Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive interstitial lung disease, wherein transforming growth factor β (TGF-β) and sphingosine-1-phosphate (S1P) contribute to the pathogenesis of fibrosis. However, the in vivo contribution of sphingosine kinase (SphK) in fibrotic processes has not been documented. Microarray analysis of blood mononuclear cells from patients with IPF and SphK1- or SphK2-knockdown mice and SphK inhibitor were used to assess the role of SphKs in fibrogenesis. The expression of SphK1/2 negatively correlated with lung function and survival in patients with IPF. Also, the expression of SphK1 was increased in lung tissues from patients with IPF and bleomycin-challenged mice. Knockdown of SphK1, but not SphK2, increased survival and resistance to pulmonary fibrosis in bleomycin-challenged mice. Administration of SphK inhibitor reduced bleomycin-induced mortality and pulmonary fibrosis in mice. Knockdown of SphK1 or treatment with SphK inhibitor attenuated S1P generation and TGF-β secretion in a bleomycin-induced lung fibrosis mouse model that was accompanied by reduced phosphorylation of Smad2 and MAPKs in lung tissue. In vitro, bleomycin-induced expression of SphK1 in lung fibroblast was found to be TGF-β dependent. Taken together, these data indicate that SphK1 plays a critical role in the pathology of lung fibrosis and is a novel therapeutic target.—Huang, L. S., Berdyshev, E., Mathew, B., Fu, P., Gorshkova, I. A., He, D., Ma, W., Noth, I., Ma, S.-F., Pendyala, S., Reddy, S. P., Zhou, T., Zhang, W., Garzon, S. A., Garcia, J. G. N., Natarajan, V. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis.
Keywords: TGF-β, S1P, S1P lyase
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive interstitial lung disease of unknown etiology with an average survival of 3–5 yr following diagnosis (1). The pathogenesis of IPF is not completely understood; however, IPF involves alveolar epithelial cell injury, areas of type II cell hyperplasia, accumulation of fibroblasts and myofibroblasts, and the deposition of extracellular matrix (ECM) proteins, such as fibronectin (FN) and collagen (2–4). Fibroblast accumulation gradually leads to an excessive scarring of the lung tissue and progressive and irreversible destruction of lung architecture, leading to loss of lung function, disruption of gas exchange, and respiratory failure (5). Therapies for IPF are mostly ineffective, and the only effective treatment available is lung transplantation.
Molecular and cellular mechanisms underlying the pathogenesis of IPF are incompletely defined; however, animal models have been developed that reveal some pathophysiology similar to that of human IPF. Among the various animal models of lung fibrosis, the bleomycin model in mice is the best characterized and the most widely used to investigate experimental pulmonary fibrosis (6). In bleomycin-induced pulmonary fibrosis, reactive oxygen species (ROS), pro- and anti-inflammatory cytokines, growth factors, noncollagenous ECM proteins, antifibrinolytic agents, lipid mediators, and G-protein-coupled receptors have been implicated that are also know to play a role in lung tissue remodeling and wound healing. The bioactive lipid, lysophosphatidic acid (LPA), and its G-protein-coupled receptor LPA1 were recently shown to promote the pathogenesis underlying IPF. Bronchoalveolar lavage (BAL) fluids from patients with IPF showed enhanced LPA levels compared to control subjects (7), and LPA1-deficient mice were protected against bleomycin-induced pulmonary fibrosis (8). The source of LPA and mechanisms of its generation in IPF are unclear. It can be generated from phosphatidic acid (PA) by the action of phospholipase A1or phospholipase A2 and/or from lysophosphatidylcholine by the activity of autotaxin (9). Mice with mutated cytosolic phospholipase A2 exhibited protection against bleomycin-induced pulmonary fibrosis, suggesting a pivotal role for phospholipase A2 in the fibrotic process (10).
In addition to LPA, another bioactive lipid, sphingosine-1-phosphate (S1P), may also play a role in pulmonary fibrosis. In mammalian cells, S1P is generated primarily by phosphorylation of sphingosine, catalyzed by two isoforms of sphingosine kinases (SphKs), SphK1, and SphK2 (11). The transforming growth factor β (TGF-β)-induced transdifferentiation of myoblasts to myofibroblasts was dependent on the SphK1/S1P3 axis (12), and prolonged exposure of mice to FTY720, a prodrug for S1P1agonist FTY720-phosphate, exacerbated bleomycin-induced vascular leak, lung injury, and fibrosis in mice (13). Our recent work using the mouse model of radiation-induced pulmonary fibrosis demonstrated the involvement of SphK1/S1P in radiation-induced pulmonary fibrogenesis (14). Although these studies suggest that S1P signaling via S1P receptors contribute to the development of bleomycin- and radiation-induced pulmonary fibrosis, the exact involvement of SphK1 and/or SphK2 and S1P balance in driving the fibrotic disease in vivo has not been investigated.
Our preliminary studies showed increased expression of SphK1, but not SphK2, in lung tissues from patients with IPF and bleomycin-treated animals. Further, microarray analysis of peripheral blood mononuclear cells (PBMCs) from patients with IPF showed a direct correlation between increased SphK1/2 expression and decreased diffusing capacity of the lung for carbon monoxide (DLCO) and survival time in patients with IPF. Similarly, lung tissues from bleomycin-treated mice exhibited increased S1P and dihydro-S1P (DHS1P) levels. Therefore, we hypothesized that increased S1P production by elevated SphK1 expression may play a role in lung fibrotic processes induced by bleomycin. Data reported here show for the first time in a preclinical animal model of IPF that knockdown of SphK1 or inhibition of SphK activity reduces intracellular S1P production and TGF-β secretion and attenuates bleomycin-induced lung fibrosis and mortality in mice. Together, these results represent the first direct experimental evidence that SphK1 is a novel prognostic and therapeutic target of pulmonary fibrosis, and targeting SphK1 to inhibit S1P generation may present a novel therapeutic approach to pulmonary fibrosis.
MATERIALS AND METHODS
Reagents
Bleomycin sulfate was from Hospira Inc. (Lake Forest, IL, USA), and the Sircol Collagen Assay Kit was from Accurate Chemical and Scientific Corp. (Westbury, NY, USA). The neutralizing chicken anti-TGF-β1 antibody and control chicken IgG, IL6, and TGF-β1 ELISA kits were obtained from R&D Systems (Minneapolis, MN, USA). S1P and a 17-carbon analog of S1P (C17-S1P) were obtained from Avanti Polar Lipids (Alabaster, AL, USA). Lysis buffer was purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Protease inhibitor cocktail tablets (EDTA-free Complete) and phosphatase inhibitor cocktail were from Roche Diagnostics (Indianapolis, IN, USA). Recombinant human TGF-β1 was purchased from Pepro Tech Inc. (Rocky Hill, NJ, USA). SphK inhibitor 2 (SKI-II; 4-[[4-(4-chlorophenyl)-2-thiazolyl]amino]-phenol), and polyclonal anti-SphK1 antibody (cat. no. 10006822) were procured from Cayman Chemical Co. (Ann Arbor, MI, USA). Rabbit polyclonal anti-SphK2 antibody (ab37978) was purchased from Abcam (Cambridge, MA, USA). Rabbit polyclonal anti-phospho-Smad2, anti-Smad2, anti-JNK, anti-phospho-JNK, anti-p38 MAPK, anti-phospho-p38MAPK and mouse anti-Akt, and anti-phospho-Akt antibodies were from Cell Signaling Technology. Horseradish peroxidase-linked anti-mouse IgG and anti-rabbit IgG antibodies were obtained from Bio-Rad Laboratories Inc. (Hercules, CA, USA). Rabbit anti-fibronectin and anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Mouse anti-α-smooth muscle actin (α-SMA) was from Sigma-Aldrich (St. Louis, MO, USA).
