Abstract
The large size and complex organization of the human brain makes it unique among primate brains. In particular, the neocortex constitutes about 80% of the brain, and this cortex is subdivided into a large number of functionally specialized regions, the cortical areas. Such a brain mediates accomplishments and abilities unmatched by any other species. How did such a brain evolve? Answers come from comparative studies of the brains of present-day mammals and other vertebrates in conjunction with information about brain sizes and shapes from the fossil record, studies of brain development, and principles derived from studies of scaling and optimal design. Early mammals were small, with small brains, an emphasis on olfaction, and little neocortex. Neocortex was transformed from the single layer of output pyramidal neurons of the dorsal cortex of earlier ancestors to the six layers of all present-day mammals. This small cap of neocortex was divided into 20–25 cortical areas, including primary and some of the secondary sensory areas that characterize neocortex in nearly all mammals today. Early placental mammals had a corpus callosum connecting the neocortex of the two hemispheres, a primary motor area, M1, and perhaps one or more premotor areas. One line of evolution, Euarchontoglires, led to present-day primates, tree shrews, flying lemurs, rodents and rabbits. Early primates evolved from small-brained, nocturnal, insect-eating mammals with an expanded region of temporal visual cortex. These early nocturnal primates were adapted to the fine branch niche of the tropical rainforest by having an even more expanded visual system that mediated visually guided reaching and grasping of insects, small vertebrates, and fruits. Neocortex was greatly expanded, and included an array of cortical areas that characterize neocortex of all living primates. Specializations of the visual system included new visual areas that contributed to a dorsal stream of visuomotor processing in a greatly enlarged region of posterior parietal cortex and an expanded motor system and the addition of a ventral premotor area. Higher visual areas in a large temporal lobe facilitated object recognition, and frontal cortex, included granular prefrontal cortex. Auditory cortex included the primary and secondary auditory areas that characterize prosimian and anthropoid primates today. As anthropoids emerged as diurnal primates, the visual system specialized for detailed foveal vision. Other adaptations included an expansion of prefrontal cortex and insular cortex. The human and chimpanzee-bonobo lineages diverged some 6–8 million years ago with brains that were about one-third the size of modern humans. Over the last two million years, the brains of our more recent ancestors increased greatly in size, especially in the prefrontal, posterior parietal, lateral temporal, and insular regions. Specialization of the two cerebral hemispheres for related, but different functions became pronounced, and language and other impressive cognitive abilities emerged.
Introduction
Humans have always been interested in their origins. Thus, nearly all cultures have stories about the origins of the first people. Seldom did these stories suggest that they came from other species, but instead held that our ancestors were always here in the spirit world, and they somehow became human, or that they were somehow otherwise created. However, for biological scientists, it has long been clear that we evolved from a long line of ancestors that were evermore less like ourselves as one goes back in time across previous generations. Richard Dawkins (2004) [1] provides a rough impression of what it would be like to achieve time travel and go back over the series of ancestors by considering a series of contemporary species that progressively resemble us less and less until reaching unicellular species representing the ancestors of all animal life. Unfortunately, this effort is only an approximation, as it provides little detail about the behaviors, morphologies, and nervous system organizations of the actual ancestors who were successively modified to finally produce modern humans. The task of reconstructing the actual course of the evolution of humans is extremely daunting, and perhaps not completely possible. However, it can be done in ways that provide highly probable results. Accumulated findings from several fields allow conjecture about the evolution of humans from distant relatives to be ever better informed.
Here the focus is on the evolution of the human brain. Of course, brains did not evolve in anticipation of any of the goals or aspirations of present-day humans, most of us now living in complex modern cultures and places tightly packed with others. Instead, we are here because all of our direct ancestors were successful in surviving and reproducing under different circumstances, and brains evolved that were plastic enough with the emergence of modern humans to serve us well today. But how do we reconstruct the evolution of human brains? We can learn little directly about the brains of long dead ancestors, so most of the information must come from careful, detailed studies of the brains of present-day (extant) mammals, other vertebrates, and even other animals. The logic and methods of using the results of comparative studies to infer aspects of brain (or body) evolution are now reasonably well established, and have been in use for some time [2–4]. In brief, the central premise is that characters, traits or features that are present in many current members of a phylogenetic radiation (a clade), are most parsimoniously explained as being retained from a common ancestor. The clade can be of recent or ancient origin, for example, including only primates or all vertebrates. For example, the corpus callosum, a brain structure connecting the two cerebral hemispheres, is present in all placental (eutherian) mammals, not in egg-laying prototherian mammals (monotremes), or pouch-rearing metatherian (marsupial) mammals. It follows that the corpus callosum emerged as a new structure or character in the mammalian ancestors of all placental mammals, but after the branching of the mammalian radiation that lead to marsupials and monotremes.
A great advantage for current researchers is that the phylogenetic relationships of modern mammals are now much better understood. Phylogenetic relationships have long been constructed by comparing similarities in body organization across species, with the advantage that the fossilized skeletons of long-extinct species could also be compared. However, independently evolved similarities (convergences) and long-term retentions of ancestral (plaseomorphic) anatomies confounded the construction of phylogenetic trees, until such comparisons were extended to the molecular and genetic levels. Thus, species are now compared by how similar they are genetically, and times of divergence can be estimated by assumptions about mutation rates scaled to evidence from the fossil record [5, 6]. Today we know that tenrecs from Madagascar and shrews and hedgehogs from Europe, once placed in the Insectivore Order, are not closely related, and not even in the same Super order. We also know from such studies that fruit-eating (megabats) and insecteating (microbats) are closely related, as long suggested, but that neither is in the same super order as primates, although megabats were recently thought to be primates by some, and all bats were previously thought to be close relatives of primates. These advances in understanding phylogenetic relationships are of obvious importance in comparative studies of evolution.
