammation in WAT is characterized by recruitment of macrophages, including classically activated M1 macrophages and alternatively activated M2 macrophages. Here, we show that the mRNA levels of the proinflammatory M1 macrophage markers MCP-1, IL-6, F4/80, and TNFα were elevated in the intermediate and high dose of 10,12 CLA plus linoleic acid or CLA isomer mixture in EPI (Fig. 6A1), RET (supplementary Fig. IIA), and ING (Fig. 6B1) WAT.
Fig. 6.
CLA treatments increase mRNA markers of inflammation in epididymal (EPI) and inguinal (ING) WAT. mRNA levels of markers of low-grade inflammation were measured in EPI (A) and ING (B) WAT by real-time qPCR. (A1) and (B1) are markers of classically activated M1 macrophage markers, and (A2) and (B2) are alternatively activated M2 macrophage markers. Means ± SEM without a common letter differ (P < 0.05). L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA; Arg1, arginase-1; Mrc1, mannose receptor c1.
Given the role of alternatively activated, M2 macrophages in inflammation, including clearing apoptotic cells and necrotic tissue (reviewed in Ref. 28), we examined the influence of CLA treatments on the expression of three markers of the M2 macrophage phenotype [i.e., arginase 1, mannose receptor c1 (Mrc1 or CD206), and Clec10 (CD301)] (29). In EPI WAT, only the intermediate dose of the CLA isomer mixture increased arginase 1 expression, whereas the intermediate and high doses of the CLA isomer mixture increased Mrc1 and Clec10a expression (Fig. 6A2). In RET WAT, only the high dose of the mixed CLA isomers increased arginase 1, Mrc1, and Clec10 expression (supplementary Fig. IIB). In ING WAT, the high dose of both CLA treatments increased Mrc1 and Clec10 expression (Fig. 6B2). In general, M2 macrophage markers were expressed to the greatest extent in WAT having the highest expression levels of M1 macrophage markers, except for arginase 1 in EPI WAT.
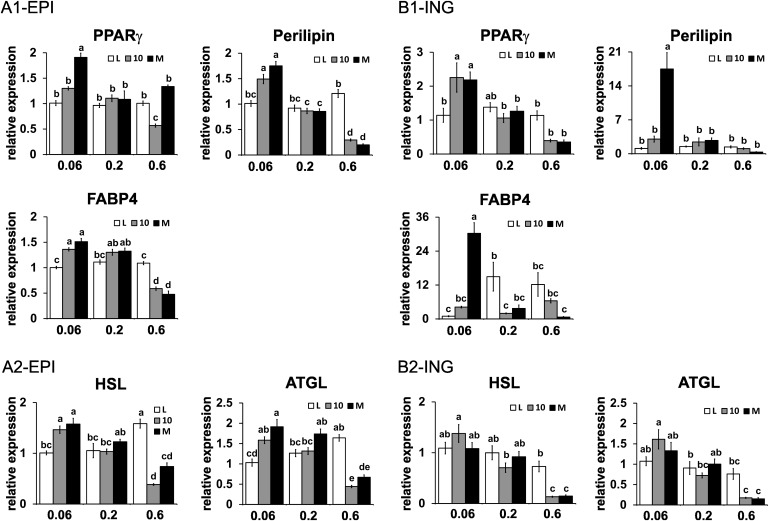
Impact of CLA treatment on mRNA markers of lipogenesis and lipolysis
Given the similar decrease in adiposity by both CLA treatments in the intermediate and high doses, we determined the extent to which this reduction correlated with changes in the expression of genes associated with lipogenesis and lipolysis. Surprisingly, only the highest dose of 10,12 CLA or the CLA isomer mixture decreased the mRNA levels of PPARγ or several of its target genes (i.e., perilipin, FABP4) in EPI WAT (Fig. 7A), RET WAT (supplementary Fig. IIIA), and ING WAT (Fig. 7B). The mRNA levels of hormone sensitive lipase and adipose tissue TG lipase, enzymes that control lipolysis, were decreased by the high dose of both CLA treatments in EPI (Fig. 7A2), RET (supplementary Fig. IIIB), and ING (Fig. 7B2). In general, the expression of genes associated with lipogenesis and lipolysis were not affected by the intermediate dose of CLA treatments.
Fig. 7.