Human subjects
The study population, consisting of a cohort of 44 patients evaluated at the University of Chicago, was included for qRT-PCR analysis. The diagnosis of IPF was established based on American Thoracic Society/European Respiratory Society (ATS/ERS) criteria, which are consistent with recent guidelines. Subjects were followed by physicians according to institutional practices. Pulmonary function tests, chest CT, and lung biopsies were performed when clinically indicated. Patients had no evidence of autoimmune syndromes, malignancies, infections, or drug or occupational exposures associated with lung fibrosis. All patient information was maintained in a relational database. The study was approved by the institutional review board at each center, and informed consent was obtained from all the patients before blood draw. Demographic and clinical characteristics of the patients, including DLCO and forced vital capacity (FVC) are provided in Table 1. The ratio of FCV% predicted is determined as (FVC/predicted FVC) × 100, and the DLCO% predicted is determined as (DLCO/predicted DLCO) × 100.
Table 1.
Characteristics of the subjects with IPF
| Variable | Overall |
|---|---|
| Case subjects | 44 |
| Age (yr) | 68.7 ± 6.8 |
| Gender (male/female) | 39/5 |
| FVC (% predicted) | 62.1 ± 15.0 |
| DLCO (% predicted) | 43.2 ± 17.4 |
FVC, forced vital capacity; DLCO, carbon monoxide diffusing capacity.
Mice
SphK1−/− and SphK2−/− mice were provided by Dr. Richard Proia (U.S. National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA). C57BL/6J mice were from Jackson Laboratories (Bar Harbor, ME, USA). The null mice were back-crossed onto the B6 background for two generations (F2 hybrid). Male wild-type, SphK1−/− or SphK2−/− mice, 8–9 wk old, weighing ∼22 g were used in all the experiments. All animal protocols were approved by the University of Illinois–Chicago Institutional Animal Care and Use Committee (IACUC).
Bleomycin-induced experimental pulmonary fibrosis model
C57BL/6J wild-type mice were purchased from Charles River Laboratory (Wilmington, MA, USA). SphK1−/− mice in C57BL/6J background were housed at the University of Illinois, Chicago, animal facility. For bleomycin instillation, 8-wk-old male mice were anesthetized with 3–5 ml/kg of a mixture of 25 mg/kg of ketamine and 2.5 ml of xylazine. They were then treated with either saline or bleomycin sulfate (2 U/kg of body weight) in saline by intratracheal (i.t.) injection in a total volume of 50 μl. Animals were euthanized for analysis after 0, 3, 7, or 14 d. BAL fluid was collected by an i.t. injection of 0.5 ml of sterile PBS solution followed by gentle aspiration. The lavage was repeated twice with 0.5 ml of sterile PBS to recover a total volume of 0.7–0.8 ml. It was then centrifuged, and the supernatant was used for protein and cytokine measurements. Lungs were removed from mice, and lobes were sectioned, embedded in paraffin, and cut into 5-μm sections. Hematoxylin and eosin (H&E) and trichrome staining were performed by the Pathology Core Facility (University of Illinois–Chicago). The studies reported here conform to the principles outlined by the Animal Welfare Act and the National Institutes of Health guidelines for the care and use of animals in biomedical research. All animal protocols were approved by the IACUC of the University of Illinois–Chicago.
Administration of SphK inhibitor to mice
C57BL/6J mice (8 wk old male) were anesthetized with 3–5 ml/kg of a mixture of 25 mg/kg of ketamine and 2.5 ml of xylazine. The mice were then treated with either saline or bleomycin sulfate (1.5 U/kg of body weight) in saline by intratracheal (i.t.) injection in a total volume of 50 μl. At 7 d after bleomycin challenge, mice were injected intraperitoneally (i.p.) with sterile PBS (100 μl) containing DMSO (5 μl) or SKI-II (5 mg/kg body weight) dissolved in DMSO (20 mg/ml) and diluted in sterile PBS (95 μl PBS+5 μl DMSO containing SKI-II) once every 2 d. At 21 d after vehicle or bleomycin challenge, animals were euthanized, and plasma, BAL fluids, and lung tissues were collected and analyzed.
Histolopathological analysis for fibrosis
The fixed lungs were sectioned, embedded in paraffin, and stained with H&E for analysis of lung injury, and with Elastica-Masson trichrome stain to check for the collagen deposition, an index of lung fibrosis. For the analysis of the fibrotic changes in lung tissue, the quantitative fibrotic scale (Ashcroft scale) was calculated as described previously (15). Briefly, the severity of the fibrotic changes in each lung section was given a mean score from the observed microscopic fields. More than 20 fields within each lung section were observed at ×100, and each field was assessed individually for severity and allotted a score from 0 (normal) to 8 (total fibrosis) (16, 17). The severity was then averaged for each lung section. To avoid bias, all histological specimens were evaluated in a blinded fashion. Each specimen was scored independently by 2 individuals, including a histopathologist; finally, the mean of their individual scores was taken as the fibrotic score.
Lipid extraction and sample preparation for LC/MS/MS
Cellular lipids were extracted by a modified Bligh and Dyer procedure with the use of 0.1 N HCl for phase separation, as described previously (18). C17-S1P (40 pmol) was employed as internal standard, and was added during the initial step of lipid extraction. The extracted lipids were dissolved in methanol/chloroform (4:1, v/v), and aliquots were taken to determine the total phospholipid content as described previously (18). Samples were concentrated under a stream of nitrogen, redissolved in methanol, transferred to autosampler vials, and subjected to electrospray ionization tandem mass spectrometry (ESI-LC/MS/MS). The instrumentation employed was an AB Sciex 5500 QTRAP hybrid triple-quadrupole linear ion-trap mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with a turboionspray ionization source interfaced with an automated Agilent 1200 series liquid chromatograph and autosampler (Agilent Technologies, Wilmington, DE, USA). S1P and DHS1P were analyzed as bis-acetylated derivatives employing reverse-phase HPLC separation, negative-ion ESI, and MRM analysis (18, 19).
Microarray profiling and analysis
The Affymetrix Human Exon 1.0 ST array (exon array; Affymetrix, Inc., Santa Clara, CA, USA) was used to profile whole-genome expression in a cohort of 44 patients with IPF. Briefly, the sample preparation and RNA isolation were based on standard molecular biology protocols. The labeling and microarray hybridization were performed at the University of Chicago Genomics Core Facility according to the manufacturer's instruction. The exon array data were then normalized and summarized using the Affymetrix Power Tools. Before summarizing gene-level expression data, probes containing known polymorphisms (based on dbSNP v131) were removed as described earlier (20). The log2-transformed gene-level (i.e., transcript cluster) expression data were then evaluated for differential expression using significance analysis of microarray (SAM; ref. 21). Gene annotations were obtained from the Affymetrix NetAffy Analysis Center (http://www.affymetrix.com/). The microarray data have been deposited in the U.S. National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (accession no. GSE38958).
