Abstract
Background
Staphylococcus aureus bacteremia results in substantial mortality. Infectious diseases specialist consultation can improve adherence to evidence-based management of S. aureus bacteremia, but its effect on mortality is unclear.
Methods
We performed a 2-year prospective cohort study of patients with S. aureus bacteremia at a large, tertiary care hospital. Patients who died within 2 days of diagnosis were excluded. Independent risk factors for 28-day mortality were determined.
Results
Among 341 patients with S. aureus bacteremia, 189 (55%) were male; 196 (58%) were Caucasian; 185 (54%) had methicillin-resistant S. aureus; 108 (32%) had nosocomial bacteremia; and 231 (68%) had a central venous catheter at the time of diagnosis. The median age was 56 years (range 22-95). One hundred eleven (33%) patients had an infectious diseases consultation. Fifty-four (16%) patients died with 28 days after diagnosis. Factors associated with mortality were intensive care unit admission ≤ 48 hours after the first positive blood culture [adjusted hazard ratio (aHR), 4.65; 95% confidence interval (CI), 2.65-8.18], cirrhosis (aHR, 4.44; 95% CI, 2.40-8.20), and advanced age (aHR, 1.27 per every 10 years of age; 95% CI, 1.08-1.50). Infectious diseases consultation was associated with a 56% reduction in 28-day mortality (aHR, 0.44; 95% CI, 0.22-0.89).
Conclusion
Only one-third of patients with S. aureus bacteremia in this cohort had an infectious diseases specialist consultation. Infectious diseases consultation was independently associated with a reduction in 28-day mortality. Routine infectious diseases consultation should be considered for patients with S. aureus bacteremia, especially those with higher severity of illness or multiple co-morbidities.
Keywords: Staphylococcus aureus bacteremia, Mortality, Infectious diseases consultation
Staphylococcus aureus bacteremia causes significant morbidity and mortality. Mortality among patients with S. aureus bacteremia ranges from 20% to 50% despite the availability of effective antimicrobial therapy. 1-3 Major factors previously associated with mortality include severity of illness at the time of S. aureus bacteremia; inappropriate empiric antimicrobial therapy; the presence of co-morbid conditions such as diabetes mellitus; retained infectious foci; and bacteremia due to methicillin-resistant S. aureus (MRSA).2, 4-6 Following an evidence-based approach is considered essential to treat S. aureus bacteremia, and consists of appropriate choice and duration of antimicrobial therapy, removal of infected foci, and detailed evaluation for metastatic infection or endocarditis.7-9 Infectious diseases consultation has been associated with increasing adherence to evidence-based treatment of S. aureus bacteremia, 1, 10 however the impact of an infectious diseases consultation on the mortality of S. aureus bacteremia is uncertain. A recent German study suggested a survival benefit, however this study was performed in a healthcare delivery system different than the United States (U.S), and may have been confounded by temporal trends. The purpose of our study was to assess the effect of an infectious diseases consultation on mortality, independent of patient co-morbidities at a U.S. tertiary care center.
Methods
Participants and Setting
A prospective cohort study of patients with S. aureus bacteremia was performed between July 2005 and July 2007 at Barnes- Jewish Hospital, a 1252-bed, academic, tertiary care center in St. Louis, Missouri. Diagnosis of S. aureus bacteremia was defined as ≥ 1 blood culture positive for S. aureus with clinical evidence of infection. Patients who were less than 18 years old at the time of diagnosis of S. aureus bacteremia, had a history of S. aureus bacteremia in the prior 3 months, or a history of S. aureus endocarditis in the prior 12 months were excluded. A total of 347 patients with S. aureus bacteremia met these inclusion criteria. Six patients (2%) who died ≤ 2 days after their first positive blood culture were also excluded, leaving 341 patients for analysis.