Impact of CLA treatment on mRNA markers of lipogenesis and lipolysis in epididymal (EPI) and inguinal (ING) WAT. mRNA levels of markers of lipogenesis (A1, B1) and lipolysis (A2, B2) were measured in EPI (A) and ING (B) WAT by real-time qPCR. Means ± SEM without a common letter differ (P < 0.05). L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA; HSL, hormone sensitive lipase; ATGL, adipose tissue TG lipase.
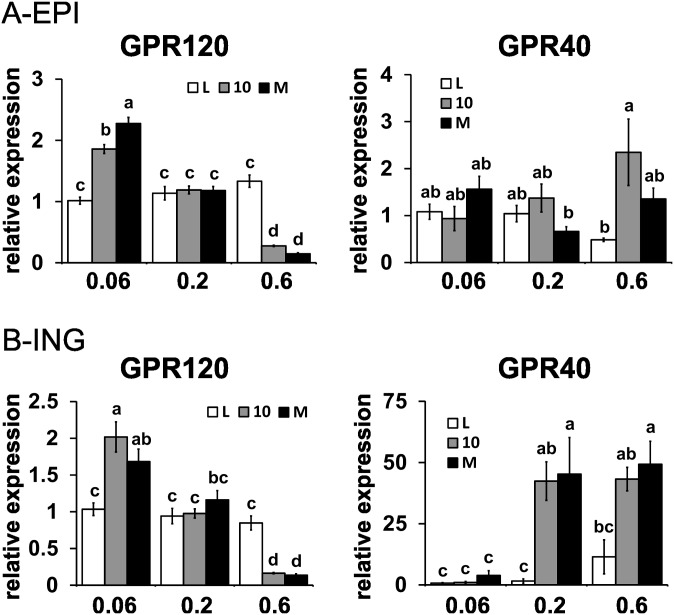
FFA receptors GPR120 and GPR40 in WAT are differentially regulated by CLA
FFA activate FFA receptors, G-protein receptors (GPR), and G-protein-coupled receptors (GPCR), which influence glucose and lipid metabolism, intracellular calcium levels, and signaling pathways (30–35). GPR40 (35, 36) and GPR120 (30, 37) are activated by long-chain FFA, and their activation is associated with increased intracellular calcium levels and extracellular signal-related kinase (ERK) activation, similar to what we have shown in human adipocytes treated with 10,12 CLA (38). Although we were unable to measure the activities of GPR120 and GPR40, we determined the effects of CLA on their expression levels. The low dose of both CLA treatments increased the expression of GPR120, whereas the high dose decreased GPR120 expression in all three WAT depots (Fig. 8, supplementary Fig. IV). Given the anti-inflammatory properties of GPR120 (30), our finding of the induction of proinflammatory M1 markers in the high dose of CLA treatments is consistent with decreased GRP120 expression in WAT. Although the expression levels of GRP40 were extremely low in WAT, the high dose of 10,12 CLA plus linoleic acid or the CLA isomer mixture induced the expression of GPR40 (Fig. 8, supplementary Fig. IV), particularly in the ING depot (Fig. 8B). Taken together, these data suggest that the expression levels of the FFA receptors GPR120 and GPR40 in WAT are differentially regulated by CLA isomers and may play a role in CLA-mediated downstream signaling in WAT.
Fig. 8.
FFA receptors GPR120 and GPR40 in epididymal (EPI) and inguinal (ING) WAT are differentially regulated by CLA. mRNA levels of markers of GPR 120 and GPR40 in EPI (A) and ING (B) were measured by real-time qPCR. Means ± SEM without a common letter differ (P < 0.05). L, linoleic acid control; 10, 10,12 CLA plus linoleic acid; M, 10,12 CLA plus 9,11 CLA.
DISCUSSION
We conducted this study to resolve contradictions in the literature concerning the dose- and isomer-specific effects of CLA on i) incorporation in WAT, liver, and muscle; ii) adiposity; iii) steatosis, hyperlipidemia, and insulin resistance; and iv) browning and inflammation in WAT. We used the 10,12 CLA isomer because it is the main isomer reported to reduce adiposity (reviewed in Ref. 3) and the CLA isomer mixture because it is used commercially in supplements and fortified foods and in most human studies. We purchased relatively pure 10,12 CLA and 9,11 CLA isomers to avoid issues related to chemical manipulation and storage associated with commercially available supplements. Linoleic acid was used as a control fatty acid, because it contains the same number of carbons and carbon-carbon double bonds as CLA. We chose the 129Sv mouse model because of its capacity to induce thermogenesis when exposed to cold or β-3 agonists compared with the C57Bl6J mouse (39). Young, growing mice fed a normal mouse diet were used in order to examine the ability of CLA to prevent the accumulation of body fat. Thus, we did not examine the ability of CLA to treat adult mice that were already overweight or obese. A 7-week feeding period was used based on work by Parra et al. (6).
