Abstract
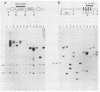
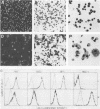
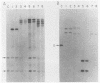
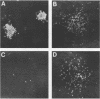
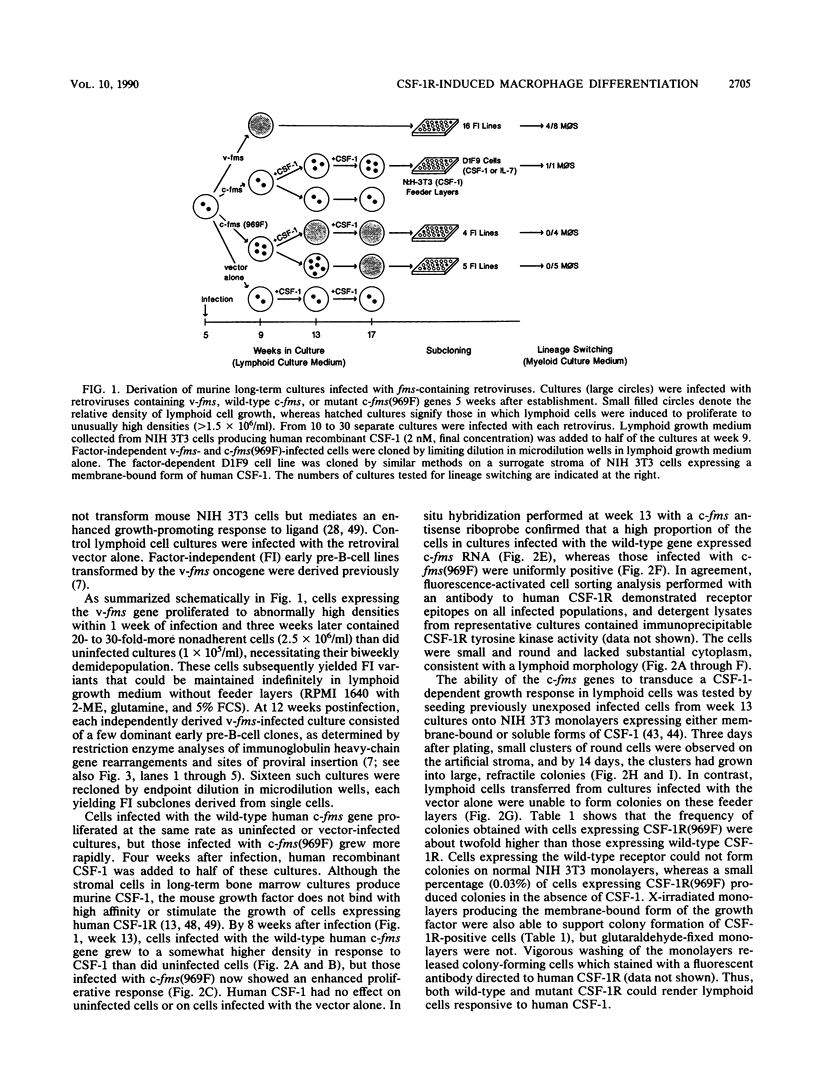
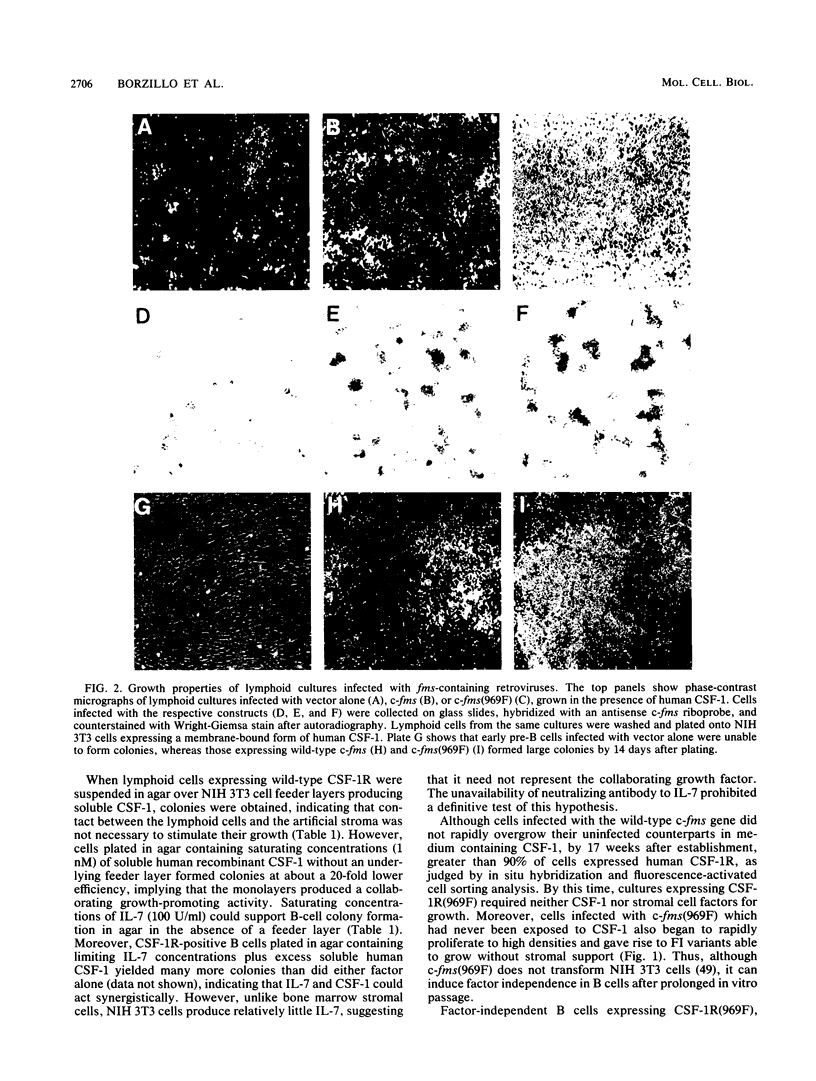
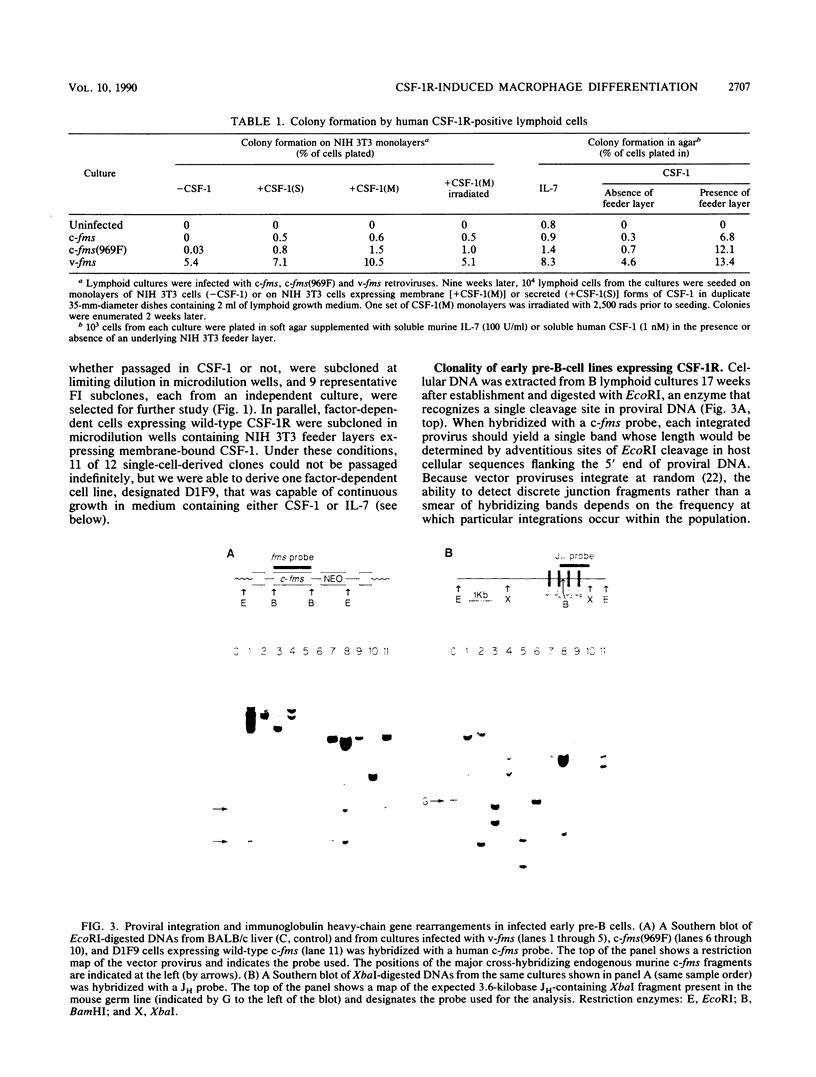
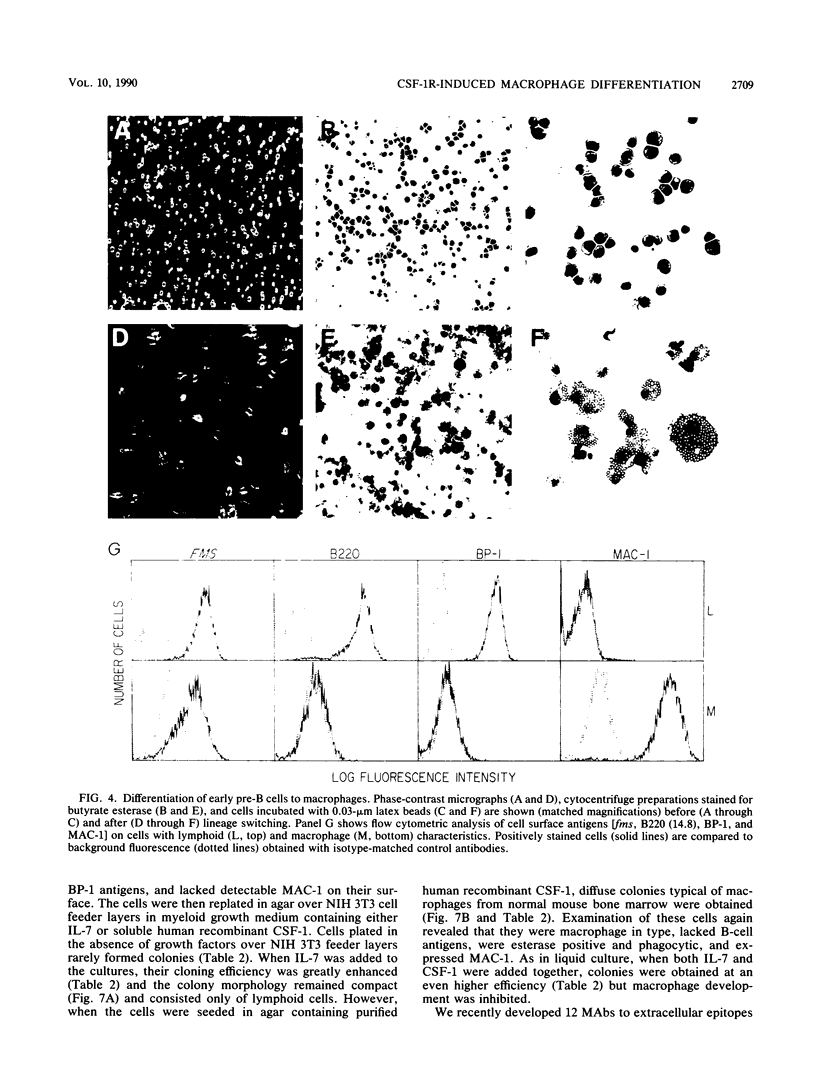
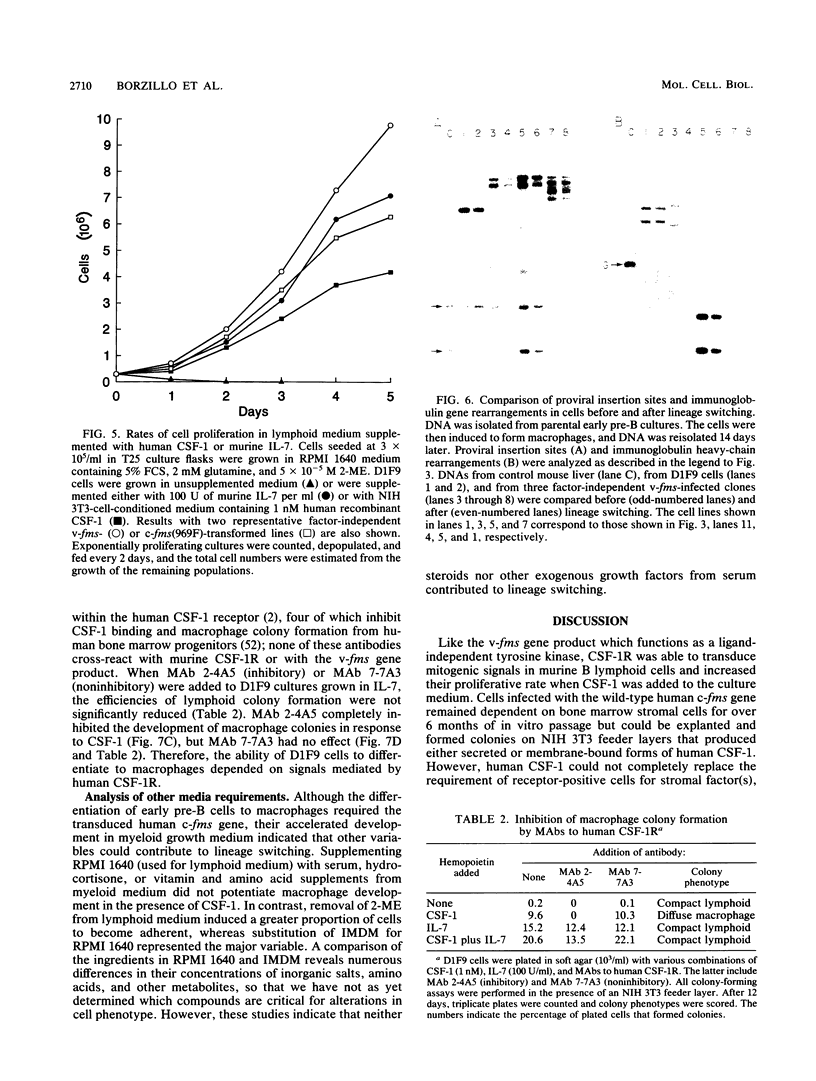
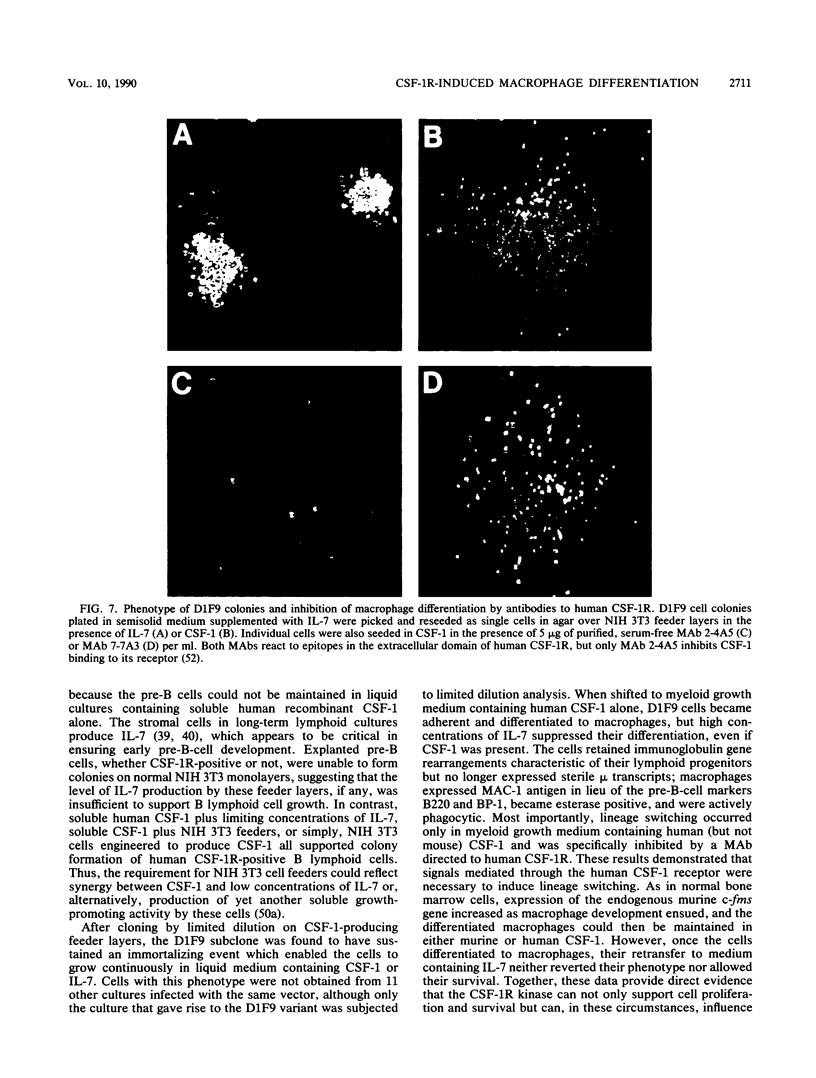
fms genes encoding either wild-type or constitutively activated colony-stimulating factor 1 receptors (CSF-1R) were introduced by retroviral infection into long-term mouse lymphoid cultures. Four early pre-B-cell lines transformed by the feline v-fms oncogene underwent spontaneous and irreversible differentiation to macrophages when transferred from RPMI 1640 to Iscove modified Dulbecco medium. Expression of wild-type human CSF-1R in early pre-B cells conferred no proliferative advantage unless human CSF-1 was added to the culture medium. A clonal, factor-dependent early pre-B-cell line (D1F9), selected for continuous growth on NIH 3T3 cell feeder layers producing human CSF-1, could be maintained in RPMI 1640 medium containing interleukin-7 (IL-7) but also differentiated to macrophages when grown in Iscove modified Dulbecco medium containing human CSF-1. The macrophages retained parental immunoglobulin gene rearrangements and proviral insertions, lost B-cell antigens, expressed butyrate esterase and MAC-1, were actively phagocytic, and no longer survived in IL-7. Unlike factor-independent v-fms transformants, the irreversible commitment of D1F9 cells to differentiate in the macrophage lineage could be suppressed by IL-7, depended on human (but not mouse) CSF-1, and was inhibited by an antibody