Variables of interest and data collection
A 28-day all-cause mortality and 365-day all-cause mortality was used to assess the efficacy of infectious diseases consultation for S. aureus bacteremia. We tracked mortality for one year after the first positive blood culture of S. aureus by reviewing both medical records and the Social Security Death Index.11 If there was no mortality data was available in medical chart, patients with any readmission beyond 28 days and 365 days after the initial diagnosis of S. aureus bacteremia were considered to be alive at 28 days and 365 days respectively. If there was no readmission data > 28 days or 365 days after diagnosis of S. aureus bacteremia available, Social Security Death Index was used to determine if patients died ≤ 28 days or between 29 and 365 days after the diagnosis of S. aureus bacteremia. Demographic characteristics, clinical data, and microbiology data were prospectively obtained from the medical record. Prolonged bacteremia was defined as the isolation of S. aureus from blood cultures on at least twice during the five days following the first positive culture.12 Metastatic S. aureus infection was defined by microbiological or radiographic evidence of remote site of infection suggesting hematogenous spread.10 Healthcare-associated, hospital onset bacteremia was defined by a positive blood culture obtained from patients who were hospitalized > 48 hours.13 Healthcare-associated, community onset of bacteremia was defined as a positive blood culture ≤ 48 hours after hospitalization with the following criteria; presence of an invasive device, history of MRSA infection or colonization, surgery, hospitalization, dialysis, or residence in a long-term care facility in the 12 months preceding the culture.13 Community-associated bacteremia was defined by a positive blood culture ≤ 48 hours without meeting the criteria of healthcare associated community-onset bacteremia.13
During the study period, two general and one transplant infectious diseases inpatient consultation services were available. All infectious diseases consultation services were staffed by American Board of Internal Medicine (ABIM) board-certified infectious disease physicians who were Washington University faculty. Infectious diseases consultations were at the discretion of the primary care team. Acquisition of infectious diseases consultation was defined as consultation for the purpose of management for S. aureus bacteremia during the index hospitalization. If S. aureus bacteremia developed while infectious diseases consultation was already in place for another reason, the date of first positive blood culture was defined as the consult initiation date. We also assessed the following aspects of evidence-based management for S. aureus bacteremia: appropriateness of antimicrobial therapy, evaluation by transesophageal echocardiogram, removal or treatment of infected foci (i.e., medical devices or abscesses), and planned duration of treatment. Appropriateness of antimicrobial therapy was defined as use of parenteral antimicrobial agents [i.e., beta-lactam antimicrobials for patients with methicillin-susceptible S. aureus (MSSA) bacteremia, vancomycin, daptomycin, or linezolid for patients with MRSA bacteremia or S. aureus bacteremia with beta-lactam allergy]. Planned duration of treatment was appropriate when parenteral antimicrobial therapy was scheduled at least ≥ 2 weeks if a trans-esophageal echocardiogram showed no evidence of endocarditis, at least ≥ 4 weeks if trans-esophageal echocardiogram was not performed, at least ≥ 4 weeks if there was evidence of endocarditis, metastatic infection, or osteomyelitis.1
Statistical analyses
Categorical variables were compared between those with and without infectious diseases consults using Fisher's exact test, while age was compared between the two groups using the Mann-Whitney test. All tests for significance were 2-tailed, with P values <.05 considered significant.
We performed multivariable survival analyses predicting a 28-day all-cause mortality and 29-365 day all-cause mortality. For the 28-day mortality model, we used the extended Cox proportional hazards model because infectious diseases consultation was treated as a time-dependent variable, using the counting process style of input (i.e., Andersen-Gill model).14 The value for consultation was 0 before the time of consultation and 1 after the consultation. Infectious diseases consultation was not modeled as a time-dependent variable for the 29-365 day mortality model because nearly all (108/111, 97%) consultations occurred within the first 28 days following the first positive culture. Potential risk factors were first assessed in bivariate analysis. The multivariable models were developed in forward stepwise fashion. Candidate variables with P < .10 in bivariate analysis were considered for inclusion in the models and variables were retained in the final models if P <.05. The proportional hazards assumption was assessed via –ln(-ln) survival curves, time-dependent covariates, and Shoenfeld residuals.15, 16 The relationship of the time-dependent variable infectious diseases consultation and mortality during 365 days of follow-up was illustrated using an extended Kaplan-Meier estimator, which can be used with time-dependent covariates.17 Extended Kaplan-Meier curves did not correspond to fixed patient cohorts, but allowed for the cohorts to be updated at each event time. Therefore, the patient without an infectious disease consultation was included in one curve, until consultation was obtained and the patient is then included in the consultation curve. The proportions illustrated should not be strictly interpreted; rather this method was intended to provide a realistic and useful graphical representation of time-varying covariate analyses. All analyses were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL) and SAS version 9.1.3 (SAS Institute, Cary, NC). The Washington University Human Research Protection approved this project.