RNA isolation and real-time RT-PCR
Total RNA was isolated from mouse lung tissue or from cells using TRIzol reagent (Life Technology, Rockville, MD, USA) according to the manufacturer's instructions. RNA was quantified spectrophotometrically, and 1 μg of RNA was reverse transcripted using the cDNA synthesis kit (Bio-Rad laboratories Inc., Hercules, CA, USA). For mouse genes, the primers were as follows. FN primers: forward, TCTGGGAAATGGAAAAGGGGAATGG, and reverse, CACTGAAGCAGGTTTCCTCGGTTGT; α-SMA primers: forward, GACGCTGAAGTATCCGATAGAACACG, and reverse, CACCATCTCCAGAGTCCAGCACAAT; TGF-β1 primers: forward, AGCGGACTACTATGCTAAAGAGGTCACCC, and reverse, CCAAGGTAACGCCAGGAATTGTTGCTATA; Col1A1 primers: forward, GGAGGGCGAGTGCTGTGCTTT, and reverse, GGGACCAGGAGGACCAGGAAGT; Col1A2 primers: forward, TGGTCTTACTGGGAACTTTGCTGC, and reverse, ACCCTGTGGTCCAACGACTCCTCTC; Col1A1 primers: forward, GGAGGGCGAGTGCTGTGCTTT, and reverse, GGGACCAGGAGGACCAGGAAGT; GAPDH primers: forward, CGACTTCAACAGCAACTCCCACTCTTCC, and reverse, TGGGTGGTCCAGGGTTTCTTACTCCTT; SphK1 primers: forward, TGTGAACCACTATGCTGGGTA, and reverse, CAGCCCAGAAGCAGTGTG; SphK2 primers: forward, AGACGGGCTGCTTTACGAG, and reverse, CAGGGGAGGACACCAATG. For human genes, the primers were as follows. GAPDH primers: forward, GCTGGCGCTGAGTACGTCGTGGAGT, and reverse, CACAGTCTTCTGGGTGGCAGTGATGG; SphK1 primers: forward, TCCTTCACGCTGATGCTCACTG, and reverse, CAGACGCCGATACTTCTCACTCTC; TGF-β1 primers: forward, TTCCCTCGAGGCCCTCCTA, and reverse, GCCGCAGCTTGGACAGGATC. GAPDH was used as an internal control. Amplification reactions were performed in triplicate with SYBR Premix Ex Taq (Takara, Otsu, Japan), and the thermal cycling conditions were as follows: 10 s at 95°C, 40 cycles of 5 s at 95°C, and 30 s at 60°C.
Collagen determination
The right lungs from mice were incised and homogenized in 5 ml 0.5 M acetic acid in PBS containing 0.6% pepsin. The extracts were rotated at 4°C overnight and cleared by centrifugation at 10,000 g for 15 min. Collagen content was measured using the Sircol Collagen Assay kit according to the manufacturer's instructions. Collagen content is presented as micrograms of acid-soluble collagen per right lung.
SDS-PAGE and Western blotting
SDS-PAGE and Western blotting were performed as described previously (14, 19). Briefly, lung tissue homogenates and human lung fibroblast lysates were prepared in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and EDTA-free complete protease inhibitor tablet. Cell lysates were cleared by centrifugation at 10,000 g for 10 min, and boiled with the Laemmli sample buffer for 5 min. Cell lysates (20–30 μg protein) were separated on 10% or 4–20% SDS-PAGE, transferred to PVDF membranes, and blocked with TBST containing 5% BSA prior to incubation with primary antibodies (1:1000 dilution, except for SphK1 and SphK2 antibodies: 1:2000 dilution) overnight. Next, PVDF membranes were washed and incubated with secondary antibodies (1:2000 dilutions, except for SphK1 and SphK2: 1:3000 dilutions). Blots were developed using an ECL chemiluminescence kit. Western blots were scanned by densitometry, and integrated density of pixels was quantified using Image Quant 5.2 software (Molecular Dynamics, Sunnyvale, CA, USA).
Isolation of primary fibroblasts from murine lungs
Mouse lung fibroblasts were isolated essentially as described previously (22). Briefly, mouse lungs from 6- to 8-wk-old mice were cut into small pieces, minced, and subjected to collagenase type III and DNase I digestion (Worthington Biochemical, Lakewood, NJ, USA) in Dulbecco's modified Eagle's medium (DMEM) with 5% FCS for 90 min. After filtration, cells were centrifuged, washed, and cultured in T-25 flasks in DMEM containing 10% fetal bovine serum (FBS) for 14 d. Fibroblasts were characterized by determining the expression of thymocyte differentiation antigen 1 (Thy-1).
Cell culture
Primary human lung fibroblasts were obtained from Lonza (Walkersville, MD, USA). Cells were grown and maintained in 6-well dishes with fibroblast growth medium (Lonza, Walkersville, MD, USA) containing 2% FBS. Murine primary lung fibroblasts were cultured in DMEM containing 10% FBS. Cells (∼80% confluent) were serum starved for 24 h prior to stimulation with S1P, bleomycin, or TGF-β1 for the indicated time. In some experiments, TGF-β neutralizing antibody was added to cover the cells 1 h before stimulation with bleomycin or TGF-β.
Statistical analysis
Data are expressed as means ± sem. All results were subjected to statistical analysis using 1-way ANOVA or 2-tailed Student's t test or Spearman's correlation test. Values of P < 0.05 were considered significant. Values are from 3 to 6 independent experiments. Survival analysis was performed using the Cox proportional hazards model, implemented in the survival library in the R Statistical Package. A log-rank test value of P < 0.05 was deemed significant.
RESULTS
Expression of SPHK1/2 correlates with disease severity and survival of patients with IPF
Microarray analysis of PBMCs of patients with IPF (n=44) for mRNA expression of S1P synthesizing enzymes (SphK1/2) correlated to the DLCO. An inverse correlation exists between DLCO and IPF disease severity (23). The expression levels of SPHK1 and SPHK2 were negatively correlated with DLCO (Fig. 1A). Kaplan-Meier survival analysis comparing patient groups demonstrated significantly reduced survival for the patients with high expression of SPHK1 or SPHK2 (Fig. 1B).
Figure 1.

Expression of SPHK1/2 correlates with the severity of disease and survival of patients with IPF. A) Expression level as a function of DLCO for SphK1 and SphK2 in IPF. Y axis denotes the relative mRNA expression level. Each point represents the value from one patient with IPF. Value of P for each gene was measured by Spearman's ank correlation test. B) Kaplan-Meier curves for patients with IPF with high and low gene expression levels in SphK1 and SphK2. Patients were classified as having high-level or low-level mRNA expression, with the median of the expression across all the patients as the threshold value. Expression level was measured by the Affymetrix Human Exon 1.0 ST Array. Values of P were measured by log-rank test.
Up-regulation of SphK1 in lung tissues from human patients with IPF and bleomycin-challenged mice
Next, the expression of SphKs from normal and IPF lungs as well as bleomycin-challenged mice was investigated. Expression of SphK1, but not SphK2, was significantly elevated in lung tissues of patients with IPF as compared to control subjects (Fig. 2A). In a murine model of pulmonary fibrosis, bleomycin challenge induced acute lung injury and fibrosis in mice (data not shown), and increased mRNA and protein expression of SphK1 but not SphK2 in lung tissues (Fig. 2B, C). The commercial SphK1 and SphK2 antibodies were found to be specific and yielded reliable results when used at dilutions of 1:2000 for the primary and 1:3000 for the secondary antibody. In addition, S1P and DHS1P levels were significantly elevated in lung tissue from bleomycin-challenged mice (Fig. 2D). These results suggest that SphK1 and S1P signaling axis may play an important role in the development of lung fibrosis in vivo.