to human CSF-1R. Signals mediated by transduced CSF-1R can therefore play a deterministic role in cell differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Gonda M. A., Rettenmier C. W., Sherr C. J. Subcellular localization of glycoproteins encoded by the viral oncogene v-fms. J Virol. 1984 Sep;51(3):730–741. doi: 10.1128/jvi.51.3.730-741.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmun R. A., Look A. T., Roberts W. M., Roussel M. F., Seremetis S., Ohtsuka M., Sherr C. J. Monoclonal antibodies to the human CSF-1 receptor (c-fms proto-oncogene product) detect epitopes on normal mononuclear phagocytes and on human myeloid leukemic blast cells. Blood. 1989 Feb 15;73(3):827–837. [PubMed] [Google Scholar]
- Bartelmez S. H., Stanley E. R. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: assay of hemopoietin-1. J Cell Physiol. 1985 Mar;122(3):370–378. doi: 10.1002/jcp.1041220306. [DOI] [PubMed] [Google Scholar]
- Baumbach W. R., Keath E. J., Cole M. D. A mouse c-myc retrovirus transforms established fibroblast lines in vitro and induces monocyte-macrophage tumors in vivo. J Virol. 1986 Aug;59(2):276–283. doi: 10.1128/jvi.59.2.276-283.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach W. R., Stanley E. R., Cole M. D. Induction of clonal monocyte-macrophage tumors in vivo by a mouse c-myc retrovirus: rearrangement of the CSF-1 gene as a secondary transforming event. Mol Cell Biol. 1987 Feb;7(2):664–671. doi: 10.1128/mcb.7.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzillo G. V., Cooper M. D., Kubagawa H., Landay A., Burrows P. D. Isotype switching in human B lymphocyte malignancies occurs by DNA deletion: evidence for nonspecific switch recombination. J Immunol. 1987 Aug 15;139(4):1326–1335. [PubMed] [Google Scholar]