Another source of new information is from comparative studies of brain development. Across species, early stages of body and brain development are likely to be more similar, with divergences in outcomes becoming progressively more obvious at later stages [7, 8]. Studies of similarities and differences in brain development can usefully fill in the gaps in understanding that are there in mature brains because of extinctions of close relatives. As a major gap, the early amniote ancestors emerged from amphibians about 340 million years ago (mya), and divided into synapsids that lead to the first mammals, but left no other surviving members, and sauropsids, which lead to the various present-day reptiles and birds. The closest comparison for mammals is with modern reptiles, lines of separate evolution for over 320 million years. Fortunately, comparative studies of brain development in reptiles, birds and mammals, provide an improved understanding of how neocortex, the hallmark of the mammalian brain, emerged [9–11]. In addition, the fossil record continues to improve. The teeth and bones, which are most often preserved, tell us much about the behaviors of long-extinct ancestors or probable ancestors, and the endocasts of the insides of the skulls tell us about brain size and shape [12]. The large temporal lobe of the skull endocasts of early primates, for example, implies the devotion of that expanded region of cortex to visual object recognition, as inferred from temporal lobe functions of modern primates. Moreover, much more can be inferred from fossil evidence of brain size, as we now know from brain scaling “rules”, based on studies of extant primates, how brains of different sizes should systematically differ in numbers of neurons [13, 14]. However, inferences about the evolution of the human brain most directly depend upon the results of studies of brain organization and function in various present-day species, whether the authors of these studies intended the results to be informative about brain evolution or not. Modern methods, together with the greatly expanded current research efforts, have greatly increased the number of observations that are relevant to the issue of brain evolution. In particular, understandings of the organization of human brains have greatly improved with the widespread use of non-invasive methods of investigation and comparative studies of primates including humans have been especially informative. Also, studies of gene expression in the brain have been very important [15, 16]. Unfortunately, the focus of research on the brains of only a few species has increased in recent years, to the detriment of inferences based on comparisons. Here we provide a brief outline of major steps in the evolution of the human brain, an understanding that is rapidly expanding. fMRI (functional magnetic resonance imaging) [17] and diffusion-tensor imaging [18].
The brains of early mammals
This review of the course of the evolution of the human brain is necessarily limited to a few major changes, so we focus on the forebrain, which is the largest part of the brain, and especially the neocortex, which constitutes the major part of the brain in most mammals, and mediates, for us at least, all conscious experience. Reptiles, birds, and mammals all have forebrains with similar major subdivisions, including an external cortex and subcortical nuclear structures [19]. Cortex includes a lateral region with olfactory functions, a dorsal region with sensory inputs, and a medial region mediating learning and memory. In reptiles, all three major subdivisions of cortex are rather similar in cellular organization, consisting of a single layer of pyramidal neurons and a scattering of smaller neurons with local, mainly inhibitory connections. The lateral cortex is olfactory in function as it receives inputs from the olfactory bulb, with inputs directly from olfactory receptors and pyramidal neurons of lateral cortex that project to subcortical structures to mediate behaviors. The apical dendrites of pyramidal neurons in dorsal cortex receive visual and somatosensory information from nuclei in the dorsal thalamus, and project to subcortical structures, and to medial cortex. In mammals, the lateral olfactory cortex, now called piriform cortex, and the medial cortex, now the hippocampus, are enlarged and have many more neurons. Yet structurally they resemble the lateral and medial cortical regions of reptiles. Dorsal cortex, now called neocortex, is not completely new, as the name suggests, but dorsal cortex has been transformed into a thick structure that is traditionally subdivided into six layers with differing functional roles [20]. Most important, the apical dendrites of pyramidal neurons are no longer the main targets of sensory inputs from the thalamus, but instead, thalamic neurons target small neurons in layer 4 of cortex that distribute information to more superficial neurons, mainly in layer 3, which then target other cortical neurons, including layer 4 neurons in other parts of neocortex. This innovation allows serial steps in cortical processing of sensory information, so that very complex computations are possible. Layer 4 information is also distributed to layer 5 neurons that provide outputs to subcortical structures, and layer 6 neurons that provide feedback connections to sources of input. How this complex, laminated neocortex emerged in evolution is not completely clear, as the synapsid relatives of mammals left no other surviving members. However, studies of cortical development have indicated that phylogenetic changes in the generation and migration of the neurons were critical to the formation of neocortex (see [9, 11]. Nevertheless, the basic laminar structure of neocortex proved to be extremely useful and adaptable, so all mammals have retained a dorsal cap of neocortex that greatly exceeds the dorsal cortex of reptiles in numbers of neurons, while varying greatly across mammalian species in absolute size, in size relative to the rest of the brain, and in number of functionally distinct cortical areas.
The endocasts of the skulls of early mammals indicate that they had little neocortex relative to brain size, with more of the forebrain devoted to piriform cortex and olfaction. However, some present-day mammals, especially humans, have huge amounts of neocortex, while others have intermediate amounts or not much more than that of early mammals. While the overall trend has been toward larger brains with more neocortex [21], variability exists for a number of reasons. Most important, large brains are metabolically costly to maintain [22], and take a long developmental time to become fully functional. The resulting cost in reproduction rate must be met by the benefits the larger brains provide in promoting long life and the successful rearing of offspring. This works for some species, to varying extents, and not for others. Thus, small brains with little neocortex suffice for some species, and these mammals are the most useful to study in order to infer how the brains of the first mammals were organized.
Early mammals evolved from mammal-like synapsids over 200 mya [6, 23]. Fossil skulls indicated that these ancestors of mammals had smaller brains with smaller olfactory bulbs, little dorsal cortex that failed to extend over the midbrain, narrow cerebral hemispheres, and massive bones of the middle ear that limited the transmission of high-frequency sounds. From such fossil evidence, [24] Rowe et al. (2011) concluded mammal-like synapsids (cynodonts) differed from mammals in having “low-resolution olfaction, poor vision, insensitive hearing, coarse tactile sensitivity, and unrefined motor coordination”, together with limited sensorimotor integration. Early mammals had much larger forebrains, greatly expanded olfactory (piriform) cortex, a dorsal cap of neocortex, an expanded cerebellum, and a thicker spinal cord. They also had auditory specializations that would allow high-frequency hearing, and possibly they alone could use high frequency communication calls [25]. Early mammals probably laid eggs, as present-day monotremes do. About 150 mya a marsupial branch emerged with a short period of uterine development followed by an extended period of post-uterine development and parental care, aided by a pouch in most present-day marsupials. Placental mammals evolved from one early branch of marsupials, as the ability to retain the embryo in the uterus for a long development time emerged. Placental (eutherian) mammals further diverged into four major branches that now constitute well over 90 percent of mammalian species. By comparing brain and especially cortical organization across current members of the six major branches, and seeing what features are common, we can infer that these features were likely retained from a common early mammal ancestor, and when some specializations were lost and others acquired [2, 26]. Common features are perhaps easiest to discover in small-brained mammals, since there is less brain and cortex to explore, but small-brained present-day mammals could also have simpler brains than their ancestors as features were lost when smaller brains sometimes evolved from ancestors with larger brains [27] [28]. In addition, early mammals needed to have some parts of their nervous systems develop very early since they hatched from eggs, as in present-day monotremes, or were born early in development, as in present-day marsupials, so that they could grasp maternal hair and nurse [29]. Since placental mammals could have long gestation periods for brain development, they thereby escaped this restriction.
Overall, the comparative evidence indicates that early mammals had on the order of 15 – 20 cortical areas (see Fig. 1) that were specialized for different functions, and therefore anatomically and physiologically distinct [26]. These areas included the primary visual, somatosensory, and auditory areas, as these areas can be easily identified in contemporary species by their histological specialization for receiving dense, direct, sensory inputs from the dorsal thalamus. For the most part, these areas have been identified in all adequately studied mammals, and a small remnant of primary visual cortex exists in even blind or nearly blind subterranean mammals [30].