Results
Demographic characteristics and clinical data of the 341 patients with S. aureus bacteremia included in this study are shown in Table 1. One hundred eighty-five patients (54%) had MRSA bacteremia and 108 patients (32%) had hospital-onset of infection. One hundred-ten patients (32%) were admitted to the intensive care units (ICUs) ≤ 48 hours after the first positive blood culture.
Table 1. Demographic Characteristics of 341 Patients with S. aureus bacteremia.
| Variable | n (%) |
|---|---|
| Age, years, median (range) | 56 (22-95) |
| Female gender | 152 (45) |
| White race | 196 (57) |
| Congestive heart failure | 63 (18) |
| Coronary artery disease | 80 (23) |
| COPD | 54 (16) |
| Renal function | |
| Normal | 243 (71) |
| CRF without dialysis | 27 (8) |
| CRF with dialysis | 71 (21) |
| Malignancy | 85 (25) |
| Chronic skin disease | 19 (6) |
| HIV | 12 (4) |
| Peripheral vascular disease | 23 (7) |
| Diabetes mellitus | 111 (33) |
| Systemic corticosteroid use last 28 days | 49 (14) |
| Cirrhosis | 28 (8) |
| History of intravenous drug use | 24 (7) |
| History of smoking | 182 (53) |
| Alcohol use | 95 (28) |
| Any transplant | 26 (8) |
| Surgery during hospitalization | 45 (13) |
| Prosthetic joint | 29 (9) |
| Other orthopedic hardware | 23 (7) |
| Vascular graft | 48 (14) |
| Prosthetic valve | 9 (3) |
| Cardiac device | 30 (9) |
| ICU admission within 48 hours after the first positive blood culture | 110 (32) |
| Central venous catheterization at the time of the first positive blood culture | 231 (68) |
| MRSA bacteremia | 185 (54) |
| Onset | |
| Community-associated | 37 (11) |
| Healthcare-associated community onset | 196 (57) |
| Healthcare-associated hospital onset | 108 (32) |
| Metastatic infection* | 59 (17) |
| Prolonged bacteremia | 93 (27) |
| Infectious diseases consultation obtained | 111 (33) |
| All-cause mortality ≤ 28 days after diagnosis | 54 (16) |
| All-cause mortality ≤ 1year after diagnosis | 140 (41) |
Note. COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; HIV, human immunodeficiency virus; ICU, intensive care unit; MRSA, methicillin-resistant S. aureus.
See methods for definition.
In this cohort, 111 patients (33%) had infectious diseases consultation for management of S. aureus bacteremia. Factors associated with obtaining infectious diseases consultation are shown in Table 2. Eighty-seven consults (78%) were obtained within 5 days after the first positive blood culture (range 0-36 days). Patients with S. aureus bacteremia who received infectious diseases consultation were more likely to be Caucasian, and more likely to have congestive heart failure, orthopedic hardware, cardiac devices (i.e., implantable pacemaker/defibrillator), prosthetic valves, human immunodeficiency virus, community-associated S. aureus bacteremia, metastatic infection, and prolonged bacteremia. Patients who did not receive infectious diseases consultation were more likely to be chronic hemodialysis recipients, have a central venous catheter, history of smoking, or a current diagnosis of malignancy. Patients with infectious diseases consultation were more likely to receive appropriate antimicrobial agents, undergo trans-esophageal echocardiogram, and have appropriate planned duration of antimicrobial therapy (Table 3).
Table 2. Comparison of S. aureus Bacteremia Patients with and without Infectious Disease Consultation.