- Borzillo G. V., Sherr C. J. Early pre-B-cell transformation induced by the v-fms oncogene in long-term mouse bone marrow cultures. Mol Cell Biol. 1989 Sep;9(9):3973–3981. doi: 10.1128/mcb.9.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A. W., Schrader J. W. Derivation of macrophage-like lines from the pre-B lymphoma ABLS 8.1 using 5-azacytidine. Nature. 1982 Jun 24;297(5868):691–693. doi: 10.1038/297691a0. [DOI] [PubMed] [Google Scholar]
- Broome J. D., Jeng M. W. Promotion of replication in lymphoid cells by specific thiols and disulfides in vitro. Effects on mouse lymphoma cells in comparison with splenic lymphocytes. J Exp Med. 1973 Sep 1;138(3):574–592. doi: 10.1084/jem.138.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P. V., Guilbert L. J., Stanley E. R. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Das S. K., Stanley E. R. Structure-function studies of a colony stimulating factor (CSF-1). J Biol Chem. 1982 Nov 25;257(22):13679–13684. [PubMed] [Google Scholar]
- Davidson W. F., Pierce J. H., Rudikoff S., Morse H. C., 3rd Relationships between B cell and myeloid differentiation. Studies with a B lymphocyte progenitor line, HAFTL-1. J Exp Med. 1988 Jul 1;168(1):389–407. doi: 10.1084/jem.168.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Lajtha L. G. Proliferation of haemopoietic stem cells in vitro. Br J Haematol. 1974 Dec;28(4):525–530. doi: 10.1111/j.1365-2141.1974.tb06671.x. [DOI] [PubMed] [Google Scholar]