Figure 1.
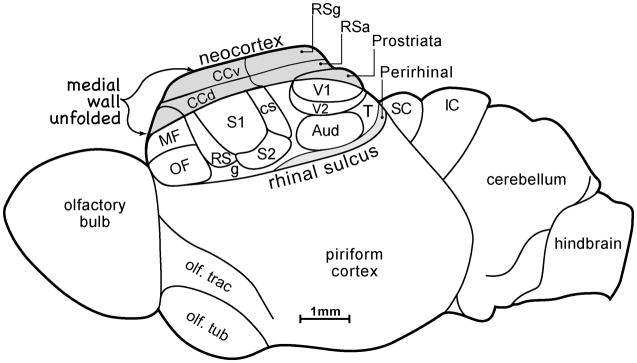
The proposed organization of the neocortex of early mammals. This reconstruction was based on a cladistic analysis of common features of neocortex of present-day mammals, and information about brain size and proportions inferred from endocasts of the skulls of early mammals. Primary somatosensory cortex, S1, was bordered by a rostral somatosensory area, SR, a caudal somatosensory area, SC, and a ventral secondary area, S2. A ventral gustatory area, g, may have been present as part of an “insula” region near the rhinal sulcus, a shallow dimple in early mammals. Perirhinal and post-rhinal areas were likely present. Auditory cortex (aud) had at least one primary area, and perhaps secondary areas, while visual cortex included a primary area, V1, the second area, V2, a temporal visual area, T, and a medial prostriata visual area. Cortex of the medial wall of the cerebral hemisphere included granular (RSg) and agranular, RSa, retrosplenial areas, and dorsal (ccd) and ventral (ccv) cingulate cortical areas. The frontal cortex included orbital frontal, OF, and medial frontal, MF, areas. A large hippocampus, connecting with entorhinal cortex, folded under caudal and medial neocortex. Piriform (olfactory) cortex was proportionately large, and the olfactory tract and bulb are shown. The small cap of neocortex failed to cover the midbrain, and the superior colliculus, SC, and inferior colliculus, IC, were exposed.
Studies of neocortex suggest that the relative positions and internal organizations of these areas are specified and maintained in early development by regional gene expression patterns that create molecular gradients that structure the basic cortical locations and internal organizations of primary sensory areas for all mammals [31]. Early mammals also likely had other visual areas including a second visual area, V2, a visual area or areas in temporal cortex, and a visual area medial to primary visual cortex, prostriata [26, 32]. Auditory cortex may have included one or more secondary areas, in addition to the primary area or areas [33]. Somatosensory cortex included somatosensory area (S) along the rostral (RS) and caudal (CS) borders of primary cortex (S1 or area 3b), and one (S2) or two (S2 plus PV) secondary areas, and possibly a gustatory area. The cortex of the medial wall between the two hemispheres contained two or more subdivisions of retrosplenial cortex, and 2 – 3 divisions of cingulate cortex. Frontal cortex had at least medial and lateral divisions. We also include perirhinal and entorhinal cortex. Surprisingly, the comparative evidence indicates that there was no separate motor or premotor cortex, as cortical motor functions were mediated by primary somatosensory cortex, S1 and the rostral somatosensory strip (corresponding to area 3a of primates). Motor and premotor cortex emerged with the evolution of placental mammals [34] together with longer intra-uterine development times. Possibly a relaxation of the need for precocious development of subcortical motor control of prehension facilitated the emergence of greater cortical motor control in placental mammals. The major cortical pathway between the two cerebral hemispheres, the corpus callosum, also emerged with placental mammals, and this shorter pathway would save axon conduction times, a savings important in especially larger brains [35].
The brains of modern monotremes, platypus and echidna, are highly specialized for somatosensation and electroreception, which evolved as a part of the somatosensory system [36], while the surviving 265 marsupial species are quite varied in behavioral adaptations, brain size, and probably brain organization, although this has not been well studied. However, modern opossums and possums have changed little in overall morphology and brain size over the last 100 million years [37], and the similar organizations of the brains of distantly related American opossums [34, 38] and Australian possums [39] suggests that the organizations of these brains closely reflect those of the brains of early mammals.
Early placental mammals emerged about 125 mya, and they did not appear to be much different in body form than early marsupials and present-day opossums [40]. They continued to have small brains with relatively little neocortex with, of course, the addition of motor cortex. Major branches of the placental radiation included the superorder Xenarthra with armadillos, sloths and anteaters, Afrotheria with tenrecs, aardvarks, elephants and others, Laurasiatheria with shrews, bats, cats, cows, whales and others, and Euarchontoglires with rodents, rabbits, tree shrews, flying lemurs, and primates [23]. Members of these branches evolved nervous systems that specialized in various ways, even in members with small brains, so that armadillos have an enlarged olfactory system, and echolocating bats have a greatly enlarged and modified auditory system [26], but whales, elephants, and humans have greatly enlarged brains that have been considerably modified from those of the early placental (eutherian) mammals, as have the brains of some carnivores and some ungulates, although to a lesser extent. In addition, there are differences in the structural organizations of shared parts of the brains of mammals that characterize major branches of the radiation of placental mammals. For example, one can identify a brain as being from a carnivore or a primate by just looking at the lateral geniculate nucleus of the visual thalamus [41, 42]. What such differences might mean functionally is beyond the scope of this review, but the existence of clade-specific traits raises the issue of what features characterize the brains of primates. Also, brains vary from no fissures to many in neocortex, with different fissure patterns characterizing different clades of mammalian evolution, and larger brains having more and deeper fissures [43]. The fissure patterns serve as useful markers relative to cortical areas, and connection patterns between cortical areas may have a role in the formation of fissures [44, 45].
The brains and neocortex of early primates
Primates provide many opportunities for comparative studies as this highly diversified Order consists of 14 families and over 350 species. Archaic primates emerged about 82 mya, and formed strepsirrhine (lemurs, lorises and galagos) and haplorhine branches about 65 mya [5]. Tarsiers soon emerged as a branch of the haplorhine radiation, while the remaining branch of anthropoid primates split into New World and Old World branches of monkeys about 35 mya. Apes emerged as a branch of the Old World anthropoids about 25 mya, while the split of bipedal ancestors of present-day humans from bonobo and chimpanzee ancestors occurred as recently as 6 mya [23]. Primates have adapted to a number of environments, and in doing so, vary greatly in size from the 40-gram mouse lemur to the 200 kg male gorilla. Primate brains also vary greatly in size from under 2 grams in mouse lemurs to as much as 16,000 grams in humans. This impressive range provides a unique opportunity for researchers to study the relationships of brain size to number of neurons and other variables [13]. Strepsirrhine primates are of special value for those interested in studying the evolution of primates because their skeletons resemble those of early primates more closely than those of tarsiers and monkeys, and their brains are closer to those of early primates in relative size and proportions.
