Abstract
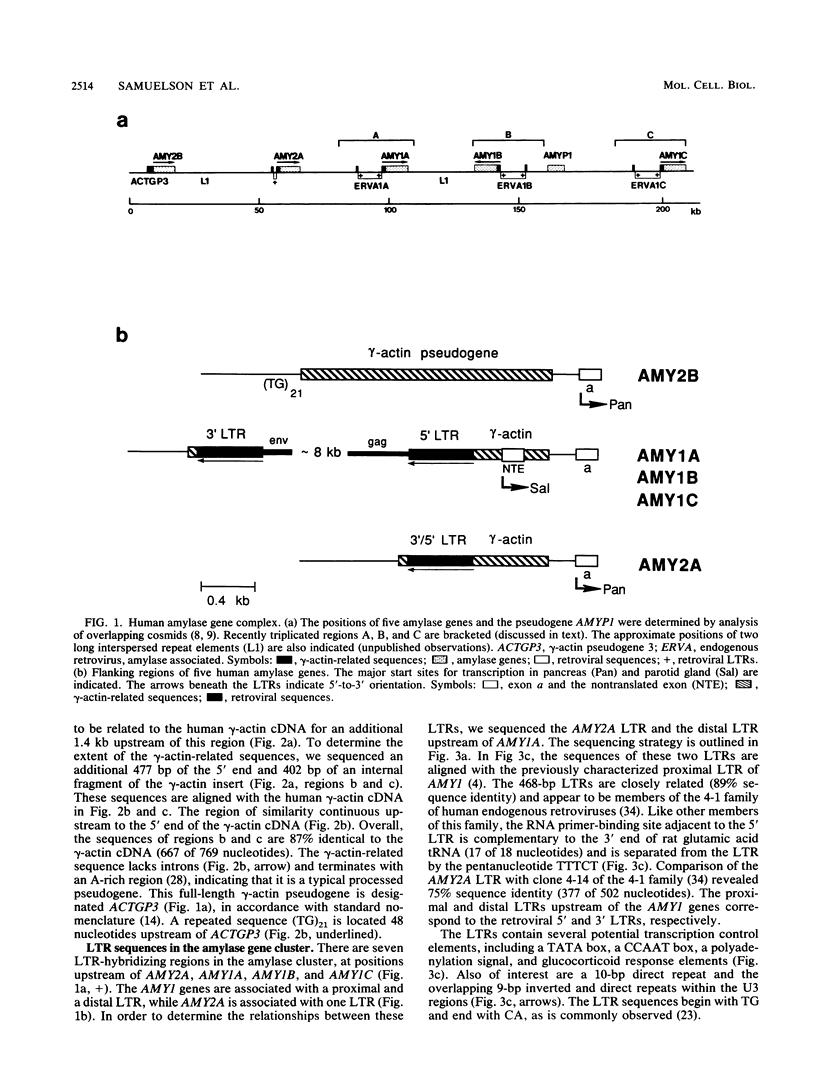
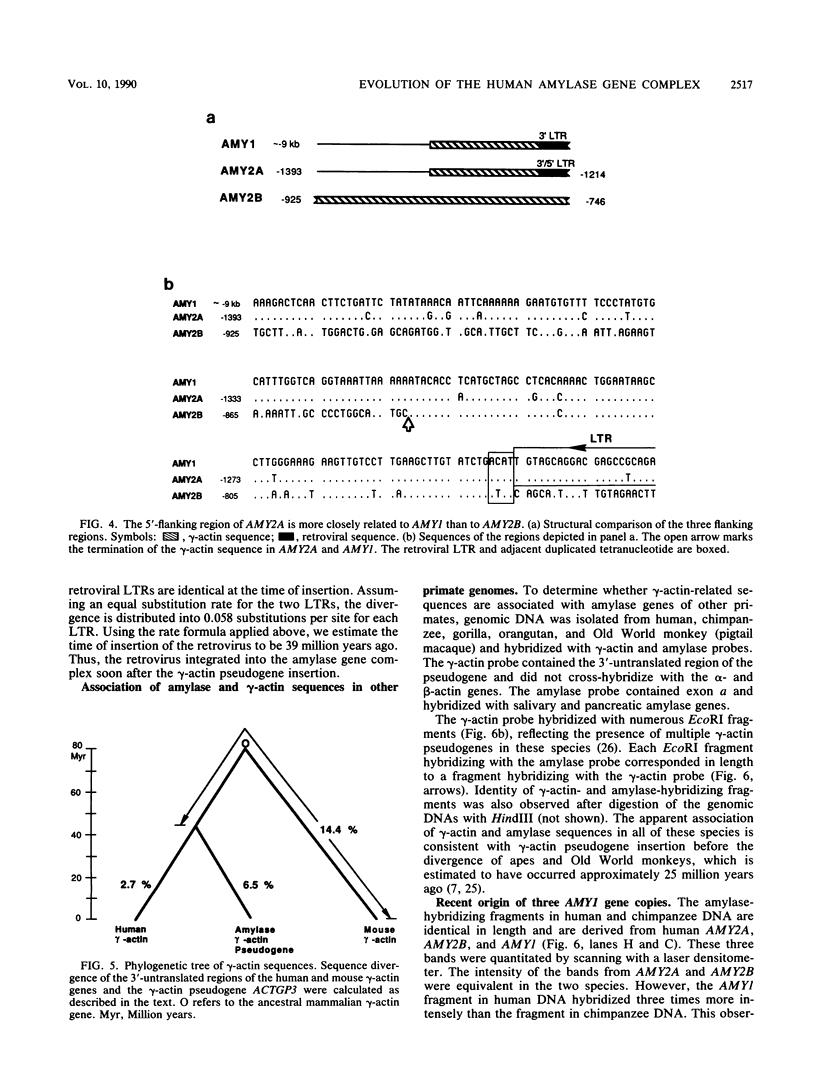
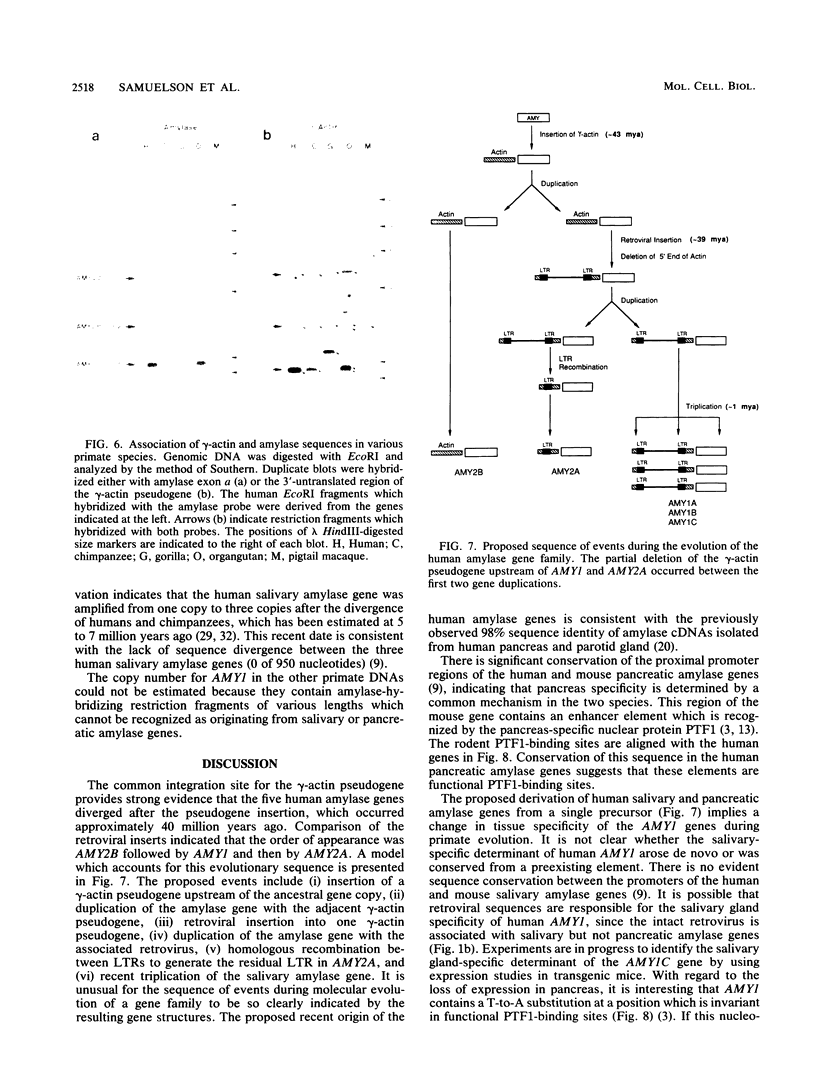
We have analyzed the junction regions of inserted elements within the human amylase gene complex. This complex contains five genes which are expressed at high levels either in the pancreas or in the parotid gland. The proximal 5'-flanking regions of these genes contain two inserted elements. A gamma-actin pseudogene is located at a position 200 base pairs upstream of the first coding exon. All of the amylase genes contain this insert. The subsequent insertion of an endogenous retrovirus interrupted the gamma-actin pseudogene within its 3'-untranslated region. Nucleotide sequence analysis of the inserted elements associated with each of the five human amylase genes has revealed a series of molecular events during the recent history of this gene family. The data indicate that the entire gene family was generated during primate evolution from one ancestral gene copy and that the retroviral insertion activated a cryptic promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulet A. M., Erwin C. R., Rutter W. J. Cell-specific enhancers in the rat exocrine pancreas. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3599–3603. doi: 10.1073/pnas.83.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Liao Y. C., Smith D. H., Barrera-Saldaña H. A., Gelinas R. E., Seeburg P. H. The human growth hormone locus: nucleotide sequence, biology, and evolution. Genomics. 1989 May;4(4):479–497. doi: 10.1016/0888-7543(89)90271-1. [DOI] [PubMed] [Google Scholar]