- Furman W. L., Rettenmier C. W., Chen J. H., Roussel M. F., Quinn C. O., Sherr C. J. Antibodies to distal carboxyl terminal epitopes in the v-fms-coded glycoprotein do not cross-react with the c-fms gene product. Virology. 1986 Jul 30;152(2):432–445. doi: 10.1016/0042-6822(86)90145-5. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Chan L. C., Furley A. J., Watt S. M., Molgaard H. V. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986 Jan;67(1):1–11. [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980 Apr;85(1):153–159. doi: 10.1083/jcb.85.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Roussel M. F., Rettenmier C. W., Sherr C. J. Synthesis, post-translational processing, and autocrine transforming activity of a carboxylterminal truncated form of colony stimulating factor-1. Oncogene Res. 1987 Sep-Oct;1(4):423–440. [PubMed] [Google Scholar]
- Holmes K. L., Pierce J. H., Davidson W. F., Morse H. C., 3rd Murine hematopoietic cells with pre-B or pre-B/myeloid characteristics are generated by in vitro transformation with retroviruses containing fes, ras, abl, and src oncogenes. J Exp Med. 1986 Aug 1;164(2):443–457. doi: 10.1084/jem.164.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Humphries R. K., Eaves A. C., Eaves C. J. Self-renewal of hemopoietic stem cells during mixed colony formation in vitro. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3629–3633. doi: 10.1073/pnas.78.6.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Burgess A. W. Molecular and biological properties of a macrophage colony-stimulating factor from mouse yolk sacs. J Cell Biol. 1978 Apr;77(1):35–47. doi: 10.1083/jcb.77.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Roussel M. F., Ashmun R. A., Sherr C. J. Transduction of human colony-stimulating factor-1 (CSF-1) receptor into interleukin-3-dependent mouse myeloid cells induces both CSF-1-dependent and factor-independent growth. Mol Cell Biol. 1989 Sep;9(9):4069–4073. doi: 10.1128/mcb.9.9.