Figure 2.
SphK1, but not SphK2, is up-regulated in lung tissues obtained from patients with IPF and bleomycin-challenged mice. A) Protein level of SphK1 and SphK2 in human lung tissue from patients with IPF and control subjects. Intensity of each band after immunostaining with anti-SphK1 or anti-SphK2 antibody was quantified and normalized with GAPDH. Data are expressed as means ± sem. **P < 0.01 vs. control, n = 8/group. B–D) mRNA level of TGF-β1, α-SMA, Col1A1, Col1A2, FN, SphK1, and SphK2 (B); protein expression of SphK1 and SphK2 (C); and C18-S1P or C18-DHS1P level (D) in mouse lungs from C57BL/6J mice after bleomycin (2 U/kg, i.t.) treatment (0, 3, 7, 14 d). Intensity of each band was quantified using anti-SphK1 or anti-SphK2 antibody and normalized to GAPDH. *P < 0.05, **P < 0.001 vs. d 0, n = 4/group.
SphK1 but not SphK2 deficiency attenuates bleomycin-induced mortality and pulmonary fibrosis in mice
To investigate the effect of SphK1 on bleomycin-induced mortality and pulmonary fibrosis, SphK1−/− and wild-type mice were challenged with bleomycin (2.0 U/kg) or sterile physiological saline and followed for 14 d. Compared to wild-type mice, SphK1−/− mice receiving same dose of bleomycin exhibited significantly lower lethality rates (Fig. 3A). Analysis of bioactive lipids showed elevated levels of S1P and DHS1P at d 14 after bleomycin challenge in wild-type mice but not in SphK1−/− mice (Fig. 3B), and the levels of total ceramides and sphingosine were not altered by bleomycin challenge in either wild-type or SphK1−/− mice (data not shown). Further, bleomycin challenge significantly increased injury and collagen deposition in the lung from wild-type mice, which was reduced in bleomycin-treated SphK1−/− mice (Fig. 3C, D). Histopathological quantification for pulmonary fibrosis (Ashcroft score) showed a significant protective effect of SphK1 deficiency in fibrogenesis (Fig. 3E). Further, bleomycin-induced increase of lung collagen content and TGF-β1 level in BAL fluid were markedly reduced in SphK1−/− mice compared to wild-type mice (Fig. 3F, G). Also, bleomycin challenge in wild-type mice increased mRNA levels of several markers of pulmonary fibrosis, including TGF-β, FN, collagen 1A1, and α-SMA, while in SphK1−/− mice, bleomycin-induced up-regulation of mRNA levels of these markers was less pronounced (Fig. 3H). At the protein level, SphK1 deficiency significantly blunted bleomycin-induced expression of FN and α-SMA (Fig. 3I). In contrast to SphK1 deficiency, SphK2 knockdown (SphK2−/− mice) had no significant effect on bleomycin-induced mortality, lung injury, and fibrosis (Supplemental Fig. S1).
Figure 3.
SphK1 deficiency attenuates bleomycin-induced mortality and pulmonary fibrosis in mouse. SphK1−/− or wild-type (WT, C57BL/6J) mice (male, 8 wk) receiving bleomycin (2 U/kg in 50 μl PBS), or PBS intratracheally were euthanized at d 14 postchallenge. Lungs were removed, embedded in paraffin, and cut into 5-μm sections for staining. A) Survival of SphK1−/− and WT mice challenged with bleomycin. B) C18-S1P and C18-DHS1P level in lung tissue from SphK1−/− and WT mice with or without bleomycin challenge. C) Representative H&E photomicrographs of lung sections obtained from SphK1−/− and WT mice with/without bleomycin challenge (black arrows indicate injury area). Original view, ×10; scale bars = 200 μm. D) Representative images of Trichrome staining of lung sections obtained from SphK1−/− and WT mice with or without bleomycin challenge (blue arrows indicate blue of collagen deposition area). Top panel, original view, ×4; scale bars = 1 mm. Bottom panel, amplification from box in the upper panel, original view, ×10; scale bars = 200 μm. E) Ashcroft score of the lung sections. F) Acid soluble collagen in lung tissue. G) TGF-β1 level in BAL fluids. H) mRNA level of TGF-β1, α-SMA, Col1A1, and FN. I) Protein levels of α-SMA and FN in lung tissue from SphK1−/− and WT mice with or without bleomycin challenge. Data are expressed as means ± sem. *P < 0.05; **P < 0.01 vs. same genotype without BLM treatment; #P < 0.05 vs. WT mice with BLM treatment, n = 4–6/group.
Next, the effect of SphK1 deficiency on bleomycin-induced lung inflammation was assessed. Bleomycin challenge of wild-type mice significantly increased lung damage and pulmonary leak. However, the increase in protein and IL-6 concentration, as well as influx of cells, including neutrophils and macrophages, in BAL fluids was similar in wild-type and SphK1−/− mice (Supplemental Fig. S2). These results suggested that the deletion of SphK1 ameliorates pulmonary fibrosis, but not inflammation on d 14 after bleomycin challenge.
Administration of SphK inhibitor reduces bleomycin-induced lung injury and fibrosis
To further investigate the potential role of SphKs in bleomycin-induced pulmonary fibrosis, we evaluated the effect of SKI-II, an inhibitor that blocks both SphK1 and SphK2 (24, 25). Initial studies showed that intraperitoneal (i.p) administration of SKI-II at 5 mg/kg body weight provided the following S1P profile in plasma [S1P (pmol/ml): 0 h, 1446±132; 1 h, 1096±84; 3 h, 1243±101; 6 h, 1048±74; 24 h, 901±62; and 48 h, 1164±69] and lung tissue [S1P (fmol/nmol lipid P): 0 h, 176±35; 1 h, 138±36; 3 h, 142±30; 6 h, 115±21; 24 h, 113±8; 48 h, 138±12]. Based on these results, wild-type mice treated with SKI-II (5 mg/kg, i.p) on d 8 after bleomycin challenge (injected once every 2 d) were dramatically protected against bleomycin-induced mortality evaluated up to 21 d postchallenge (Fig. 4A). Further, SKI-II treatment decreased S1P and DHS1P levels in lung tissue on d 21 after bleomycin challenge (Fig. 4B), but did not affect tissue levels of sphingoid bases and ceramides (data not shown). Bleomycin-induced injury, fibrosis, and collagen deposition in lungs were dramatically reduced by SKI-II (Fig. 4C–E). Further, bleomycin-induced lung collagen content and TGF-β1 level in BAL fluids were markedly reduced in SKI-II-treated mice compared to vehicle treatment (Fig. 4F, G). In addition, SKI-II treatment significantly inhibited bleomycin-induced expression of FN and α-SMA in lung tissue (Fig. 4H), protein, and IL-6 levels in BAL fluids (Fig. 5A, F), and blocked infiltration of macrophages and lymphocytes, but not neutrophils (Fig. 5B–E). Taken together, these results show that inhibition of SphK activity in vivo protected mice against bleomycin-induced lung injury and fibrosis.
Figure 4.