| Variable | Infectious diseases consultation | P | |
|---|---|---|---|
| Yes (n=111) | No (n=230) | ||
| Age, years, median (range) | 56 (23-95) | 56 (22-92) | .67 |
| Female gender | 55 (50) | 97 (42) | .20 |
| White race | 75 (68) | 121 (53) | .01 |
| Congestive heart failure | 28 (25) | 35 (15) | .04 |
| Coronary artery disease | 31 (28) | 49 (21) | .22 |
| COPD | 17 (15) | 37 (16) | .86 |
| Renal function | |||
| Normal | 90 (81) | 153 (67) | Ref |
| CRF without dialysis | 8 (7) | 19 (8) | .53 |
| CRF with dialysis | 13 (12) | 58 (26) | <.01 |
| Malignancy | 18 (16) | 67 (29) | .01 |
| Chronic skin disease | 7 (6) | 12 (5) | .80 |
| HIV | 8 (7) | 4 (2) | .02 |
| Peripheral vascular disease | 7 (6) | 16 (7) | 1.0 |
| Diabetes mellitus | 32 (29) | 79 (34) | .33 |
| Systemic corticosteroid use last 28 days | 15 (14) | 34 (15) | .87 |
| Liver cirrhosis | 6 (5) | 22 (10) | .21 |
| History of intravenous drug use | 11 (10) | 13 (6) | .18 |
| History of smoking | 49 (44) | 133 (58) | .02 |
| Alcohol use | 33 (30) | 62 (27) | .61 |
| Any transplant | 9 (8) | 17 (8) | .83 |
| Surgery during hospitalization | 16 (14) | 29 (13) | .73 |
| Prosthetic joint | 17 (15) | 12 (4) | <.01 |
| Other orthopedic hardware | 18 (16) | 5 (2) | <.01 |
| Vascular graft | 10 (9) | 38 (17) | .07 |
| Prosthetic valve | 9 (8) | 0 | <.01 |
| Cardiac device | 16 (14) | 14 (6) | .01 |
| ICU admission within 48 hours after the first positive blood culture | 35 (31) | 75 (33) | .90 |
| Central venous catheterization at the time of the first positive blood culture | 63 (57) | 168 (73) | <.01 |
| MRSA | 58 (52) | 127 (55) | .64 |
| Onset | |||
| Community-associated | 21 (19) | 16 (7) | Ref |
| Healthcare-associated community onset | 61 (55) | 135 (59) | <.01 |
| Healthcare-associated hospital onset | 29 (26) | 79 (34) | <.01 |
| Metastatic infection | 41 (37) | 18 (8) | <.01 |
| Prolonged bacteremia* | 43 (39) | 50 (22) | <.01 |
Note. COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; HIV, human immunodeficiency virus; ICU, intensive care unit; MRSA, methicillin-resistant S. aureus; Ref, reference.
See methods for definition.
Table 3. Univariate Analysis of Evidence-Based Management based on Infectious Disease Consultation.
| Variable | Infectious diseases consultation | P | |
|---|---|---|---|
| Yes (n=111) | No (n=230) | ||
| Appropriate antimicrobial choicea | 100 (90) | 182 (79) | .01 |
| Appropriate planned duration of antimicrobial therapy (n=292)b | 84/104 (81) | 54/188 (29) | <.001 |
| Retained infected focus present | 14 (13) | 34 (15) | .50 |
| Trans-esophageal echocardiogram, performed | 38 (34) | 18 (8) | <.001 |
Note.
See methods for definition.
Duration of therapy was unspecified for 49 patients.
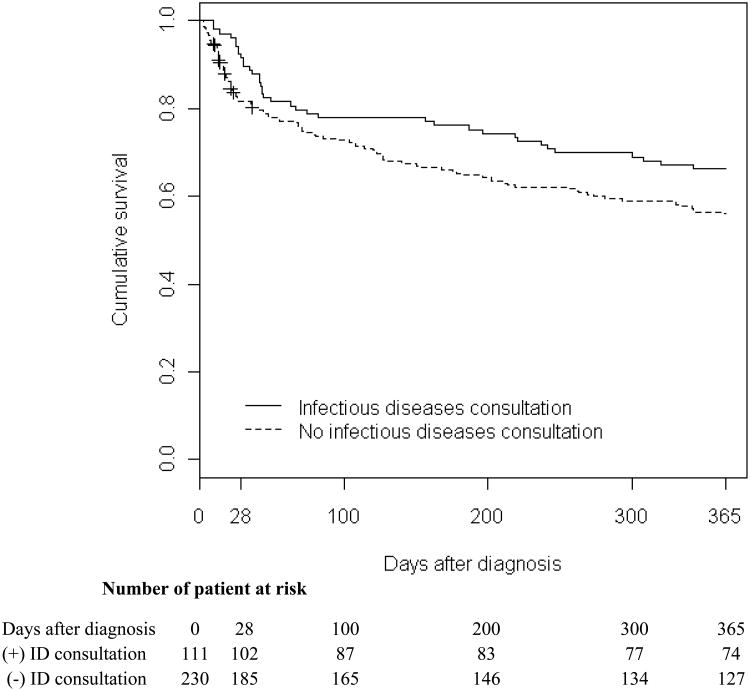
The overall all-cause 1-year mortality rate for patients with S. aureus bacteremia was 41% [140/341; 95% confidence interval (CI), 36-46 (%)]. Median length of time from diagnosis to death was 46.5 days. All-cause 28-day mortality was 16% [54/341; 95% CI, 12-20 (%)]. The extended Kaplan-Meier survival curve for patients with S. aureus bacteremia stratified by infectious disease consultation is shown in Figure 1. Patients with S. aureus bacteremia who died within 28 days of first positive blood culture were more likely to be older and treated with vancomcycin, had chronic renal failure, cirrhosis, MRSA bacteremia, and have been admitted to the ICU ≤ 48 hours after the first positive blood culture (Table 4). In the multivariate model, factors independently associated with 28-day mortality among patients with S. aureus bacteremia were ICU admission ≤ 48 hours after the first positive blood culture [adjusted hazard ratio (aHR), 4.65; 95% confidence interval (CI), 2.65-8.18, P <.001], cirrhosis (aHR, 4.44; 95% CI, 2.40-8.20, P <.001), and increasing age (aHR, 1.27; per every 10 years of age; 95% CI, 1.08-1.50, P =.004). Infectious diseases consultation was associated with decreased risk of 28-day mortality (aHR, 0.44; 95% CI, 0.22-0.89, P =.022) (Table 4).
Figure 1. Extended Kaplan-Meier Curve of S. aureus Bacteremia Based on Infectious Diseases Consultation.
Note. ID, infectious diseases.
Extended Kaplan-Meier curves did not correspond to fixed patient cohorts, but allowed for the cohorts to be updated at each event time. The patient without an infectious disease consultation was included in one curve, until consultation was obtained and the patient is then included in the consultation curve. Adjusted hazard ratio for 28-day mortality was 0.44 (P =.022) and the crude hazard ratio for 29-365 day mortality was 0.88 (P =.579).
Table 4. Predictors of Mortality ≤ 28 Days after Diagnosis of S. aureus Bacteremia for 341 Patients.
| Variable | Died ≤ 28days after the diagnosis (n=54) | Survived > 28days after the diagnosis (n=287) | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Age years, median, (range) | 62 (22-91) | 55 (23-95) | 1.26 (1.07-1.49) | 1.27 (1.08-1.50) |
| Female gender | 27 (50) | 125 (44) | 1.31 (0.77-2.23) | |
| White race | 35 (65) | 161 (56) | 1.39 (0.80-2.44) | |
| Congestive heart failure | 13 (24) | 50 (17) | 1.41 (0.75-2.63) | |
| Coronary artery disease | 14 (26) | 66 (23) | 1.15 (0.62-2.11) | |
| COPD | 12 (22) | 42 (15) | 1.60 (0.84-3.04) | |
| Renal function | ||||
| Normal | 36 (67) | 207 (72) | 1.00 | |
| CRF without dialysis | 11 (20) | 16 (6) | 3.22 (1.64-6.34) | |
| CRF with dialysis | 7 (13) | 64 (22) | 0.65 (0.29-1.48) | |
| Malignancy | 13 (24) | 72 (25) | 0.96 (0.51-1.79) | |
| Chronic skin disease | 2 (4) | 17 (6) | 0.66 (0.16-2.65) | |
| HIV | 1 (2) | 11 (4) | 0.48 (0.07-3.44) | |
| Peripheral vascular disease | 3 (6) | 20 (7) | 0.80 (0.25-2.56) | |
| Diabetes mellitus | 14 (26) | 97 (34) | 0.71 (0.39-1.31) | |
| Systemic corticosteroid use last 28 days | 9 (17) | 40 (14) | 1.23 (0.60-2.51) | |
| Cirrhosis | 14 (26) | 14 (5) | 4.72 (2.56-8.68) | 4.44 (2.40-8.20) |
| History of intravenous drug use | 4 (7) | 20 (7) | 1.03 (0.37-2.85) | |
| History of smoking | 32 (59) | 150 (52) | 1.32 (0.77-2.28) | |
| Alcohol use | 14 (26) | 81 (28) | 0.90 (0.49-1.66) | |
| Any transplant | 4 (7) | 22 (8) | 0.95 (0.34-2.64) | |
| Surgery during hospitalization | 5 (9) | 40 (14) | 0.66 (0.26-1.65) | |
| Prosthetic joint | 3 (6) | 26 (9) | 0.60 (0.19-1.93) | |
| Prosthetic valve | 2 (4) | 7 (2) | 1.33 (0.32-5.45) | |
| Other orthopedic hardware | 4 (8) | 19 (7) | 1.11 (0.40-3.08) | |
| Vascular graft | 3 (6) | 45 (16) | 0.34 (0.11-1.10) | |
| Cardiac device | 8 (15) | 22 (8) | 1.81 (0.86-3.84) | |
| ICU admission ≤ 48 hours after the first positive blood culture | 36 (67) | 74 (26) | 4.94 (2.81-8.71) | 4.65 (2.65-8.18) |
| Central venous catheterization at the time of the first positive blood culture | 41 (76) | 190 (66) | 1.58 (0.85-2.95) | |
| Vancomycin use | 47 (87) | 214 (75) | 2.13 (0.96-4.71) | |