- Cockell M., Stevenson B. J., Strubin M., Hagenbüchle O., Wellauer P. K. Identification of a cell-specific DNA-binding activity that interacts with a transcriptional activator of genes expressed in the acinar pancreas. Mol Cell Biol. 1989 Jun;9(6):2464–2476. doi: 10.1128/mcb.9.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi M., Horii A., Tomita N., Nishide T., Ogawa M., Mori T., Matsubara K. Overlapping two genes in human DNA: a salivary amylase gene overlaps with a gamma-actin pseudogene that carries an integrated human endogenous retroviral DNA. Gene. 1988;62(2):229–235. doi: 10.1016/0378-1119(88)90561-6. [DOI] [PubMed] [Google Scholar]
- Erba H. P., Eddy R., Shows T., Kedes L., Gunning P. Structure, chromosome location, and expression of the human gamma-actin gene: differential evolution, location, and expression of the cytoskeletal beta- and gamma-actin genes. Mol Cell Biol. 1988 Apr;8(4):1775–1789. doi: 10.1128/mcb.8.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erba H. P., Gunning P., Kedes L. Nucleotide sequence of the human gamma cytoskeletal actin mRNA: anomalous evolution of vertebrate non-muscle actin genes. Nucleic Acids Res. 1986 Jul 11;14(13):5275–5294. doi: 10.1093/nar/14.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot P. C., Bleeker M. J., Pronk J. C., Arwert F., Mager W. H., Planta R. J., Eriksson A. W., Frants R. R. The human alpha-amylase multigene family consists of haplotypes with variable numbers of genes. Genomics. 1989 Jul;5(1):29–42. doi: 10.1016/0888-7543(89)90083-9. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Wiebauer K., Caldwell R. M., Samuelson L. C., Meisler M. H. Concerted evolution of human amylase genes. Mol Cell Biol. 1988 Mar;8(3):1197–1205. doi: 10.1128/mcb.8.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy D. E., Larsen S. H., Karn R. C., Hodes M. E. Identification of a human salivary amylase gene. Partial sequence of genomic DNA suggests a mode of regulation different from that of mouse, Amy1. Mol Biol Med. 1987 Jun;4(3):145–155. [PubMed] [Google Scholar]