Administration of SphK1 inhibitor (SKI-II) attenuates bleomycin-induced lung injury and fibrosis. WT (C57BL/6J) mice (male, 8 wk) received bleomycin (1.5 U/kg in 50 μl PBS) or PBS intratracheally. On d 8, the mice were injected with SKI-II (i.p., 5 mg/kg, 2.5 μl DMSO plus 47.5 μl PBS) or vehicle (2.5 μl DMSO plus 47.5 μl PBS), and repeated every 2 d. Mice were euthanized on d 21 after bleomycin challenge. Lungs were excised, embedded, and cut into 5-μm sections and stained. A) Survival of bleomycin-challenged mice with or without SKI-II treatment. B) Effect of SKI-II on C18-S1P or C18-DHS1P level in mouse lung tissue. C) Representative H&E photomicrographs of lung sections obtained from PBS- or bleomycin (BLM)-challenged mice with or without SKI-II treatment (black arrows, injury area). Original view, ×10; scale bars = 200 μM. D) Representative images of Trichrome staining of lung sections obtained from PBS- or bleomycin-challenged mice with or without SKI-II treatment (blue arrows, collagen deposition area). Top panel, original view, ×4; scale bars = 1 mm. Bottom panel, amplification from box in top panel, original view, ×10; scale bars = 200 μM. E) Ashcroft score of the lung sections. F) Acid soluble collagen in lung tissue. G) TGF-β1 level in BAL fluids. H) Protein of α-SMA and FN from PBS- or bleomycin-challenged mice with or without SKI-II treatment. Data are expressed as means ± sem. *P < 0.05 vs. mice injected with PBS solution; #P < 0.05 vs. bleomycin-challenged mice without SKI-II treatment, n = 5–8/group.
Figure 5.

Effects of SKI-II on bleomycin-mediated inflammatory changes in mouse lung. WT (C57BL/6J) mice (male, 8 wk) received bleomycin (BLM; 1.5 U/kg in 50 μl PBS) or PBS intratracheally and were fed for 7 d, followed by injection of SKI-II (5 mg/kg, 2.5 μl DMSO plus 47.5 μl PBS, i.p.) or vehicle solution at d 8 after bleomycin challenge every 2 d. The mice were euthanized at d 21 after bleomycin challenge. Lungs were lavaged by PBS solutions, and BAL fluids were analyzed as described in Materials and Methods. A) Total protein levels. B) Total cell number. C) Neutrophils. D) Macrophages. E) Lymphocytes. F) IL-6. levels in BAL fluids. Data are expressed as means ± sem. *P < 0.05 vs. PBS-challenged mice with injection of vehicle solution; #P < 0.05 vs. BLM-challenged mice without SKI-II treatment, n = 5–8/group.
SphK1 deficiency attenuates bleomycin-induced phosphorylation of Smad, Akt, and MAPKs
Next, we evaluated the effect of SphK1 deficiency on signaling pathways linked to fibrosis. The increased phosphorylation of Smad, Akt, JNK, and p38 MAPK in lung tissue of the wild-type mice at 14 d after bleomycin challenge was significantly reduced in SphK1−/− mice (Fig. 6) and SKI-II treated mice (data not shown). Interestingly, SphK1 deficiency in mouse lung fibroblasts attenuated TGF-β-induced expression of FN, α-SMA, and acid-soluble collagen (Supplemental Fig. S3A–D), and phosphorylation of Smad, Akt, JNK, and p38 MAPK (Supplemental Fig. S3E–I). S1P also increased the expression of FN and α-SMA in mouse lung fibroblasts (Fig. 7A), and S1P-induced phosphorylation of Smad, JNK, and p38 MAPK in mouse lung fibroblast was also blocked by SphK1 knockdown (Fig. 7B–E). These results demonstrate that knockdown or inhibition of SphK activity attenuated the phosphorylation of Smad, Akt, JNK, and p38 MAPK in vivo and in vitro.
Figure 6.

SphK1 deficiency attenuates bleomycin-induced phosphorylation of Smad, Akt, and MAPKs in mouse lung. SphK1−/− or WT (C57BL/6J) mice (male, 8 wk) receiving bleomycin (BLM; 2 U/kg in 50 μl PBS) or PBS i.t. were euthanized on d 14 after bleomycin administration. A) Whole-lung homogenates were subjected to SDS-PAGE and Western blotting. B–E) Intensity of bands probed with anti-P-Smad2 (B), anti-P-JNK1 (C), anti-P-Akt (D), or anti-P-p38 (E) antibodies, quantified by densitometry and normalized to the corresponding total protein. Data are expressed as means ± sem. *P < 0.05, **P < 0.01 vs. same genotype without BLM treatment; #P < 0.05 vs. WT mice with BLM treatment, n = 4–6/group.
Figure 7.

SphK1 deficiency attenuates S1P-induced activation of Smad2 and JNK/p38 in mouse lung fibroblast. A) S1P and TGF-β1 induce FN and α-SMA expression in mouse lung fibroblast. Mouse lung fibroblast cells (∼90% confluence) were treated with S1P (0, 0.1, or 1 μM) or TGF-β1 (0, 1, or 5 ng/ml) for 24h, and cell lysates (20 μg protein) were subjected to SDS-PAGE of Western blotting. Intensity of the bands with anti-FN or anti-α-SMA antibody was quantified and normalized to GAPDH. Data are expressed as means ± sem of 3 independent experiments. *P < 0.05 vs. cells without treatment. B–E) SphK1 deficiency attenuates S1P-induced activation of Smad2 and JNK/p38 in mouse lung fibroblasts. After serum starvation, primary mouse lung fibroblast cells were stimulated by S1P (1 μM) for 1h. B) Cell lysates were subjected to SDS-PAGE and Western blotting. C–E) Intensity of bands observed with anti-P-Smad2 (C), anti-P-JNK1 (D), or anti-P-p38 (E) antibodies was quantified and normalized to corresponding total protein. Data are expressed as means ± sem of 3 independent experiments. *P < 0.05 vs. same cells without S1P treatment; #P < 0.05 vs. WT cells after S1P treatment.
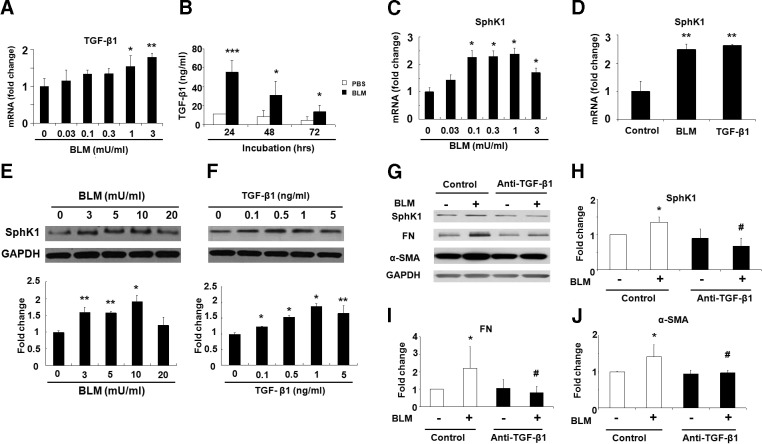
Bleomycin-induced expression of SphK1 is TGF-β dependent
Having demonstrated a role for SphK1 in TGF-β- and S1P-mediated expression of fibrogenic proteins in lung fibroblasts, we next investigated whether bleomycin-induced SphK1 expression is TGF-β dependent. As shown in Fig. 8A, B, bleomycin dose-dependently elevated TGF-β release into the culture medium in human lung fibroblasts, with maximum secretion at 24 h postchallenge. Interestingly, both bleomycin and TGF-β stimulated SphK1 mRNA and protein expression in human lung fibroblasts (Fig. 8C–F). Furthermore, preincubation with anti-TGF-β antibody attenuated bleomycin-mediated increase of SphK1, FN, and α-SMA expression in human lung fibroblasts (Fig. 8G–J). These data suggest that bleomycin mediated up-regulation of SphK1 is TGF-β dependent in human lung fibroblasts.