| Gentamicin use | 3 (6) | 28 (10) | 0.55 (0.17-1.76) | |
| MRSA | 35 (65) | 150 (52) | 1.61 (0.92-2.82) | |
| Onset | ||||
| Community-associated | 3 (6) | 34 (12) | 1.00 | |
| Healthcare-associated community onset | 29 (54) | 167 (58) | 1.88 (0.57-6.16) | |
| Healthcare-associated hospital onset | 22 (41) | 86 (30) | 2.63 (0.79-8.79) | |
| Any metastatic infection | 9 (17) | 50 (17) | 0.95 (0.47-1.95) | |
| Prolonged bacteremia | 13 (24) | 80 (28) | 0.84 (0.45-1.57) | |
| Infectious diseases consultation (time-dependent, covariate) | 9 (17) | 102 (36) | 0.46 (0.23-0.93) | 0.44 (0.22-0.89) |
Note. CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; HIV, human immunodeficiency virus; ICU, intensive care unit; MRSA, methicillin-resistant S. aureus; Ref, reference.
Variables considered but not retained in the final model were chronic renal failure without dialysis, vascular graft, vancomycin use, and MRSA bacteremia.
For the 287 patients who survived 28 days after the initial positive culture, predictors of 1-year mortality after 28 days (i.e., 29-365 day) of the diagnosis of S. aureus bacteremia were elucidated. Factors independently associated with 1-year mortality for this group were malignancy at the time of bacteremia (aHR, 3.06; 95% CI, 1.97-4.74, P <.001), cirrhosis (aHR, 4.11; 95% CI, 2.02-8.33, P <.001), peripheral vascular disease (aHR, 2.58; 95% CI, 1.29-5.18, P = .008), advanced age (aHR, 1.16 per every 10 years of age, 95% CI, 1.01-1.35, P = .041), and ICU admission ≤ 48 hours after the first positive blood culture (aHR, 1.72; 95% CI, 1.09-2.73, P =.021). There was a no statistical difference in 1 year mortality after the diagnosis of S. aureus bacteremia between patients who received infectious diseases consultation and those who did not receive infectious diseases consultation (crude HR, 0.88; 95% CI, 0.56-1.38, P =.579).
Discussion
In this cohort of patients with S. aureus bacteremia, infectious diseases consultation was independently associated with a reduction in 28-day mortality, even after adjusting for preexisting co-morbidities and severity of disease. We determined 1-year mortality for the entire cohort using Social Security Death Index which has been validated for detecting deceased individuals including the date of death.18, 19 Although previous studies conducted in US hospitals have described the positive effect of infectious diseases consultation on adherence to evidence-based management of S. aureus bacteremia, these studies failed to demonstrate a survival benefit.1, 10 A recent retrospective study by Reig, et al. conducted in Germany noted a 40% reduction of in-hospital mortality of S.aureus bacteremia patients who received infectious diseases consultation. 20 Germany has a different healthcare delivery system compared to the United States, which may affect generalizability. Moreover, significant changes in practice might have occurred in their study period since the duration of study was longer (a 6-year period from 2002-2007). In contrast, our study demonstrated the benefit of infectious diseases consultation in mortality in a 2-year period. The German study only examined the difference in short-term mortality (i.e., in-hospital and at 90-day mortality) in combined population of retrospective and prospective subset. Our study investigated short-term and long-term mortality in an exclusively prospective cohort population.