CLA isomers incorporate into WAT and decrease adiposity
We demonstrated that 10,12 and 9,11 CLA isomers incorporated into WAT (Table 1) and liver (Table 2) and that 9,11 CLA in the 0.2% dose of the mixed CLA treatment incorporated into muscle (supplementary Table IV), consistent with reports of incorporation into rodent liver (40, 41) or human WAT and muscle (42). WAT was the preferred storage organ for CLA isomers, consistent with work by Andreoli et al. (40). As demonstrated in many rodent studies, the high level (0.6%) of both CLA treatments robustly decreased adiposity in growing mice (Fig. 1). This reduction was not due to decreased food intake (supplementary Table V). Consistent with work by Parra et al. (6), who fed 0.1% and 0.3% (w/w) mixed CLA isomers to young C57BL6 mice consuming a low-fat diet for 35 days, 129Sv mice fed the intermediate dose of both CLA treatments (i.e., 0.1% 10,12 CLA plus 0.1% linoleic acid or 0.2% of mixed CLA isomers) had decreased adiposity. In general, the intermediate and high doses of 10,12 CLA (i.e., 0.1% and 0.3%, respectively) were equally effective as the CLA isomer mixture (i.e., 0.2 and 0.6%, respectively) in reducing adiposity, suggesting the 9,11 CLA isomer had no additive or synergistic effect on reducing body fat when combined with 10,12 CLA. In contrast, the low dose of both CLA treatments did not reduce adiposity. Thus, there appears to be a threshold dose of 10,12 CLA alone (i.e., 0.1%) and mixed CLA isomers (i.e., 0.2%) that significantly reduces adiposity in young growing mice over a 7-week feeding period without causing steatosis. Strikingly, increasing the 10,12 CLA dose by 3-fold (i.e., 0.1% to 0.3%) caused steatosis, demonstrating the small window of opportunity for fat loss without causing steatosis.
Increase in steatosis and markers of inflammation
Consistent with several studies using 0.5% or more 10,12 CLA or a CLA isomer mixture in the diet (reviewed in Ref. 3), the high dose of both CLA treatments increased steatosis, as demonstrated by overt appearance of lipid in the liver (Fig. 2A), increased liver weight and TG content (Fig. 2B), and saturated and monounsaturated FA content (Table 2). In contrast, the low and intermediate doses of both CLA treatments did not cause steatosis (Fig. 2), hepatomegaly (Fig. 2B), or markedly affect the FA profile. This increase in steatosis and lack of fasting hyperglycemia at the 0.6% level of CLA suggest insulin resistance and could be due to increased insulin levels (36, 43, 44), given insulin's lipogenic actions. However, CLA did not increase serum insulin levels or cause insulin resistance (i.e., no increase in HOMA-IR score) in our study. In parallel with steatosis, serum levels of MCP-1 and IL-6 (Fig. 3A) and ING WAT mRNA levels of MCP-1 (Fig. 6B1) were highest in the high-CLA groups. These data show that a relatively high dose of 10,12 CLA (0.3%) and an equal CLA isomer mixture (0.6%), equivalent to 10 times one of the maximum doses consumed by humans when expressed per kilogram of body weight (19), causes lipoatrophy, inflammation, and steatosis.
Alternatively activated M2 macrophages attenuate inflammation and protect against metabolic diseases, including insulin resistance (reviewed in Ref. 45). We found that the high dose of CLA treatments increased the expression of several M2 markers in WAT (i.e., Mrc, Clec10; Fig. 6), along with markers of classically activated M1 macrophages (i.e., TNFα, MCP-1, F4/80; Fig. 6). Thus, the increase in M2 markers in WAT of mice in the high-CLA dose may be due to their role in clearance of apoptotic cells or necrotic tissue in inflamed WAT (reviewed in Refs. 28, 30).