The clade Euarchonta includes flying lemurs (Dermoptera), tree shrews (Scandentia), primates, and their extinct relatives including the once widespread stem primates. The fossil evidence suggests that the ancestral euarchontans were small (10 – 20g), arboreal, and insectivorous, much like the nocturnal, modern-day tree shrew, Ptilocercus [46]. Stem primates (Plesiadapiforms) had grasping hands and feet, and were thus adapted to feed in the terminal branches of trees and bushes on fruit, buds, and insects [47]. They had convergent orbits, indicating the importance of frontal vision. They had a large brain relative to body size, but not as large as present-day strepsirrhines, and an expanded temporal lobe, suggesting an emphasis on vision.
Brain organization has been studied extensively in strepsirrhine galagos, as well as in both New World and Old World monkeys. Although galago and monkey brains differ considerably in a number of brain features, as well as overall brain size relative to body size, they also share a remarkable number of brain features, which distinguish them from non-primates. This is particularly apparent in the visual system, where the dorsal lateral geniculate nucleus of the visual thalamus has a lamination pattern of parvocellular and magnocellular layers that identify all primates, but differ from all non-primates [48]. Likewise, galagos and monkeys have a similar arrangement of nuclei in the pulvinar of the visual thalamus [49, 50], and the superior colliculus of the midbrain represents only the contralateral visual field, in contrast to other mammals where it represents the complete visual field of the contralateral eye [51]. The representation of the hand in the somatosensory thalamus of galagos and monkeys is large, with subdivisions for each finger, and primates have two somatosensory nuclei, ventroposterior superior, and ventroposterior inferior, that are not easily identified in most non-primates [41].
In the neocortex of galagos, a number of cortical areas have been identified that are different or well defined only in primates [52]. In all primates, primary visual cortex has separate processing modules, the so-called blob and non-blob regions, which process color or stimulus orientation information. However, lamination patterns vary in distinctiveness and in anatomical structure [53], and genes that are highly expressed in layer 4 of primary visual cortex and are activity dependent are less expressed in prosimian primates and New World monkeys [15]. Thus, levels of expression are higher in primates more closely related to humans and are very low in rodents and carnivores. The second visual area has modular bands of cortex with different inputs and outputs. A third visual area, V3, is well defined. A middle temporal visual area, MT, and associated visual areas (MTc, MST, FST), and a dorsomedial visual area (DM), all contribute to the visual guidance of motor behavior. The temporal lobe is large and has several proposed visual areas. Auditory cortex also has an arrangement of primary and secondary areas that is similar in all studied primates, but different from non-primates [33]. In motor cortex, primary (M1), ventral and dorsal premotor, supplementary motor, and cingulate motor areas are all well defined, and similar in galagos and monkeys [54]. Non-primate placental mammals may have a dorsal premotor or supplementary motor area, but the ventral premotor area appears to be unique to primates [55]. Prefrontal cortex is large, and includes a large granular subdivision that may not exist in mammals other than primates [56]. Unlike tree shrews and other close relatives of primates, galagos and other primates (see Fig. 2) have a large region of posterior parietal cortex, and this cortex has a number of subregions or domains where reaching, grasping, defensive, eye, and other movements can be evoked by electrical stimulation [57]. This large region of cortex appears to be involved in the selection, planning and guidance of movement patterns via sensory inputs and outputs to motor and premotor cortex.
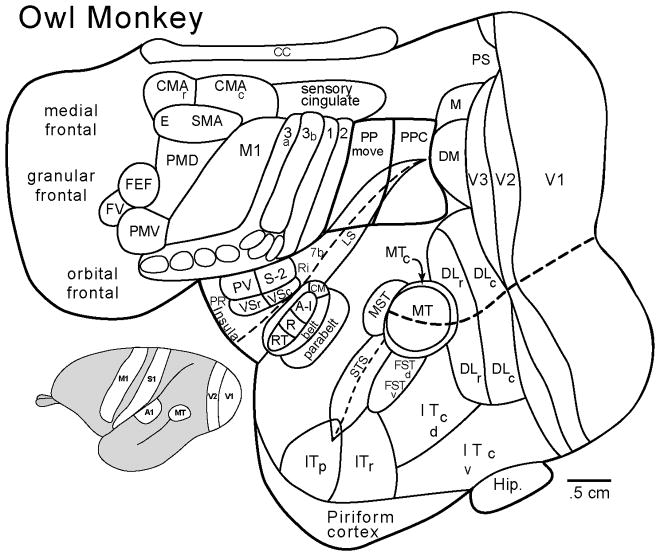
Figure 2.
The organization of neocortex in a New World owl monkey. Nearly all of the cortical areas shown here are those shared by all primates. Macaque monkeys, apes, and humans have larger brains with more cortical areas. Compared to early mammals, primates have an array of visual areas, including V1, V2, and V3, rostral and caudal dorsolateral areas, DLr and DLc, a dorsomedial area, DM, a medial area, M, a prostriatal area, PS, a middle temporal area, MT, and associated areas, MTc, MST, FSTd and FSTv, and perhaps four subdivisions of inferior temporal cortex, IT. Anterior parietal cortex includes a primate area, 3b or S1, bordering areas 3a and 1 (compare with 5R and SC of early mammals) and an area 2. Somatosensory areas of the lateral sulcus (LS) include S2, the parietal ventral area, PV, and rostral and caudal divisions of ventral somatosensory cortex, VSr and VSc. Primary auditory cortex includes A1, the rostral area, R, and the rostrotemporal area, RT. A belt of secondary auditory areas includes the caudomedial area, CM. The parabelt represents a third level of cortical auditory processing. Motor cortex includes the primary area, M1, dorsal and ventral premotor areas, PMD and PMV, a supplementary motor area, SMA, with a eye (E) movement region, a frontal eye field, FEF, adjoined by a frontal visual area, FV, and rostral and caudal cingulate motor areas, CMAr and CMAc. The insular cortex has a gustatory field in the region of the parietal rostral, PR, cortex. Posterior parietal cortex, PPC, contains a more rostral half divided into domains for specific movements, PPC move, and a caudal half dominated by visual inputs. As for all eutherian mammals, there is a corpus callosum, cc. Only part of the hippocampus, Hip, is shown where it adjoins postrhinal cortex.