In our study, infectious diseases consult was associated with 56% reduction in all-cause mortality among S. aureus bacteremic patients within 28 days of initial positive blood culture. This result may be due to improved evidence-based management after infectious diseases consultation. Patients in our cohort who had an infectious diseases consultation were significantly more likely to have received appropriate antimicrobial therapy, be evaluated by a trans-esophageal echocardiogram, and have an appropriate planned duration of antimicrobial therapy. We found patient-related factors including advanced age, cirrhosis, and ICU admission ≤ 48 hours after the first positive blood culture representing severity of illness were independently associated with 28-day mortality Our findings are consistent with previous studies. Elderly patients with S. aureus bacteremia are at higher risk of mortality21, 22 A previous study demonstrated that cirrhosis has been associated with approximately a 2-fold increased risk of death in patients with S. aureus bacteremia.23 Severity of illness, including septic shock, high Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and high Severity of Illness score are predictors of mortality of S. aureus bacteremia.2, 5, 24 Our study is consistent with these prior reports in that patients with ICU admission ≤ 48 hours of the first positive blood culture were 4.6 times more likely to die.
In this study, the 28-day mortality rate after the diagnosis of S. aureus bacteremia was 15.8%, (the 28-day mortality rate reached 17.3% if 6 patients who died within 2 days of a positive blood culture being obtained were included) similar to published mortality rates (8-23%) for patients with S. aureus bacteremia in US tertiary care hospitals.1, 2, 10 We found that 39 % of all deaths during the one year follow-up period occurred in the first 28 days after diagnosis of S. aureus bacteremia. This finding is consistent with previous studies that death after S. aureus bacteremia the most frequently occurred in the first month after the diagnosis.2, 25 We found a significant difference in survival in the first 28 days between patients who had infectious diseases consultation and those who did not have infectious diseases consultation. Infectious diseases consultation did not confer additional survival benefit after 28 days, and patient comorbidities had a greater impact on mortality after the first four weeks after infection onset.
Prolonged bacteremia, metastatic infection, and the presence of prosthetic material were more likely to be associated with infectious diseases consultation in our study, suggesting that more complicated bacteremia resulted in infectious diseases consultation in our study hospital. Non-Caucasian patients with S. aureus bacteremia were less likely to receive infectious diseases consultation in our cohort; however, this was not significant after stratifying by receipt of chronic hemodialysis (data not shown). A previous study showed a low rate of adherence to infectious disease consultant recommendations among hemodialysis-dependent patients with S. aureus bacteremia.1 Given the high frequency of central venous catheterization among cancer and hemodialysis patients, and the increased risk of complications 26, 27 due to S. aureus catheter-related bloodstream infections, our data suggests that theses two patients populations may benefit from infectious diseases consultation.
Our study had several limitations. Because our study was conducted at a single tertiary, academic medical center, our data may not be generalizable to other hospitals. Our result may be affected by referral bias since our institution is a tertiary academic medical center. Because our hospital serves an adult population, the survival benefit from infectious diseases consultation in this cohort may not apply to pediatric population with S. aureus bacteremia. Receipt of an infectious diseases consult was a non-random event among patients with S. aureus bacteremia in our study. Given limitation in clinical information at the time of diagnosis, we used ICU admission ≤ 48 hours at the time of diagnosis as a predictor of the severity of illness. Although this variable revealed highly correlated with mortality, more objective measures such as the APACHE II score and the Pitt bacteremia score may be more appropriate for assessing the severity of illness. 28 While we attempted to adjust for other risk factors for mortality in our study, we cannot exclude the possibility that unmeasured factors may have accounted for differences in mortality. Larger, multicenter studies in which methods such as propensity score modeling could be used to address these limitations.
Our study demonstrated a relationship between infectious diseases consultation and reduced short-term mortality. Patients who received an infectious diseases consultation in our study were more likely to receive evidence-based management. With rising prevalence of methicillin-resistant S. aureus and increasing use of medical devices, treatment of S. aureus bacteremia continues to be a challenge. Infectious diseases consultation may help guide comprehensive evaluation, adherence to evidence-based management, and improve clinical outcomes for these patients.