Increase in browning of WAT
Energy expenditure is a function of basal metabolic rate (BMR), adaptive thermogenesis, and physical activity. CLA has been proposed to reduce adiposity by enhancing energy expenditure via increasing BMR or thermogenesis, thereby increasing fat oxidation (46–50). Enhanced thermogenesis is associated with an upregulation of UCP1, which facilitates proton transport across the inner mitochondrial membrane, thereby diverting energy from ATP synthesis to heat production. UCP1 is expressed primarily in brown adipocytes, but it is inducible in brite adipocytes (14, 15, 39, 51–54) and linked to thermogenesis, fatty acid oxidation, and decreased adiposity (reviewed in Ref. 55). Brite or beige adipocytes in WAT can arise from mesenchymal stem cells that express PRDM16 (52) upon stimulation of PGC-1α by PPARγ agonists like rosiglitazone (14, 39). Alternatively, they can (trans)differentiate from mature white adipocytes (39, 53, 54). Notably, activation of COX-2 (14, 15) is a key initiator of brite adipocyte recruitment. COX-2 produces PGs that enhance mitochondrial biogenesis and increase the uncoupling capacity when activated with adrenergics or cold exposure (53).
Several studies have shown that high doses of a mixture of CLA isomers (1.5%) (16) or 10,12 alone (1%) (17) decreased adiposity and increased UCP1 and CPT-1b expression in EPI or RET WAT, respectively, supporting the hypothesis that CLA decreases body fat, at least in part, by uncoupling mitochondria in WAT. Consistent with our hypothesis, the intermediate dose of 10,12 CLA or the CLA isomer mixture increased mRNA or protein markers of browning (i.e., UCP1, Cidea, Elovl3, CPT-1b, Cox8b, PPARα); fatty acid oxidation (i.e., PPARα, CPT-1b); cytochrome c oxidase activity; and PG production (i.e., COX-2, PGF2α synthase) in EPI WAT (Figs. 4, 5) without causing steatosis (Fig. 2). Unexpectedly, mRNA markers of browning were not increased by the high dose of CLA treatments in WAT, even though this dose decreased adiposity the most. Indeed, LaRosa et al. (18) showed that the expression of UCP1 in 10,12 CLA-treated mice gradually increased after 2, 4, 7, and 10 days of treatment, and then decreased 70% by day 17. Thus, it is possible that the high dose of CLA treatment increased WAT browning acutely, causing the most rapid depletion of lipid in WAT (i.e., lipoatrophy). Subsequently, the depletion of WAT caused a downregulation of browning and other genes associated with energy metabolism by the end of the 7-week study. Alternatively, the robust decrease in WAT by the high dose of CLA could be due to general dedifferentiation or adipocyte apoptosis (reviewed in Ref. 3).
Effects of CLA on GPR40 and GPR120
10,12 CLA, but not 9,11 CLA, has been shown to inhibit fatty acid transport and cAMP content in tumors and ING WAT by activating an inhibitory GPCR, which was blocked using pertussis-toxin, an inhibitor of heptahelical GPCR associated with CXCR1 and CXCR2 receptors that are coupled to calcium mobilization and ERK activation (56). Moreover, CLA activates GPR40 and increases intracellular calcium accumulation in pancreatic β cells, thereby stimulating insulin secretion (36). Previously, we showed that 10,12 CLA-mediated decrease in glucose and fatty acid uptake in primary adipocytes was prevented by pertussis-toxin, possibly via inhibition of MEK/ERK signaling (57). Furthermore, we demonstrated that 10,12 CLA's activation of inflammatory signaling and suppression of PPARγ and glucose uptake were dependent on increased intracellular calcium levels (38). Consistent with these data, we found in this study that the high dose of CLA treatments increased the expression of GPR40 in ING WAT (Fig. 8B), suggesting a potential role of this FFA receptor in mediating some of the proinflammatory effects of CLA in WAT. GPR40 inhibitor or silencing experiments are needed to test this hypothesis.
The expression of the long-chain FFA receptor GPR120, which has been shown to be activated by omega-3 fatty acids and to repress macrophage-mediated inflammation (30), was increased by the low dose of CLA treatments in EPI (Fig. 8A), RET (supplementary Fig. IV), and ING (Fig. 8B) WAT, and decreased by the high dose of CLA treatments. Consistent with these data, we found that a relatively high dose of 10,12 CLA, but not 9,11 CLA or oleic acid, decreased GPR120 expression in human adipocytes (58). Thus, it is tempting to speculate that CLA suppression of GPR120 contributes to the severe proinflammatory status of mice consuming the high dose of CLA.