Primate brains also differ from those of other mammals in average neuron packing density and average neuron size [13]. In the non-primates that have been studied, neuron densities decrease and neurons get bigger as species with larger brains are considered, while as primate species with larger brains are considered, the neuron densities and average neuron sizes remain the same, resulting in greater numbers of neurons for especially larger brain sizes in primates compared to non-primates. This neuron advantage may be part of the explanation for greater cognitive abilities in primates, especially as much of this result depends on the high neuron packing density that is maintained as neocortex increases in size across primate species. Furthermore, neuron packing density is especially high in primary sensory cortex of primates, but not non-primates [58]. This is especially the case for primary visual cortex, where neuron-packing densities can be 3–5 times higher than in other regions of cortex. As densely packed neurons are overall small, they have more limited activating inputs, preserving the details of information while being less integrative in function [59].
A special feature of neocortex in primates is that it is subdivided into more functionally distinct areas than in non-primates (or most non-primates since more study is needed), and this allows for the greater specialization of some areas, with morphological consequences. Thus, some areas in primates, such as V1, are anatomically specialized for preserving and analyzing small bits of information, while other areas, prefrontal cortex for example, are specialized for broad integrative functions. As large cortical areas, such as V1, are better for detail, and small cortical areas for integrating diverse inputs, primates have few large areas and many small areas [59].
The evolution of human brains: conclusions from the fossil record and comparative studies of monkeys, apes and humans
Three major features characterize human brains from those of early monkey and ape ancestors. First, the human brain has increased tremendously in size, especially in the amount of neocortex which came to constitute 80% of the human brain [60]. Second, some parts of neocortex have greatly enlarged relative to the rest of neocortex, most notably prefrontal, insular, posterior parietal, and temporal cortex. These are the same regions that continue to grow the most as they mature during human postnatal development [61]. In contrast, some parts of the cortex did not expand as the large human brain evolved, notably the primary sensory and motor areas [62]. Third, although the evidence is limited, the number of cortical areas, the fundamental functional divisions of cortex, greatly increased with the evolution of human brains. Neocortex in early mammals had roughly 20 cortical areas while neocortex in humans likely has roughly 200 areas, a ten-fold increase. Evidence for these conclusions comes in part from the fossil evidence of the great increase in brain size from early ape-like ancestors to modern humans, and from comparative studies of monkey, ape and human brains [48].
Apes emerged as a branch of the anthropoid primate radiation some 30 million years ago (mya). They formed an initially large and varied radiation, which was subsequently reduced to the surviving lesser apes, gibbons and siamangs, and the great apes, and orangutans, gorillas, chimpanzees and bonobos. Early apes had body and brain sizes that overlapped those of the larger Old World monkeys (~ 100 cm3), which is considerably smaller than the great ape range of 275 –752 cm3 [63]. Our early bipedal ancestors, including archaic hominins, separated from the ape ancestors of chimpanzees and bonobos, our closest living relatives, some 6 mya. As expected, our early hominin ancestors had brains the sizes of great apes, but they were distinguished by modifications in the skeleton that indicated that they were bipedal, an adaptation to the savanna that provided more distant vision, less exposure to the overhead sun, and allowed modifications of the hominin hand that provided advantages in tool use and carrying food and offspring [64]. Over the last 2 million years, the brains of or ancestors increased greatly in size from the 400 – 600 cm3 range to the 1200 – 1600 cm3 range of modern humans [65, 66]. This increase in brain size came at a considerable cost in metabolic requirements [22] and deferred reproduction while brains matured. These requirements were met in part by an emphasis on obtaining higher-grade foods, and the processing of foods to promote efficient digestion by grinding, and over the last one million years or more, by cooking [67]. Simply put, chimpanzees and gorilla foods would not maintain us. Of course, human children’s slow maturation of the brain limits their motor and cognitive skills so that they are long dependent on their parents and other adults for food that is high in calories (energy), lipids and proteins and easy to digest, as well as protection. Our early ancestors (and present-day chimpanzees) were dependent on their mothers up to five years, and this increased to mid-teens in humans [68]. Fortunately, large brains are associated with longer life-spans in primates [69]. Nevertheless, of the more than twenty hominin species that emerged over the last 6 million years, ours is the only one that exists today. Neanderthals with brains the size of Homo Sapiens persisted until ~ 30,000 years ago, while the small Homo floresienses with small brains, ~ 420 cm3 died out some 1200 years ago [65]. The evolution of large, costly, slowly developing brains did not always work out.
Unfortunately, we know little about the organization of the brains of extant apes, other than from a few older experimental studies of limited value, studies of the proportions of parts of brains and a few more recent studies of brain architecture and patterns of gene expression from brains obtained after natural death[16] [70]. Much more is now known about human brains, due to the widespread use of functional magnetic resonance imaging (fMRI) and other non-invasive ways of investigating human brain organization and function. Some of the imaging methods have been adapted for limited use in anesthetized chimpanzees [18, 71]. Thus, most comparisons of human brain organization have been with the brains of Old World macaque monkeys [72], which have been extensively studied with experimental and histological methods. Yet, we know from histological and imaging approaches that primary sensory and motor areas in chimpanzees are about the same size as in humans, although our brains are three or more times larger. We also know that some of the differences in the right and left cerebral hemispheres associated with language areas in the human brain also occur in chimpanzee brains [73], although chimpanzees do not have language. However, MRI results suggest that pathways from temporal to frontal areas in humans that are involved in language are differently organized in chimpanzees [18]. Nevertheless, asymmetries in the shapes of the two cerebral hemispheres associated with functional differences in humans are seen in great apes and in fossil hominins, suggesting that hemispheric specializations emerged long in the past, more than 6 mya, although they are more pronounced in humans. Hemispheric specializations would reduce the need for large numbers of thick, rapidly conducting axons between the two hemispheres, which otherwise could be costly in terms of their bulk, and reduce the problem of interconnecting distant populations of neurons [35]. This is an increasing issue as brains get bigger. While axon diameters are thicker and faster conducting in the corpus callosum of chimpanzees than in macaque monkeys, no further increase in axon thickness occurs in the corpus callosum of humans [74]. This suggests that hemispheric specializations in hominins reduced the need for large axons in the corpus callosum. Studies of split-brain patients have revealed that many different specializations exist in the right and left cerebral hemispheres of humans [75].