Figure 8.
Bleomycin-induced SphK1 expression via TGF-β1 secretion in human lung fibroblasts. A–C) mRNA level of TGF-β1 (24 h; A), secreted TGF-β1 level (24–72 h; B), and mRNA level of SphK1 (24 h; C) in human lung fibroblasts after bleomycin (BLM; 0–3 mU/ml) treatment. *P < 0.05, **P < 0.01 vs. cells without BLM treatment. D) mRNA levels of SphK1 in human lung fibroblasts after BLM (3 mU/ml) or TGF-β1 (5 ng/ml) challenge for 24 h. **P < 0.01 vs. cells without treatment. E) Protein levels of SphK1 in human lung fibroblasts after BLM (0–20 mU/ml) challenge for 48 h. *P < 0.05, **P < 0.01 vs. cells without BLM treatment. F) Protein level of SphK1 in human lung fibroblasts in response to TGF-β1 (0–5 ng/ml) challenge for 48 h. *P < 0.05, **P < 0.01 vs. cells without TGF-β1 treatment. G–J) Inhibition of BLM-induced expression of SphK1, FN, and α-SMA. Human lung fibroblast cells (∼90% confluence) were starved with serum-free medium followed by pretreatment with neutralizing anti-TGF-β1 or control IgG antibody (5 μg/ml) for 1 h, and cells were challenged with BLM (3 mU/ml, 48 h). G) Cell lysates (20 μg protein) were subjected to SDS-PAGE and Western blotting. H–J) Intensity of bands with anti-SphK1 (H), anti-FN (I), or anti-α-SMA (J) antibody was quantified and normalized to GAPDH. Data are means ± sem of 3 independent experiments. *P < 0.05 vs. same antibody treatment without BLM challenge; #P < 0.05 vs. control antibody plus bleomycin challenge.
DISCUSSION
IPF is a fatal lung disease for which no effective pharmacological treatment is available. Therefore, identification of new therapeutic targets for drug development to ameliorate or improve prognosis in patients with IPF is critical. The major findings of our study are the following: SphK1 correlated with the pathogenesis of IPF and survival of patients; bleomycin challenge increased expression of SphK1, but not SphK2, and S1P levels in mouse lung tissues; bleomycin-challenged SphK1−/− mice had greater survival, less fibrosis, decreased phosphorylation of Smad2, Akt, JNK1, and p38 MAPK, TGF-β secretion, and accumulation of S1P in lung tissue; administration of SphK1 inhibitor attenuated bleomycin-induced pulmonary fibrosis and mortality. In vitro, TGF-β and S1P induced expression of FN, α-SMA, and collagen and enhanced phosphorylation of Smad2, JNK1, and p38 MAPK in lung fibroblasts, which was attenuated by SphK1 knockdown. These results concur with our recent findings regarding the potential link between radiation-induced pulmonary fibrosis, up-regulation of SphK1 expression and activity, and S1P levels (14). The present study provides evidence for the first time of a potential link between bleomycin-induced S1P production and increased profibrogenic responses in vivo, and demonstrates that SphK1 is a novel prognostic and therapeutic target of pulmonary fibrosis.
Molecular mechanisms regulating dysregulated wound repair in pulmonary fibrosis are incompletely understood. Bleomycin increased the production of proinflammatory and fibrogenic cytokines that stimulate excessive secretion of ECM proteins in the lung parenchyma. TGF-β, a key cytokine linking inflammation and fibrogenesis, is up-regulated in IPF lungs (26–28) and in animal models of lung fibrosis (29, 30). In addition, we currently observed that bleomycin induced secretion of TGF-β was attenuated by blocking S1P generation via knockdown of SphK1 expression or inhibiting SphK activity in vivo. Further, we also proved that bleomycin- induced SphK1 expression is TGF-β dependent, and TGF-β regulated S1P level in lung fibroblast by inducing the expression of SphK1 (22). Together, these data indicate a link between bleomycin-mediated TGF-β expression and intracellular S1P level in pulmonary fibrosis.
An important and novel finding of the present study is that SphK1 is a novel biomarker for IPF diagnosis and prognosis, since transcript levels of SphK1 in PBMCs from patients with IPF showed very strong correlation to the progress and survival, and the expression of SphK1 was dramatically increased in lung tissues from patients with IPF and bleomycin-challenged mice. Further, bleomycin-induced lung fibrosis, secretion of TGF-β1, accumulation of S1P, and expression of ECM proteins were attenuated in SphK1−/− mice. S1P generation from sphingosine in mammalian cells and tissues is a balance between its synthesis catalyzed by either SphK1 and/or SphK2 and degradation mediated by S1P phosphatases and S1P lyase (S1PL) (31). S1P levels are elevated in BAL fluids from patients with IPF and mRNA expression of SphK1 in alveolar macrophages and SphK1 protein expression in lung tissue from patients with IPF (32). Down-regulation of SphK1, S1PR2, and S1PR3, but not S1PR1, with siRNA decreased TGF-β mediated up-regulation of α-SMA and FN in human lung fibroblasts, suggesting regulation of TGF-β-induced differentiation of fibroblasts to myofibroblasts via S1P signaling cascade in vitro (22). Interestingly, SphK1-transgenic mice with overexpressed SphK1 in the heart developed cardiac fibrosis through reactive oxygen species mediated S1P/S1PR3/SphK1 signaling axis (33). In addition, SphK1/S1P signaling is involved in TGF-β-induced epithelial mesenchymal transition (EMT) in alveolar type II cells by the activation of p-Smad3, Rho-GTP, and oxidative stress (32). Our results also show that SphK2 deficiency has no effect on bleomycin-induced pulmonary fibrosis and injury (Supplemental Fig. S1) and suggest that SphK1, but not SphK2, plays major role in the development of fibrogenesis in the bleomycin murine model of pulmonary fibrosis.
The role of intracellular S1P, regulated by SphKs, in pulmonary diseases is complex. As evident from the bleomycin-induced pulmonary fibrosis increased S1P levels in lung tissue seems to play an important role in driving fibrogenesis/lung injury. However, in LPS-induced murine model of acute lung injury, SphK1 and SphK2 deficiency potentiated the lung injury (34, 35), while S1PL suppression ameliorated inflammatory responses and barrier disruption by LPS in vivo and in vitro. The present study with SphK1−/− mice shows a profibrotic role for S1P in bleomycin-induced pulmonary fibrosis. Bleomycin challenge up-regulated acid sphingomyelinase and acid ceramidases activities in fibrotic lungs of mice, and knockdown of acid sphingomyelinase attenuated bleomycin-induced lung inflammation and fibrosis (36). Further, bleomycin-induced acid sphingomyelinase and acid ceramidase activation increased S1P levels in NIH 3T3 cells due to enhanced production of ceramide and sphingosine and phosphorylation of sphingosine to S1P by SphKs (36). These findings are supported by our results, demonstrating that increased SphK1 expression in bleomycin-challenged mice and increased S1P levels in lung tissues are critical for fibrogenesis.