In summary, our data showed in young 129Sv mice that i) CLA isomers incorporate into WAT and liver in a dose-dependent manner; ii) there is an intermediate, threshold dose of 10,12 CLA (i.e., 0.1% 10,12 CLA plus 0.1% linoleic acid) and mixed CLA isomers (i.e., 0.1% 10,12 CLA plus 0.1% 9,11 CLA) that reduces adiposity without causing steatosis or elevating serum FFA, TG, glucose, or insulin levels; iii) an equal isomer mixture of CLA reduced adiposity to the same extent as 10,12 CLA plus linoleic acid, demonstrating that the 9,11 isomer of CLA does not further reduce body fat; iv) an intermediate dose of CLA treatments increases mRNA and protein markers of browning and cytochrome c oxidase activity in EPI WAT but not in BAT or muscle; v) an intermediate or high dose of CLA treatments increase the expression of several markers of low-grade inflammation in WAT; and vi) a high dose of CLA causes lipoatrophy, leading to steatosis and marked inflammation. Future kinetic studies are needed to examine how and when intermediate doses of CLA isomers impact exogenous and endogenous glucose and fatty acid utilization in WAT and liver over time and whether these isomers alone or in combination increase energy expenditure in vivo. A greater understanding of how important GPR120 and GPR40 are as mediators of CLA signaling is needed.
Supplementary Material
Acknowledgments
The authors thank Stephanie Marshall for assistance with the hepatic TG assay. The authors appreciate the critical review of this manuscript by Dr. Susanne Mandrup at the University of Southern Denmark. The authors are grateful for all of the helpful advice and thoughtful discussions with Dr. Ron Morrison at the University of North Carolina at Greensboro.
Footnotes
Abbreviations:
- AUC
- area under the curve
- BAT
- brown adipose tissue
- BMR
- basal metabolic rate
- 9,11 CLA
- cis-9, trans-11 conjugated linoleic acid
- 10,12 CLA
- trans-10, cis-12 conjugated linoleic acid
- Cidea
- cell death-induced DNA fragmentation factor-a-like effector A
- Cox8b
- cytochrome c oxidase subunit VIII b
- COX-2
- cyclooxygenase-2
- CPT
- carnitine palmitoyltransferase
- Elovl3
- elongation of very long chain fatty acids 3 protein
- EPI
- epididymal
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinase
- FABP
- fatty acid binding protein
- GPCR
- G protein-coupled receptor
- GPR
- G protein receptor
- GTT
- glucose tolerance test
- ING
- inguinal
- IL
- interleukin
- MAPK
- mitogen-activated protein kinase
- MCP
- monocyte chemoattractant protein
- MEK
- MAPK kinase
- Mrc
- mannose receptor c
- NF-κB
- nuclear factor kappa B
- PPAR
- peroxisome proliferator activated receptor
- PG
- prostaglandin
- RET
- retroperitoneal
- TBP
- TATA-binding protein
- TG
- triglyceride
- TMEM 26
- transmembrane protein 26
- TNFα
- tumor necrosis factor α
- UCP
- uncoupling protein
- WAT
- white adipose tissue
This work was supported by National Institutes of Health Grant 5R01-DK-063070-09 to M.M.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures and two tables.
REFERENCES
- 1.World Health Organization. Obesity and overweight. Accessed January 25, 2013, at www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Centers for Disease Control and Prevention. US obesity trends. Accessed January 25, 2013, at http://www.cdc.gov/obesity/data/trends.html.