- Horii A., Emi M., Tomita N., Nishide T., Ogawa M., Mori T., Matsubara K. Primary structure of human pancreatic alpha-amylase gene: its comparison with human salivary alpha-amylase gene. Gene. 1987;60(1):57–64. doi: 10.1016/0378-1119(87)90213-7. [DOI] [PubMed] [Google Scholar]
- Howard G., Keller P. R., Johnson T. M., Meisler M. H. Binding of a pancreatic nuclear protein is correlated with amylase enhancer activity. Nucleic Acids Res. 1989 Oct 25;17(20):8185–8195. doi: 10.1093/nar/17.20.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. H., Tanimura M., Sharp P. M. An evaluation of the molecular clock hypothesis using mammalian DNA sequences. J Mol Evol. 1987;25(4):330–342. doi: 10.1007/BF02603118. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Man Y. M., Delius H., Leader D. P. Molecular analysis of elements inserted into mouse gamma-actin processed pseudogenes. Nucleic Acids Res. 1987 Apr 24;15(8):3291–3304. doi: 10.1093/nar/15.8.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., Rasheed S., Khan A. S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishide T., Nakamura Y., Emi M., Yamamoto T., Ogawa M., Mori T., Matsubara K. Primary structure of human salivary alpha-amylase gene. Gene. 1986;41(2-3):299–304. doi: 10.1016/0378-1119(86)90110-1. [DOI] [PubMed] [Google Scholar]
- Osborn L., Rosenberg M. P., Keller S. A., Meisler M. H. Tissue-specific and insulin-dependent expression of a pancreatic amylase gene in transgenic mice. Mol Cell Biol. 1987 Jan;7(1):326–334. doi: 10.1128/mcb.7.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban A. T. Retroviral DNA integration. Cell. 1985 Aug;42(1):5–6. doi: 10.1016/s0092-8674(85)80092-1. [DOI] [PubMed] [Google Scholar]
- Peter B., Man Y. M., Begg C. E., Gall I., Leader D. P. Mouse cytoskeletal gamma-actin: analysis and implications of the structure of cloned cDNA and processed pseudogenes. J Mol Biol. 1988 Oct 5;203(3):665–675. doi: 10.1016/0022-2836(88)90200-8. [DOI] [PubMed] [Google Scholar]