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinken S. P., Alexander W. S., Adams J. M. Hemopoietic lineage switch: v-raf oncogene converts Emu-myc transgenic B cells into macrophages. Cell. 1988 Jun 17;53(6):857–867. doi: 10.1016/s0092-8674(88)90309-1. [DOI] [PubMed] [Google Scholar]
- Koike K., Stanley E. R., Ihle J. N., Ogawa M. Macrophage colony formation supported by purified CSF-1 and/or interleukin 3 in serum-free culture: evidence for hierarchical difference in macrophage colony-forming cells. Blood. 1986 Apr;67(4):859–864. [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Matsui T., Heidaran M., Miki T., Popescu N., La Rochelle W., Kraus M., Pierce J., Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989 Feb 10;243(4892):800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- McCulloch E. A. Stem cells in normal and leukemic hemopoiesis (Henry Stratton Lecture, 1982). Blood. 1983 Jul;62(1):1–13. [PubMed] [Google Scholar]
- Metcalf D., Burgess A. W. Clonal analysis of progenitor cell commitment of granulocyte or macrophage production. J Cell Physiol. 1982 Jun;111(3):275–283. doi: 10.1002/jcp.1041110308. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Clonal analysis of proliferation and differentiation of paired daughter cells: action of granulocyte-macrophage colony-stimulating factor on granulocyte-macrophage precursors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5327–5330. doi: 10.1073/pnas.77.9.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Schmierer A. E., March C. J., Overell R. W., Park L. S., Urdal D. L., Mochizuki D. Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F. H., Ray P., Brown K., Barker P. E., Jhanwar S., Ruddle F. H., Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988 Apr;7(4):1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Chen J. H., Roussel M. F., Sherr C. J. The product of the c-fms proto-oncogene: a glycoprotein with associated tyrosine kinase activity. Science. 1985 Apr 19;228(4697):320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Roussel M. F., Ashmun R. A., Ralph P., Price K., Sherr C. J. Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol Cell Biol. 1987 Jul;7(7):2378–2387. doi: 10.1128/mcb.7.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Roussel M. F. Differential processing of colony-stimulating factor 1 precursors encoded by two human cDNAs. Mol Cell Biol. 1988 Nov;8(11):5026–5034. doi: 10.1128/mcb.8.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Look A. T., Roussel M. F., Sherr C. J. Tandem linkage of human CSF-1 receptor (c-fms) and PDGF receptor genes. Cell. 1988 Nov 18;55(4):655–661. doi: 10.1016/0092-8674(88)90224-3. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R., Metcalf D. Induction of macrophage colony-stimulating factor-dependent growth and differentiation after introduction of the murine c-fms gene into FDC-P1 cells. Mol Cell Biol. 1989 Nov;9(11):5081–5092. doi: 10.1128/mcb.9.11.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell V. M., Rohrschneider L. R. Murine c-fms cDNA: cloning, sequence analysis and retroviral expression. Oncogene Res. 1987 Sep-Oct;1(4):311–324. [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Look A. T., Sherr C. J. Cell surface expression of v-fms-coded glycoproteins is required for transformation. Mol Cell Biol. 1984 Oct;4(10):1999–2009. doi: 10.1128/mcb.4.10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P. A., Dorshkind K., Witte O. N. Clonal lymphoid progenitor cell lines expressing the BCR/ABL oncogene retain full differentiative function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1908–1912. doi: 10.1073/pnas.87.