- 3.Kennedy A., Martinez K., Schmidt S., Mandrup S., Lapoint K., McIntosh M. 2010. Antiobesity mechanisms of action of conjugated linoleic acid. J. Nutr. Biochem. 21: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whigham L. D., Watras A. C., Schoeller D. A. 2007. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am. J. Clin. Nutr. 85: 1203–1211. [DOI] [PubMed] [Google Scholar]
- 5.Poirier H., Niot I., Clement L., Guerre-Millo M., Besnard P. 2005. Development of conjugated linoleic acid (CLA)-mediated lipoatrophic syndrome in the mouse. Biochimie. 87: 73–79. [DOI] [PubMed] [Google Scholar]
- 6.Parra P., Palou A., Serra F. 2010. Moderate doses of conjugate linoleic acid reduce fat gain, maintain insulin sensitivity without impairing inflammatory adipose tissue status in mice fed a high-fat diet. Nutr. Metab. (Lond.) 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risérus U., Arner P., Brismar K., Vessby B. 2002. Treatment with dietary trans-10, cis-12 conjugated linoleic acid causes isomer specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 25: 1516–1521. [DOI] [PubMed] [Google Scholar]
- 8.Tholstrup T., Raff M., Staarup E., Lund P., Basu S., Bruun J. M. 2008. An oil mixture with trans-10, cis-12 conjugated linoleic acid increases markers of inflammation and in vivo lipid peroxidation compared with cis-9, trans-11 conjugated linoleic acid in post-menopausal women. J. Nutr. 138: 1445–1451. [DOI] [PubMed] [Google Scholar]
- 9.Moloney F., Toomey S., Noone E., Nugent A., Allan B., Loscher C. E., Roche H. M. 2007. Antidiabetic effects of cis-9, trans-11 conjugated linoleic acid may be mediated via anti-inflammatory effect in white adipose tissue. Diabetes. 56: 574–582. [DOI] [PubMed] [Google Scholar]
- 10.Halade G. V., Rahman M. M., Fernandes G. 2010. Differential effects of conjugated linoleic acid isomers in insulin-resistant female C57bl/6j mice. J. Nutr. Biochem. 21: 332–337. [DOI] [PubMed] [Google Scholar]
- 11.Almind K., Manieri K., Sivitz W., Cinti S., Kahn C. R. 2007. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA. 104: 2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cypress A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., et al. 2009. Indentification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez K., Kennedy A., West T., Milatovic D., Aschner M., McIntosh M. 2010. Trans-10,cis-12 conjugated linoleic acid instigates inflammation in human adipocytes compared with preadipocytes. J. Biol. Chem. 285: 17701–17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vegiopoulos A., Muller-Decker K., Strzoda D., Schmitt I., Chichelnitskiy E., Ostertag A., Diaz M. B., Rozman J., Angelis M. H. D., Nusing R. M., et al. 2010. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 328: 1158–1161. [DOI] [PubMed] [Google Scholar]
- 15.Madsen L., Pedersen L. M., Lillefosse H. H., Fjaere E., Bronstad I., Hao Q., Petersen R. K., Hallenborg P., Ma T., Matteis R. D., et al. 2010. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS ONE. 5: e11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wendel A. A., Purushotham A., Liu L. F., Belury M. A. 2009. Conjugated linoleic acid induces uncoupling protein 1 in white adipose tissue of ob/ob mice. Lipids. 44: 975–982. [DOI] [PubMed] [Google Scholar]
- 17.House R. L., Cassady J. P., Eisen E. J., Eling T. E., Collins J. B., Grissom S. F., Odle J. 2005. Functional genomic characterization of delipidation elicited by trans-10, cis-12 conjugated linoleic acid (t10c12-CLA) in a polygenic obese line of mice. Physiol. Genomics. 21: 351–361. [DOI] [PubMed] [Google Scholar]
- 18.LaRosa P. C., Miner J., Xia Y., Zhou Y., Kachman S., Fromm M. E. 2006. Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice: a microarray and histological analysis. Physiol. Genomics. 27: 282–294. [DOI] [PubMed] [Google Scholar]
- 19.Raff M., Tholstrup T., Brunn J., Lund P., Straarup E., Christensen R., Sandberg M., Mandrup S. 2009. Conjugated linoleic acids reduce body fat in health post-menopausal women for 12 weeks increases lean body mass in obese humans. J. Nutr. 139: 1347–1352. [DOI] [PubMed] [Google Scholar]
- 20.Li Z., Kaplan M., Hachey D. 2000. Hepatic microsomal and peroxisomal docosahexaenoate biosynthesis during piglet development. Lipids. 35: 1325–1333. [DOI] [PubMed] [Google Scholar]