It is also known that some cortical regions of the large human brain have increased in size more than others. A mark of the human brain is the large frontal lobe, the cortex that is in front of the “central sulcus”, a sulcus that is more frontal than central in most primates. While humans do have the largest amount of frontal cortex, 5 – 6 times larger than in a chimpanzee, the frontal lobes are not disproportionately large, relative to the rest of the brain, in humans compared to great apes [76]. What is proportionately larger in humans is the prefrontal cortex, especially area 10 [76] which is involved in higher cognitive functions, such as the planning of future actions, undertaking initiatives, and attention. Granular frontal cortex in humans is not only large, but it has pyramidal neurons with more complex dendritic arbors and more spines for synapses [77]. Passingham and Wise (2012) [55] proposed that granular prefrontal cortex evolved in anthropoid primates as it promoted more effective foraging by reducing risky and unproductive behaviors. There are also many other differences that characterize the frontal cortex of humans. We have long known that part of the left frontal lobe, Broca’s area or region, is specialized for language production. Other specialized features of frontal cortex have been reviewed by Passingham and Wise (2012)[55], and Semendeferi et al. (2011). [78]
The amount of cortex in the lateral fissure varies greatly across primates, from a rather shallow fissure in some prosimians to a deep fissure with a large fundus or floor, the insula, which looks much like a mushroom cap with the banks of the fissure forming the stem. The upper or rostral bank of the lateral sulcus is largely devoted to somatosensory areas and functions, while the lower or caudal bank is associated with auditory areas and functions. The fundal region or insula is generally subdivided into three regions on the basis of differences in the development of layer 4, the granular cell layer. Thus, there is a posterior granular region followed by more anterior dysgranular and agranular regions [79]. A most rostral region of the insula is distinguished in humans and apes by the presence of an unusual type of neuron, the von Economo or spindle shaped neurons, which are more common in the right than the left insula [80]. The human insula is amazing in absolute size. Even in proportion to the rest of the brain, the insula in humans is larger than those in apes and other primates [81]. Overall, the insula has regions and areas involved in processing information about taste, pain, temperature, touch and internal state, but in the greatly enlarged human insula, new regions have emerged, including those mediating empathy and social awareness [82, 83].
Another region of special enlargement is the posterior parietal cortex. The non-primate ancestors of primates had little posterior parietal cortex, but all primates have a large posterior parietal region where somatosensory and visual inputs influence areas or domains of cortex that are involved in planning, imitation, and executing reaching, grasping, self-protection, and eye movements via their projections to motor and premotor cortex [57]. Posterior parietal cortex is exceptionally large in humans, where functional areas identified in monkeys have also been identified, but additional regions of posterior parietal cortex have expanded the scope of the original functions to help mediate functions, such as the skilled use of many types of tools that are particularly human. All primates have domains, small subdivisions of posterior parietal cortex, where electrical stimulation can enable eye, defense of the head, reaching, grasping, and other ethologically relevant movements via connections with motor and premotor cortex [57], but humans appear to have more subdivisions [17, 72]. In addition, the right and left posterior parietal regions are differently organized in humans. Thus the right hemisphere networks have a dominant role in visuospatial attention, so that lesions often produce visuospatial neglect of the opposite visual hemifield [84].
Human brains also differ from all other primate brains in having more cortical areas, and this may be more important than overall brain size [48]. There is compelling evidence that Old World macaque monkeys have more cortical areas than prosimian galagos that apes probably have more areas than monkeys, and that humans have far more than monkeys. However, the exact numbers are missing as areas can be very difficult to identify, and thus we are left with estimates. The cortical areas proposed for macaque monkeys, for instance, are drawn from a number of studies, with some of these areas now well established as valid by various types of evidence, and others based on limited evidence, such as perceived differences in histological appearance or in apparent differences in connections with other parts of the brain. As evidence accumulates, borders will change and proposed areas will change and proposed areas will be subdivided, so that present estimates may be too small. However, galagos appear to have at least 50 cortical areas [85], and as many as 129 areas have been proposed for macaques [86]. Human brains (where less is known experimentally, but evidence is accumulating) may have as many as 150 – 200 or more cortical areas [87, 88]. Consistent with this premise is the evidence that in the three-times larger human brain, the well-defined primary sensory and motor areas are about the same size as in a chimpanzee brain. Thus, all areas did not simply get bigger, as more areas were likely added, providing new functions and more levels of analysis.
Prologue
While the emphasis of this review has been on neocortex, as this part expanded so much in the evolution of the human brain, changes in any part of the brain effects and modifies many other parts. As one example, the superior colliculus of the visual midbrain receives cortical projections in all mammals, but the information relayed to the superior colliculus changes as neocortex adds areas and modifies others. As another example, the number of neurons in the cerebellum scales with the number of neurons in the neocortex of primates, providing evidence that they are functionally interrelated [89]. Changes in the organization and size of neocortex also are reflected in cortical projections to the basal ganglia, the dorsal thalamus, the amygdala, and the hippocampus. In addition, primates and especially humans have lost some brain functions. Most notably, monkeys, chimpanzees, and humans have progressively fewer functional genes for olfactory receptors, representing a progressive loss of olfactory function [90]. Yet, some types of olfactory processing in orbital frontal cortex may be enhanced in humans [91]. Thus, we have only covered a small part of a complex story.
References
- 1.Dawkins R. The Ancestor’s Tale. New York: Houghton Mifflin Co; 2004. [Google Scholar]
- 2.Kaas JH. Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav Evol. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- 3.Hennig W. Phylogenetic Systematics. Urbana: University of Illinois Press; 1966. [Google Scholar]
- 4.Albert JS. Phylogenetic character reconstruction. In: Striedter GF, Rubenstein JLR, Kaas JH, editors. Evolution of Nervous Systems. Vol. 1. London: Elsevier; 2007. pp. 41–53. [Google Scholar]