In addition to the SphK1/S1P/S1PR signaling axis (32), changes in intracellular S1P also regulated the expression of TGF-β. In vivo, we found that suppressed S1P accumulation in lung tissue via knockdown of SphK1 expression or inhibition of SphK activity attenuated the expression and secretion of TGF-β after bleomycin challenge. Administration of SphK1 inhibitor, SKI-II, blocked bleomycin-induced mortality and fibrosis in mice, as well as activation of TGF-β signaling in lung tissue. SKI-II inhibits both SphK1 and SphK2 in mammalian cells (24, 25), and in vivo SKI-II has been reported to exhibit a Ki value between 8 and 35 μM (37, 38). SKI-II is also an orally active SphK inhibitor, and at a dose of 100 mg/kg, a concentration of 0.8 μM of the drug in blood was achieved at 1 h (39). In the present study, we used SKI-II at a dose of 5 mg/kg body weight (i.p.) in mice that is 10 times lower compared to previous reports (24, 39). Even at this relatively low dose, SKI-II was effective in lowering S1P levels in plasma and lung tissue by ∼20–40% over a period of 48 h after injection of the drug, which is comparable to S1P levels in plasma of SphK1-null mice (∼50% less compared to wild type; ref. 40). In the present study, we did not achieve the desired inhibition of ∼50% of S1P levels in plasma or lung tissue; however, the SKI-II inhibitor even at 5 mg/kg body weight offered protection against bleomycin-induced pulmonary fibrosis in mice. Although, it is well known that S1P is a potent endothelial barrier-enhancing agent and prevents vascular leak by tightening the vascular barrier (41–44), it is still unclear how S1P regulates TGF-β expression during fibrogenesis.
Mass spectrometry quantification of S1P and DHS1P requires use of internal standards, such as C17-S1P or deuterated C17- or C18-S1P. A recent report showed recovery of the C17-S1P internal standard was >100% in whole blood from SphK2−/− mice, and this was attributed to the presence of endogenous C17-S1P in the samples (45). The present study used C17-S1P as an internal standard for quantification of endogenous S1P in biological samples, and is therefore of some concern. However, analysis of lung tissues without the addition of exogenous C17-S1P revealed no signal for the presence of C17-S1P in the anylates from wild-type, SphK1−/− and SphK2−/− mice. Similarly, plasma anylates did not show significant levels of endogenous C17-S1P in our analysis; however, the presence of C17-S1P cannot be ruled out in the plasma. Additional studies in human, and mouse tissues as well as cell culture systems are warranted for quantification and verification of C17-S1P in plasma using deuterated S1P as an internal standard.
In summary, we have identified S1P metabolizing enzymes as novel biomarkers of IPF, and SphK1 as an essential mediator of pulmonary injury and fibrogenesis by regulating S1P accumulation in lung tissue. Increased expression of SphK1 in lung tissues was observed in samples obtained from patients with IPF and bleomycin-challenged mice, and a profibrogenic role for SphK1 in pulmonary fibrosis was demonstrated. In addition, targeting SphK1 by administration of SphK1 inhibitor dramatically blocked bleomycin-induced mortality, lung injury, and fibrosis in mice. Together, our current study indicates that SphK1 is a novel prognostic and therapeutic target of pulmonary fibrosis, and targeting SphK1 with small molecule inhibitors may facilitate development of therapies for pulmonary fibrosis.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grants HL P01 98050 and P01 58064 to V.N. and J.G.N.G, and HL66109 and ES11863 to S.P.R.
The authors thank Dr. Prasad Kanteti for helpful discussion and manuscript preparation. The authors thank Dr. Richard L Proia (NIH/National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA) for providing breeding pairs of the SphK1−/− and SphK2−/− mice. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- α-SMA
- α-smooth muscle actin
- BAL
- bronchoalveolar lavage
- DHS1P
- dihydro-sphingosine-1-phosphate
- DLCO
- diffusing capacity of the lung for carbon monoxide
- ECM
- extracellular matrix
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- FN
- fibronectin
- FVC
- forced vital capacity
- H&E
- hematoxylin and eosin
- IPF
- idiopathic pulmonary fibrosis
- LPA
- lysophosphatidic acid
- PA
- phosphatidic acid
- PBMC
- peripheral blood mononuclear cell
- ROS
- reactive oxygen species
- S1P
- sphingosine-1-phosphate
- S1PL
- S1P lyase
- SKI-II
- sphingosine kinase inhibitor 2
- SphK
- sphingosine kinase
- TGF-β
- transforming growth factor β
REFERENCES
- 1. King T. E., Jr. (1998) Update in pulmonary medicine. Ann. Intern. Med. 129, 806–812 [DOI] [PubMed] [Google Scholar]
- 2. Sappino S.P., Schurch W., Gabbiani G. (1990) Differentiation repertoire of fibroblastic cells: expression of cytoskeletal protein as marker of phenotypic modulations. Lab. Invest. 63, 144–161 [PubMed] [Google Scholar]
- 3. Phan S. H. (2002) The myofibroblast in pulmonary fibrosis. Chest 122, 286S–289S [DOI] [PubMed] [Google Scholar]
- 4. Cucoranu I., Clempus R., Dikalova A., Phelan P. J., Ariyan S., Dikalov S., Sorescu D. (2005) NAD(P)H oxidase 4 mediates transforming growth factor b1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 97, 900–907 [DOI] [PubMed] [Google Scholar]
- 5. Selman M., King T. E., Pardo A. (2001) Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 134, 136–151 [DOI] [PubMed] [Google Scholar]
- 6. Mouratis M. A., Aidinis V. (2011) Modeling pulmonary fibrosis with bleomycin. Curr. Opin. Pulm. Med. 17, 355–361 [DOI] [PubMed] [Google Scholar]
- 7. Funke M., Zhao Z., Xu Y., Chun J., Tager A. M. (2012) The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am. J. Respir. Cell Mol. Biol. 46, 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tager A. M., LaCamera P., Shea B. S., Campanella G. S., Selman M., Zhao Z., Polosukhin V., Wain J., Karimi-Shah B. A., Kim N. D., Hart W. K., Pardo A., Blackwell T. S., Xu Y., Chun J., Luster A. D. (2008) The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 14, 45–54 [DOI] [PubMed] [Google Scholar]
- 9. Aoki J., Inoue A., Okudaira S. (2008) Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta 1781, 513–518 [DOI] [PubMed] [Google Scholar]
- 10. Ghosh M., Stewart A., Tucker D. E., Bonventre J. V., Murphy R. C., Leslie C. C. (2004) Role of cytosolic phospholipase A(2) in prostaglandin E(2) production by lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 30, 91–100 [DOI] [PubMed] [Google Scholar]
- 11. Hla T. (2004) Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 15, 513–520 [DOI] [PubMed] [Google Scholar]