- 21.Lin X., Bo J., Oliver S. A., Corl B. A., Jacobi S. K., Oliver W. T., Harrell R. J., Odle J. 2011. Dietary conjugated linoleic acid alters long chain polyunsaturated fatty acid metabolism in brain and liver of neonatal pigs. J. Nutr. Biochem. 22: 1047–1054. [DOI] [PubMed] [Google Scholar]
- 22.Potteiger J. A., Jacobsen D. J., Donnelly J. E. 2002. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int. J. Obes. Relat. Metab. Disord. 26: 87–89. [DOI] [PubMed] [Google Scholar]
- 23.Xu X., Ying Z., Cai M., Xu Z., Li Y., Jiang S. Y., Tzan K., Wang A., Parthasarathy S., He G., et al. 2011. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progeitor cell population in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300: R1115–R1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell C. R., Harris M. B., Cordaro A. R., Starnes J. W. 2002. Effect of body temperature during exercise on skeletal muscle cytochrome c oxidase content. J. Appl. Physiol. 93: 526–530. [DOI] [PubMed] [Google Scholar]
- 25.Wu J., Bostrum P., Sparks L., Ye L., Choi J., Giang A., Khandekar M., Virtanen K., Nuutila P., Schaart G., et al. 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 150: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang T., Zhang J., Yin J., Staszkiewicz J., Gawronska-Kozak B., Jung D. Y., Ko H. J., Ong H., Kim J. K., Mynatt R., et al. 2010. Uncoupling of inflammation and insulin resistance by NF-κB in transgenic mice through elevated energy expenditure. J. Biol. Chem. 285: 4637–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao P., Feng B., Ma J., Nie Y., Paul E., Li Y., Xu H. 2012. Constituitive activation of IKKβ in adipose tisssue prevents diet-induced obesity in mice. Endocrinology. 153: 154–165. [DOI] [PubMed] [Google Scholar]
- 28.Odegaard J. I., Chawla A. 2011. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 6: 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen K. D., Qiu Y., Cui X., Goh Y. P., Mwang J., David T., Mukundan L., Brombacher F., Locksley R. M., Chawla A. 2011. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 480: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-Inflammatory and insulin-sensitizing effects. Cell. 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotarsky K., Nilsson N. E., Flodgren E., Owman C., Olde B. 2003. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem. Biophys. Res. Commun. 301: 406–410. [DOI] [PubMed] [Google Scholar]
- 32.Soto-Guzman A., Robledo T., Lopez-Perez M., Salazar E. P. 2008. Oleic acid induces ERK1/2 activation and AP-1 DNA binding activity through a mechanism involving Src kinase and EGFR transactivation in breast cancer cells. Mol. Cell. Endocrinol. 294: 81–91. [DOI] [PubMed] [Google Scholar]
- 33.Briscoe C. P., Tadayyon M., Andrews J. L., Benson W. G., Chambers J. K., Eilert M. M., Ellis C., Elshourbagy N. A., Goetz A. S., Minnick D. T., et al. 2003. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278: 11303–11311. [DOI] [PubMed] [Google Scholar]
- 34.Qanbar R., Bouvier M. 2003. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol. Ther. 97: 1–33. [DOI] [PubMed] [Google Scholar]
- 35.Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., et al. 2003. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 422: 173–176. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt J., Liebscher K., Merten N., Grundmann M., Mielenz M., Sauerwein H., Christiansen E., Due-Hansen M. E., Ulven T., Ullrich S., et al. 2011. Conjugated linoleic acids mediate insulin release through islet G protein-coupled receptor FFA1/GPR40. J. Biol. Chem. 286: 11890–11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. 2005. Free fatty acids regulate gut incretin glucagon-like-peptide-1 secretion through GPR120. Nat. Med. 11: 90–94. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy A., Martinez K., Chung S., LaPoint K., West T., Hopkins R., Schmidt S., Andersen K., Mandrup S., McIntosh M. 2010. Inflammation and insulin resistance induced by trans-10, cis-12 conjugated linoleic acid are dependent on intracellular calcium levels in primary cultures of human adipocytes. J. Lipid Res. 51: 1906–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. 2010. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285: 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreoli M. F., Illesca P. G., Gonzalez M. A., Bernal C. A. 2010. Conjugated linoleic acid reduces hepatic steatosis and restores liver triacylglycerol secretion and the fatty acid profile during protein repletion in rats. Lipids. 45: 1035–1045. [DOI] [PubMed] [Google Scholar]