- 5.Steiper ME, Seiffert ER. Evidence for a convergent slowdown in primate molecular rates and its implications for the timing of early primate evolution. Proc Natl Acad Sci U S A. 2012;109:6004–6011. doi: 10.1073/pnas.1119506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bininda-Emonds ORP, Cardillo M, Jones KE, MacPhee RDE, Beck RMD, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- 7.Fraser GJ, Britz R, Hall AG, Johanson Z, Smith MM. Replacing the first-generation dentition in pufferfish with a unique beak. Proc Natl Acad Sci U S A. 2012;109:8179–8184. doi: 10.1073/pnas.1119635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould JS. Ontogeny and Phylogeny. Cambridge: Harvard Univ Press; 1977. [Google Scholar]
- 9.Molnár Z. Evolution of cerebral cortical development. Brain Behav and Evol. 2011;78:94–107. doi: 10.1159/000327325. [DOI] [PubMed] [Google Scholar]
- 10.Molnár Z, Tavare A, Cheurq AFP. The origin of neocortex: lessons from comparative embryology. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems. Vol. 3. Oxford: Elsevier; 2007. pp. 13–26. Mammals. [Google Scholar]
- 11.Puelles L. Pallio-pallial tangential migrations and growth signaling: New scenario for cortical evolution. Brain Behav Evol. 2011;78:108–127. doi: 10.1159/000327905. [DOI] [PubMed] [Google Scholar]
- 12.Tattersall I. The Fossil Trail. 2. Oxford: Oxford Univ Press; 2009. [Google Scholar]
- 13.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci U S A. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herculano-Houzel S. Not all brains are made the same; new views on brain scaling in evolution. Brain Behav and Evol. 2011;78:22–36. doi: 10.1159/000327318. [DOI] [PubMed] [Google Scholar]
- 15.Takahata T, Shukla R, Yamamori T, Kaas JH. Differential expression patterns of striate-cortex-enriched genes among Old World, New World, and prosimian primates. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr308. [E-pub ahead of print] November 7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preuss TM. Human brain evolution: From gene discovery to phenotype discovery. Proc Natl Acad Sci U S A. 2012;109:10709–10716. doi: 10.1073/pnas.1201894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand J-B, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Rilling JK, Glasser MF, Jbabdi S, Andersson J, Preuss TM. Continuity, divergence, and the evolution of brain language pathways. Front Evol Neurosci. 2012;3:1–6. doi: 10.3389/fnevo.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. 2. Hoboken: John Wiley & Sons; 2005. [Google Scholar]
- 20.Shepherd GM. The microcircuit concept applied to cortical evolution: from three-layer to six-layer cortex. Front Neuroanat. 2011;5:1–15. doi: 10.3389/fnana.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerison HJ. What fossils tell us about the evolution of the neocortex. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems. Vol. 3. London: Elsevier; 2007. pp. 1–12. Mammals. [Google Scholar]
- 22.Aiello LC, Wheeler P. The expensive tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol. 1995;36:199–221. [Google Scholar]
- 23.Murphy WJ, Pevzner PA, O’Brien JO. Mammalian phylogenomics comes of age. Trends in Genetics. 2004;20:631–639. doi: 10.1016/j.tig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Rowe TB, Macrini TE, Luo Z-X. Fossil evidence on origin of the mammalian brain. Science. 2011;332:955–957. doi: 10.1126/science.1203117. [DOI] [PubMed] [Google Scholar]
- 25.Allman J. Evolving Brains. New York: W.H. Freeman & Co; 1999. [Google Scholar]
- 26.Kaas JH. Reconstructing the areal organizatioin of the neocortex of the first mammals. Brain Behav and Evol. 2011;78:7–21. doi: 10.1159/000327316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catania KC, Lyon DC, Mock OB, Kaas JH. Cortical organization in shrews: evidence from five species. J Comp Neurol. 1999;410:55–72. [PubMed] [Google Scholar]
- 28.Naumann RK, Anjum F, Roth-Alpermann C, Brecht M. Cytoarchitecture, areas, and neuron numbers of the Etruscan Shrew cortex. J Comp Neurol. 2012;520:2512–2530. doi: 10.1002/cne.23053. [DOI] [PubMed] [Google Scholar]
- 29.Ashwell KWS. Development of the spinal cord and peripheal nervous system in platypus (Ornithorhynchus anatinus) and short-beaked echidna (Tachyglossus aculeatus) Somato Mot Res. 2012;29:13–27. doi: 10.3109/08990220.2012.662185. [DOI] [PubMed] [Google Scholar]
- 30.Cooper HM, Herbin M, Nevo E. Visual system of a naturally microphthalmic mammal: the blind mole rat, Spalax ehrenbergi. J Comp Neurol. 1993;328:313–350. doi: 10.1002/cne.903280302. [DOI] [PubMed] [Google Scholar]
- 31.Assimacopoulos S, Kao T, Issa NP, Grove EA. Fibroblast Growth Factor 8 organizes the neocortical area map and regulates sensory map topography. J Neurosci. 2012;32:7191–7201. doi: 10.1523/JNEUROSCI.0071-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosa MGP, Krubitzer LA. The evolution of visual cortex: where is V2? TINS. 1999;22:242–248. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- 33.Kaas JH. The evolution of auditory cortex: the core areas. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. New York: Springer-Verlag; 2011. pp. 407–427. [Google Scholar]
- 34.Beck PD, Pospichal MW, Kaas JH. Topography, architecture, and connections of somatosensory cortex in opossums: evidence for five somatosensory areas. J Comp Neurol. 1996;366:109–133. doi: 10.1002/(SICI)1096-9861(19960226)366:1<109::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- 36.Krubitzer L, Manger P, Pettigrew J, Calford M. Organization of somatosensory cortex in monotremes: In search of the prototypical plan. J Comp Neurol. 1995;351:261–306. doi: 10.1002/cne.903510206. [DOI] [PubMed] [Google Scholar]
- 37.Kemp E. The endocranial cavity of a nonmammalian eucynodont (Chiniquodon theotenicus) and its implications for the origin of the mammalian brain. J Vert Paleontology. 2009;29:1188–1198. [Google Scholar]
- 38.Catania KC, Jain N, Franca JG, Volchan E, Kaas JH. The organization of somatosensory cortex in the short-tailed opossum (Monodelphis domestica) Somatosens Mot Res. 2000;17:39–51. doi: 10.1080/08990220070283. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson MA, Churakov G, Sommer M, Van Tran N, Zemann A, Brosius J, Schmitz J. Tracking marsupial evolution using archaic genomic retroposon insertions. PLoS Bio. 2010;8:1–9. doi: 10.1371/journal.pbio.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji Q, Luo ZX, Yuan CX, Wible JR, Zhang JP, Georgi JA. The earliest known eutherian mammal. Nature. 2002;416:816–822. doi: 10.1038/416816a. [DOI] [PubMed] [Google Scholar]
- 41.Kaas JH. The evolution of the dorsal thalamus in mammals. In: Kaas JH, Krubitzer LA, Kaas JH, editors. Evolution of Nervous Systems. Vol. 3. London: Elsevier; 2007. pp. 500–516. [Google Scholar]
- 42.Kaas JH, Guillery RW, Allman JM. Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav Evol. 1972;6:253–299. doi: 10.1159/000123713. [DOI] [PubMed] [Google Scholar]
- 43.Welker WI. Why does cerebral cortex fissure and fold? In: Jones EG, Peters A, editors. Cerebral Cortex. New York: Plenum Pub; 1990. pp. 3–136. [Google Scholar]
- 44.Van Essen DC. Cerebral cortical folding patterns in primates: why they vary and what they signify. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4. London: Elsevier; 2007. pp. 267–276. Primates. [Google Scholar]
- 45.Kaas JH. Cerebral Fissure Patterns. In: Squire LR, editor. Encyclopedia of Neuroscience. 3. Oxford: Elsevier; 2009. pp. 793–800. [Google Scholar]