Acknowledgments
Financial support. Center for Disease Control and Prevention Epicenter (5U01CI000033302). M.J.K is funded by the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research (UL1 RR024992)
Funding source: Center for Disease Control and Prevention Epicenter (5U01CI000033302)
Footnotes
Conflict of interest: None for all authors
We verify that all authors had access to the data and a role in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fowler VG, Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998 Sep;27(3):478–86. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 2.Mylotte JM, Tayara A. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis. 2000 Nov;31(5):1170–4. doi: 10.1086/317421. [DOI] [PubMed] [Google Scholar]
- 3.Beeston CJ, Gupta R, Chadwick PR, Young RJ. Methicillin-resistant Staphylococcus aureus bacteraemia and mortality in a teaching hospital. Eur J Clin Microbiol Infect Dis. 2009 Jun;28(6):585–90. doi: 10.1007/s10096-008-0675-3. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000 Jul;118(1):146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 5.Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhoj P, Frimodt-Moller N. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med. 2002 Jan 14;162(1):25–32. doi: 10.1001/archinte.162.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med. 2002 Oct 28;162(19):2229–35. doi: 10.1001/archinte.162.19.2229. [DOI] [PubMed] [Google Scholar]
- 7.Chang FY, Peacock JE, Jr, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003 Sep;82(5):333–9. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 8.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009 Jul 1;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005 Jun 14;111(23):e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins TC, Price CS, Sabel AL, Mehler PS, Burman WJ. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis. 2008 Apr 1;46(7):1000–8. doi: 10.1086/529190. [DOI] [PubMed] [Google Scholar]
- 11.Roots Web. Social Security Death Index. [Last accessed 30 Aug 2009]; Available at: http://ssdi.genealogy.rootsweb.com/
- 12.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006 Aug 17;355(7):653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 13.Klevens RM, Morrison MA, Fridkin SK, et al. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg Infect Dis. 2006 Dec;12(12):1991–3. doi: 10.3201/eid1212.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen PK, Gill RD. Cox regression model for counting processes: A large sample study. Annals of Statistics. 1982;(10):1100–20. [Google Scholar]
- 15.Kleinbaum DG, Klein M. Survival Analysis: A self-learning text. Second. NY: Springer; 2005. [Google Scholar]
- 16.Schoenfeld D. Partial residuals for the proportional hazards model. Biometrika. 1982;69:51–55. [Google Scholar]
- 17.Snappin SM, Jiang Q, Iglewicz B. Illustrating the Impact of a Time-Varying Covariate With an Extended Kaplan-Meier Estimator. The American Statistician. 2005;59(4):301–307. [Google Scholar]
- 18.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004 Mar 5;2(1):2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn J, Kramer N, McDermott D. Validation of the Social Security Death Index (SSDI): An Important Readily-Available Outcomes Database for Researchers. West J Emerg Med. 2008 Jan;9(1):6–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Rieg S, Peyerl-Hoffmann G, de With K, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation - a study of 521 patients in Germany. J Infect. 2009 Aug 1; doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Malani PN, Rana MM, Banerjee M, Bradley SF. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J Am Geriatr Soc. 2008 Aug;56(8):1485–9. doi: 10.1111/j.1532-5415.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 22.McClelland RS, Fowler VG, Jr, Sanders LL, et al. Staphylococcus aureus bacteremia among elderly vs younger adult patients: comparison of clinical features and mortality. Arch Intern Med. 1999 Jun 14;159(11):1244–7. doi: 10.1001/archinte.159.11.1244. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Park WB, Lee KD, et al. Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci. Clin Infect Dis. 2003;37(6):794–9. doi: 10.1086/377540. [DOI] [PubMed] [Google Scholar]
- 24.Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J. 2001 Mar;31(2):97–103. [PubMed] [Google Scholar]
- 25.Harbarth S, Rutschmann O, Sudre P, Pittet D. Impact of methicillin resistance on the outcome of patients with bacteremia caused by Staphylococcus aureus. Arch Intern Med. 1998 Jan 26;158(2):182–9. doi: 10.1001/archinte.158.2.182. [DOI] [PubMed] [Google Scholar]
- 26.Crowley AL, Peterson GE, Benjamin DK, et al. Venous thrombosis in patients with short-and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit Care Med. 2008;36(2):385–90. doi: 10.1097/01.CCM.0B013E3181611F914. [DOI] [PubMed] [Google Scholar]
- 27.Fowler VG, Jr, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis. 2005;40:695–703. doi: 10.1086/427806. [DOI] [PubMed] [Google Scholar]
- 28.Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009 Feb;31(2):146–150. doi: 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]