- Pilbeam D. The descent of hominoids and hominids. Sci Am. 1984 Mar;250(3):84–96. doi: 10.1038/scientificamerican0384-84. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Steele P. E., O'Neill R. R., Rabson A. B., Martin M. A. Nucleotide sequence of a full-length human endogenous retroviral segment. J Virol. 1985 Jun;54(3):764–772. doi: 10.1128/jvi.54.3.764-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson L. C., Wiebauer K., Gumucio D. L., Meisler M. H. Expression of the human amylase genes: recent origin of a salivary amylase promoter from an actin pseudogene. Nucleic Acids Res. 1988 Sep 12;16(17):8261–8276. doi: 10.1093/nar/16.17.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Immunological time scale for hominid evolution. Science. 1967 Dec 1;158(3805):1200–1203. doi: 10.1126/science.158.3805.1200. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Kuchino Y., Nishimura S. Mammalian tRNA genes: nucleotide sequence of rat genes for tRNAAsp, tRNAGly and tRNAGlu. Nucleic Acids Res. 1981 May 25;9(10):2239–2250. doi: 10.1093/nar/9.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. The phylogeny of the hominoid primates, as indicated by DNA-DNA hybridization. J Mol Evol. 1984;20(1):2–15. doi: 10.1007/BF02101980. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Steele P. E., Rabson A. B., Bryan T., Martin M. A. Distinctive termini characterize two families of human endogenous retroviral sequences. Science. 1984 Aug 31;225(4665):943–947. doi: 10.1126/science.6089336. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Wu C. I., Li W. H., Shen J. J., Scarpulla R. C., Limbach K. J., Wu R. Evolution of cytochrome c genes and pseudogenes. J Mol Evol. 1986;23(1):61–75. doi: 10.1007/BF02100999. [DOI] [PubMed] [Google Scholar]