- 41.Porsgaard T., Xu X., Mu H. 2006. The form of conjugated linoleic acid does not influence plasma and liver triacylglycerol conentrations in Syrian gold hampsters. J. Nutr. 136: 2201–2206. [DOI] [PubMed] [Google Scholar]
- 42.Goedecke J. H., Rae D., Smuts C., Lambert E., O'Shea M. 2009. Conjugated linoleic acid isomers, t10,c12 and c9,t11, are differentially incorporated into adipose tissue and skeletal muscle in humans. Lipids. 44: 983–988. [DOI] [PubMed] [Google Scholar]
- 43.Tsuboyama-Kasaoka N., Takahashi M., Tanemura K., Kim H., Tange T., Okuyama H., Kasai M., Ikemoto S., Ezaki O. 2000. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes. 49: 1534–1542. [DOI] [PubMed] [Google Scholar]
- 44.Delany J. P., Bhohm E., Truett A., Scimeca J., West D. 1999. Conjugated linoleic acid rapidly reduces body fat content in mice without altering food intake. Am. J. Physiol. 276: R1172–R1179. [DOI] [PubMed] [Google Scholar]
- 45.Chawla A., Nguyen K. D., Goh Y. P. S. 2011. Macrophage mediated inflammation in metabolic disease. Nat. Rev. Immunol. 11: 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West D. B., Blohm F., Truett A., DeLany J. 2000. Conjugated linoleic acid persistently increases total energy expenditure in AKR/J mice without increasing uncoupling protein gene expression. J. Nutr. 130: 2471–2477. [DOI] [PubMed] [Google Scholar]
- 47.Terpstra A. H., Beynen A., Everts H., Kocsis S., Katan M., Zock P. 2002. The decrease in body fat in mice fed conjugated linoleic acid is due to increases in energy expenditure and energy loss in the excreta. J. Nutr. 132: 940–945. [DOI] [PubMed] [Google Scholar]
- 48.Terpstra A. H., Javadi M., Beynen A., Kocsis S., Lankhorst A., Lemmens A., Mohede I. 2003. Dietary conjugated linoleic acids as free fatty acids and triacylglycerols similarly affect body composition and energy balance in mice. J. Nutr. 133: 3181–3186. [DOI] [PubMed] [Google Scholar]
- 49.Ohnuki K., Haramizu S., Oki K., Ishihara K., Fushiki T. 2001. A single oral administration of conjugated linoleic acid enhanced energy metabolism in mice. Lipids. 36: 583–587. [DOI] [PubMed] [Google Scholar]
- 50.Nagao K., Wang Y. M., Inoue N., Han S. Y., Buang Y., Noda T., Kouda N., Okamatsu H., Yanagita T. 2003. The trans-10, cis-12 isomer of conjugated linoleic acid promotes energy metabolism in OLETF rats. Nutrition. 19: 652–656. [DOI] [PubMed] [Google Scholar]
- 51.Ishibashi J., Seale P. 2010. Beige can be slimming. Science. 328: 1113–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seale P., Conroe H., Estall J., Kajimura S., Frontini A., Ishibashi J., Cohen P., Cinti S., Spiegelman B. 2011. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., Cinti S. 2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298: E1244–E1253. [DOI] [PubMed] [Google Scholar]
- 54.Frontini A., Cinti S. 2010. Distribution and development of brown adipocytes in the murine and human adipose tissue organ. Cell Metab. 11: 253–256. [DOI] [PubMed] [Google Scholar]
- 55.Langin D. 2010. Recruitment of brown fat and conversion of white into brown adipocytes: strategies to fight the metabolic complications of obesity? Biochim. Biophys. Acta. 1801: 372–376. [DOI] [PubMed] [Google Scholar]
- 56.Sauer L. A., Dauchy R. T., Blask D. E., Krause J. A., Davidson L. K., Dauchy E. M., Welham K. J., Coupland K. 2004. Conjugated linoleic acid isomers and trans fatty acids inhibit fatty acid transport in hepatoma 7288ctc and inguinal fat pads in Buffalo rats. J. Nutr. 134: 1989–1997. [DOI] [PubMed] [Google Scholar]
- 57.Brown J. M., Boysen M. S., Chung S., Fabiyi O., Morrison R. F., Mandrup S., McIntosh M. 2004. Conjugated linoleic acid induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J. Biol. Chem. 279: 26735–26747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reardon M., Gobern S., Martinez K., Chuang C., Reid T., McIntosh M. 2012. Oleic acid attenuates trans-10, cis-12 conjugated linoleic acid (10,12 CLA)-mediated inflammatory signaling in primary human adipocytes. Lipids. 47: 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.