- 46.Block JI, Silcox MT, Boyer DM, Sargis EJ. New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proc Natl Acad Sci U S A. 2007;104:1159–1164. doi: 10.1073/pnas.0610579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Block JI, Boyer DM. Grasping primate origins. Science. 2002;298:1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- 48.Kaas JH. Why does the brain have so many visual areas? J Cogn Neurosci. 1989;1:121–135. doi: 10.1162/jocn.1989.1.2.121. [DOI] [PubMed] [Google Scholar]
- 49.Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007;55:285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldwin MKL, Balaram P, Kaas JH. Superior colliculus connections with visual thalamus in prosimian galagos (Otolemur garnetti) 2012 Submitted. [Google Scholar]
- 51.Lane RH, Allman JM, Kaas JH, Miezin FM. The visuotopic organization of the superior colliculus of the owl monkey (Aotus trivirgatus) and the bush baby (Galago senegalensis) Brain Res. 1973;60:335–349. doi: 10.1016/0006-8993(73)90794-4. [DOI] [PubMed] [Google Scholar]
- 52.Kaas JH. Evolution of neocortex in primates. Prog Brain Res. 2012;5:91–102. doi: 10.1016/B978-0-444-53860-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preuss TM, Qi H, Kaas JH. Distinctive compartmental organization of human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:11601–11606. doi: 10.1073/pnas.96.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Passingham RE, Wise SP. The Neurobiology of Prefrontal Cortex. Oxford: Oxford Univ Press; 2012. [Google Scholar]
- 56.Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Front Neuroanat. 2011;5:1–7. doi: 10.3389/fnana.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci U S A. 2010;107:15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain and Mind. 2000;1:7–23. [Google Scholar]
- 60.Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REJ, Leite REP, Filho WJ, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 61.Hill J. Proc Natl Acad Sci U S A. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preuss TM. The human brain: rewired and running hot. Ann N Y Acad Sci. 2011;1225:182–191. doi: 10.1111/j.1749-6632.2011.06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Falk D. Endocranial costs and their significance for primate brain evolution. In: Swindler DR, Erwin JM, editors. Comparative Primate Biology: Vol 1, Systematics, Evolution, and Anatomy. Vol. 1. New York: Alan R. Liss; 1986. pp. 477–490. [Google Scholar]
- 64.Hunt KD. The evolution of human bipedality. J Hum Evol. 1994;26:181–202. [Google Scholar]
- 65.de Sousa A, Wood B. The hominin fossil record and the emergence of the modern human central nervous system. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4. London: Elsevier; 2007. pp. 291–336.pp. 291–336. [Google Scholar]
- 66.McHenry HM. Tempo and mode in human evolution. Proc Natl Acad Sci U S A. 1994;91:6780–6786. doi: 10.1073/pnas.91.15.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wrangham R. Catching fire: how cooking made us human. New York: Basic Books; 2009. [Google Scholar]
- 68.Bogin B. The evolution of Human brain and body growth patterns. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4. London: Elsevier; 2007. pp. 337–345. [Google Scholar]
- 69.Allman JM, McLaughlin T, Hakeem A. Brain weight and life-span in primate species. Proceedings of National Academy of Science, USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aldridge K. Patterns of differences in brain morphology in humans as compared to extant apes. J Hum Evol. 2011;60:94–105. doi: 10.1016/j.jhevol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keller SS, Roberts N, Hopkins W. A comparative magnetic resonance imaging study of the anatomy, variability, and asymmetry of Broca’s area in the human and chimpanzee brain. J Neurosci. 2009;29:14607–14616. doi: 10.1523/JNEUROSCI.2892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. TRENDS in Cog Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Gannon PJ, Kheck NM, Braun AR, Holloway RL. Planum parietale of chimpanzees and orangutans; a comparative resonance of human-like planum temporale asymmetry. Anat Rec. 2005;287A:1128–1141. doi: 10.1002/ar.a.20256. [DOI] [PubMed] [Google Scholar]
- 74.Caminiti R, Ghaziri H, Galuske R, Hof PR, Innocenti GM. Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates. Proc Natl Acad Sci U S A. 2009;106:19551–19556. doi: 10.1073/pnas.0907655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gazzaniga MS. Organization of the Human Brain. Science. 1989;245:947–952. doi: 10.1126/science.2672334. [DOI] [PubMed] [Google Scholar]
- 76.Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nature. 2002 doi: 10.1038/nn814. On-Line, February, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Elston GN, Benavides-Piccione R, Elston A, Zietsch B, Defelipe J, Manger P, Casagrande V, Kaas JH. Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec. 2006;288A:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- 78.Semendeferi K, Teffer K, Buxhoeveden DP, Park MS, Bludau S, Amunts K, Travis K, Buckwalter J. Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb Cortex. 2011;21:1485–1497. doi: 10.1093/cercor/bhq191. [DOI] [PubMed] [Google Scholar]
- 79.Gallay DS, Gallay MN, Jeanmonod D, Rouiller EM, Morel A. The Insula of Reil revisited: multiarchitectonic organization in macaque monkeys. Cereb Cortex. 2012;22:175–190. doi: 10.1093/cercor/bhr104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park SJ, Goubert V, Hof PR. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- 81.Semendeferi K, Damasio H. The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. J Hum Evol. 2000;38:317–332. doi: 10.1006/jhev.1999.0381. [DOI] [PubMed] [Google Scholar]
- 82.Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- 83.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 84.Mountcastle VB. The parietal system and some higher brain functions. Cereb Cortex. 1995;5:377–390. doi: 10.1093/cercor/5.5.377. [DOI] [PubMed] [Google Scholar]
- 85.Wong P, Kaas JH. Architectonic subdivisions of neocortex in the Galago (Otolemur garnetti) Anat Rec (Hoboken) 2010;293:1033–1069. doi: 10.1002/ar.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Essen DC, Glasser MF, Dierker DL, Harwell J. Cortical Parcellations of the macaque monkey analyzed on surface-based atlases. Cereb Cortex. 2011 Nov 2; doi: 10.1093/cercor/bhr290. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Changizi MA, Shimojo S. Parcellation and area-area connectivity as a function of neocortex size. Brain Behav Evol. 2005;66:88–98. doi: 10.1159/000085942. [DOI] [PubMed] [Google Scholar]
- 88.Kaas JH. Reconstructing the organization of the neocortex of the first mammals and subsequent modifications. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems. Vol. 3. London: Elsevier; 2007. pp. 27–48. [Google Scholar]
- 89.Herculano-Houzel S, Kaas JH. Gorilla and orangutan brains conform to the primate cellular scaling rules: implications for human evolution. Brain Behav and Evol. 2011;77:33–44. doi: 10.1159/000322729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gilad Y, Man O, Pääbo S, Lancet D. Human specific loss of olfactory receptor genes. Proc Natl Acad Sci U S A. 2003;100:3324–3327. doi: 10.1073/pnas.0535697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shepherd GM. Neurogastronomy. New York: Columbia Univ. Press; 2012. [Google Scholar]