- 12. Cencetti F., Bernacchioni C., Nincheri P., Donati C., Bruni P. (2010) Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol. Biol. Cell 21, 1111–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shea B. S., Brooks S. F., Fontaine B. A., Chun J., Luster A. D., Tager A. M. (2010) Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am. J. Respir. Cell Mol. Biol. 43, 662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorshkova I., Zhou T., Mathew B., Jacobson J. R., Takekoshi D., Bhattacharya P., Smith B., Aydogan B., Weichselbaum R. R., Natarajan V., Garcia J. G., Berdyshev E. V. (2012) Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J. Lipid Res. 53, 1553–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inage M., Nakamura H., Saito H., Abe S., Hino T., Takabatake N., Terashita K., Ogura M., Kato S., Hosokawa T., Sata M., Tomoike H. (2009) Vesnarinone represses the fibrotic changes in murine lung injury induced by bleomycin. Int. J. Biol. Sci. 5, 304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ashcroft T., Simpson J. M., Timbrell V. (1988) Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 41, 467–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hübner R. H., Gitter W., El Mokhtari N. E., Mathiak M., Both M., Bolte H., Freitag-Wolf S., Bewig B. (2008) Standardized quantification of pulmonary fibrosis in histological samples. BioTechniques 44, 507–511 [DOI] [PubMed] [Google Scholar]
- 18. Vaskovsky V. E., Kostetsky E. Y., Vasendin I. M. (1975) A universal reagent for phospholipid analysis. J. Chromatogr. 114, 129–141 [DOI] [PubMed] [Google Scholar]
- 19. Berdyshev E. V., Gorshkova I., Usatyuk P., Kalari S., Zhao Y., Pyne N. J., Pyne S., Sabbadini R. A., Garcia J. G., Natarajan V. (2011) Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS One 6, e16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duan S., Zhang W., Bleibel W. K., Cox N. J., Dolan M. E. (2008) SNPinProbe_1.0: a database for filtering out probes in the Affymetrix GeneChip human exon 1.0 ST array potentially affected by SNPs. Bioinformation 2, 469–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tusher V. G., Tibshirani R., Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 98, 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kono Y., Nishiuma T., Nishimura Y., Kotani Y., Okada T., Nakamura S., Yokoyama M. (2007) Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am. J. Respir. Cell Mol. Biol. 37, 395–404 [DOI] [PubMed] [Google Scholar]
- 23. Hamada K., Nagai S., Tanaka S., Handa T., Shigematsu M., Nagao T., Mishima M., Kitaichi M., Izumi T. (2007) Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 131, 650–656 [DOI] [PubMed] [Google Scholar]
- 24. Chiba Y., Takeuchi H., Sakai H., Misawa M. (2010) SKI-II, an inhibitor of sphingosine kinase, ameliorates antigen-induced bronchial smooth muscle hyperresponsiveness, but not airway inflammation, in mice. J. Pharmacol. Sci. 114, 304–310 [DOI] [PubMed] [Google Scholar]
- 25. French K. J., Schrecengost R. S., Lee B. D., Zhuang Y., Smith S. N., Eberly J. L., Yun J. K., Smith C.D. (2003) Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 63, 5962–5969 [PubMed] [Google Scholar]
- 26. Khalil N., O'Connor R. N., Unruh H. W., Warren P. W., Flanders K. C., Kemp A., Bereznay O. H., Greenberg A. H. (1991) Increased production and immunohistochemical localization of transforming growth factor-β in idiopathic lung fibrosis. Am. J. Respir. Cell Mol. Biol. 5, 155–162 [DOI] [PubMed] [Google Scholar]
- 27. Limper A. H., Broekelmann T. J., Colby T. V., Malizia G., McDonald J. A. (1991) Analysis of local mRNA expression for extracellular matrix proteins and growth factors using in situ hybridization in fibroproliferative lung disorders. Chest 99, 55S–56S [DOI] [PubMed] [Google Scholar]
- 28. Bartram U., Speer C. P. (2004) The role of transforming growth factor beta in lung development and disease. Chest 125, 754–765 [DOI] [PubMed] [Google Scholar]
- 29. Liu L., Carron B., Yee H. T., Yie T. A., Hajjou M., Rom W. (2009) Wnt pathway in pulmonary fibrosis in the bleomycin mouse model. J. Environ. Pathol. Toxicol. Oncol. 28, 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu G., Friggeri A., Yang Y., Milosevic J., Ding Q., Thannickal V. J., Kaminski N., Abraham E. (2010) miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207, 1589–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oskouian B., Saba J. (2007) Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle 6, 522–527 [DOI] [PubMed] [Google Scholar]
- 32. Milara J., Navarro R., Juan G., Peiró T, Serrano A., Ramón M., Morcillo E., Cortijo J. (2012) Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax 67, 147–156 [DOI] [PubMed] [Google Scholar]
- 33. Takuwa N., Ohkura S-I., Takashima S-I., Ohtani K., Okamoto Y., Tanaka T., Hirano K., Usui S., Wang F., Du W., Yoshioka K., Banno Y., Sasaki M., Ichi I., Okamura M., Sugimoto N., Mizugishi K., Nakanuma Y., Ishii I., Takamura M., Kaneko S., Kojo S., satouchi K., Mitumori K., Chun J., Takuwa Y. (2010) S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovac. Res. 85, 484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di A., Kawamura T., Gao X. P., Tang H., Berdyshev E., Vogel S. M., Zhao Y. Y., Sharma T., Bachmaier K., Xu J., Malik A. B. (2010) A novel function of sphingosine kinase 1 suppression of JNK activity in preventing inflammation and injury. J. Biol. Chem. 285, 15848–14857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zemann B., Urtz N., Reuschel R., Mechtcheriakova D., Bornancin F., Badegruber R., Baumruker T., Billich A. (2007) Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol. Lett. 109, 56–63 [DOI] [PubMed] [Google Scholar]
- 36. Dhami R., He X., Schuchman E. H. (2010) Acid sphingomyelinase deficiency attenuates bleomycin-induced lung inflammation and fibrosis in mice. Cell. Physiol. Biochem. 26, 749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kharel Y., Mathews T. P., Kennedy A. J., Houck J. D., Macdonald T. L., Lynch K. R. (2011) A rapid assay for assessment of sphingosine kinase inhibitors and substrates. Anal. Biochem. 411, 230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao P., Peterson Y. K., Smith R. A., Smith C. D. (2012) Characterization of isoenzymes-selective inhibitors of human sphingosine kinases. PLoS ONE 7, e44543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. French K. J., Upson J. J., Keller S. N., Zhuang Y., Yun J. K., Smith C. D. (2006) Antitumor activity of sphingosine kinase inhibitors. J. Pharmacol. Exp. Ther. 318, 596–603 [DOI] [PubMed] [Google Scholar]
- 40. Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 [DOI] [PubMed] [Google Scholar]
- 41. Dudek S. M., Jacobson J. R., Chiang E. T., Birukov K. G., Wang P., Zhan X., Garcia J. G. (2004) Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J. Biol. Chem. 279, 24692–24700 [DOI] [PubMed] [Google Scholar]
- 42. Garcia J. G., Liu F., Verin A. D., Birukova A., Dechert M. A., Gerthoffer W. T., Bamberg J. R., English D. (2001) Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McVerry B. J., Peng X., Hassoun P. M., Sammani S., Simon B. A., Garcia J. G. (2004) Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am. J. Respir. Crit. Care Med. 170, 987–993 [DOI] [PubMed] [Google Scholar]
- 44. Peng X., Hassoun P. M., Sammani S., McVerry B. J., Burne M. J., Rabb H., Pearse D., Tuder R. M., Garcia J. G. (2004) Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 169, 1245–1251 [DOI] [PubMed] [Google Scholar]
- 45. Kharel Y., Raje M., Gao M., Gellett A. M., Tomsig J. L., Lynch K. R., Santos W. L. (2012) Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem. J. 447, 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]