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Ashmun R. A., Downing J. R., Ohtsuka M., Quan S. G., Golde D. W., Roussel M. F. Inhibition of colony-stimulating factor-1 activity by monoclonal antibodies to the human CSF-1 receptor. Blood. 1989 May 15;73(7):1786–1793. [PubMed] [Google Scholar]
- Sherr C. J. Colony-stimulating factor-1 receptor. Blood. 1990 Jan 1;75(1):1–12. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Bartocci A., Patinkin D., Rosendaal M., Bradley T. R. Regulation of very primitive, multipotent, hemopoietic cells by hemopoietin-1. Cell. 1986 Jun 6;45(5):667–674. doi: 10.1016/0092-8674(86)90781-6. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2520–2524. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of murine bone marrow precursors of B lymphocytes. Methods Enzymol. 1987;150:275–286. doi: 10.1016/0076-6879(87)50085-4. [DOI] [PubMed] [Google Scholar]
- Woolford J., McAuliffe A., Rohrschneider L. R. Activation of the feline c-fms proto-oncogene: multiple alterations are required to generate a fully transformed phenotype. Cell. 1988 Dec 23;55(6):965–977. doi: 10.1016/0092-8674(88)90242-5. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Y. G., Jubinsky P. T., Sengupta A., Yeung D. C., Stanley E. R. Purification of the colony-stimulating factor 1 receptor and demonstration of its tyrosine kinase activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1268–1271. doi: 10.1073/pnas.84.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]