Abstract
Lumbar spine osteoarthritis (OA) is very common, with estimates of prevalence ranging from 40–85 %. The process of degeneration of the spine has commonly been classified as OA (disc space narrowing together with vertebral osteophyte formation); however, anatomically, the facet joint is the only synovial joint in the spine that has a similar pathological degenerative process to appendicular joints. Low back pain (LBP) is also a common condition, with nearly 80 % of Americans experiencing at least one episode of LBP in their lifetime. The complex relationship between spine radiographs and LBP has many clinical and research challenges. Specific conservative treatments for spine degeneration have not been established; there has, however, been recent interest in use of exercise therapy, because of some moderate benefits in treating chronic LBP. An understanding of the relationship between spine degeneration and LBP may be improved with further population-based research in the areas of genetics, biomarkers, and pain pathways.
Keywords: Low back pain, Osteoarthritis, Spine osteoarthritis, Disc space narrowing, Intervertebral disc degeneration, Vertebral osteophytes, Facet joint osteoarthritis, Epidemiology, Treatment, Risk factors, Prevalence, Gender, Race
Introduction
Osteoarthritis (OA) is the most common form of arthritis, affecting an estimated 27 million adults in the US [1]. The prevalence of OA has increased over the past two decades, and increases in life expectancy and obesity, both risk factors for OA, have led to concerns over the public health consequences of OA [1]. Arthritis in general is also a leading cause of disability and a significant cause of reduced quality of life [2, 3]. Commonly thought of as a disease of the peripheral joints (i.e., hips, knees, and hands), spine OA is often ignored in discussions of the prevalence and effect of OA on disability and function [4]. However, estimates of lumbar spine OA are high, ranging from 40–85 %, the large range being primarily because of study differences in definitions, distribution of age and other demographic factors, and recruitment of subjects [5].
Approximately 80 % of Americans experience at least one episode of LBP during their lifetime, making frequency of LBP second only to the common cold [6, 7]. Utilization of health care services resulting from LBP is high, and total social cost is estimated to be greater than 100 billion dollars per year in the United States [8]. Aside from prevalence and cost, the effect of LBP on the workplace is substantial. Approximately 149 million workdays per year are lost as a result of LBP [9], making it the most common reason for time off from work [10,11]. There is no doubt that LBP has a substantial effect on the US health care and workforce system, affecting more than 30 % of community-dwelling adults in a given year [12] and remaining one of the most common reasons for physician visits [13]. Because of its high prevalence and effect on health services, the spinal anatomical origins of LBP are of great interest to researchers and clinicians. To address prevention and treatment, understanding the etiology of LBP is important, because it may differ within the large majority of patients in whom we describe the etiology as mechanical or non-specific origin.
Although many imaging techniques can be used to diagnose and quantify OA, plain film radiographs are a commonly utilized technique because they are relatively inexpensive and easily administered [14]. Specific clinical reasons for use of plain film radiographs for individuals seeking care for LBP include addressing danger signals, for example malignancy, fracture, or infection, improving patient satisfaction, and improving understanding of the origin of LBP. In the absence of radicular symptoms, current guidelines suggest plain film radiography is a reasonable initial imaging option for LBP [15]. A steady rise in the use of plain film radiographs in spine care has also occurred [16]; this may be because of the increasing prevalence of LBP [17*]. Use of plain films of the spine is common, irrespective of the stage or chronicity of LBP. Carey and colleagues[18], in a stratified probability sample of North Carolina residents, found that 45.8 % reported having spinal radiographs in the previous year, despite a mean duration of 9.8 years with chronic impairing LBP. The clinical decisions correlating radiographic findings and LBP are complex and poorly understood.
The association between radiographic spine degeneration and LBP has been debated since the 1970s [19]. Interest in spine OA and its effect on pain, function, and disability are increasing, and new research improving our understanding of spine OA’s prevalence, risk factors, and associations with LBP has been conducted in recent years. The purpose of this review is to summarize this current evidence while describing the challenges of inconsistent spine OA definitions and to report recent conservative treatment approaches for LBP. This review concludes by highlighting recommendations for future spine OA and LBP research to address these two commonly related conditions with substantial public health importance.
Pathophysiology and/or Anatomy
Individual radiographic features of the spine, commonly studied and referred to as the “three-joint complex”, are the structures of vertebral osteophytes (OST), facet joint OA (FOA), and disc space narrowing (DSN) from intervertebral disc degeneration [20]. All of these spinal structures have adequate nerve supply capable of generating LBP.
The vertebral facet joints (zygapophyseal joints) are synovial joints with the typical features of hyaline cartilage over subchondral bone, a synovial membrane, and a joint capsule [21]. Facet joint OA is a multifactorial process, and it has been thought that the presence of intervertebral disc degeneration leads to a greater load and motion at the facet joint, resulting in degenerative changes similar to those seen in other synovial joints [20]. However, the presence of facet joint OA has been found to be present even in the absence of intervertebral disc degeneration [22].
Situated between two vertebral bodies, the intervertebral disc is made up of two main regions: the soft inner nucleus pulposus and the firm outer collagenous annulus fibrosis [23]. The collagen content of the intervertebral disc consists of both type I and II collagen, with the nucleus containing only type II whereas the annulus contains both types I and II [24]. Changes to the disc collagen content can occur naturally with aging, a process commonly referred to as intervertebral disc degeneration. These aging-related changes include a decrease in aggrecan, water, and collagen content [25], resulting in DSN on plain film radiographs.
A vertebral osteophyte is a bony outgrowth that arises from the periosteum at the junction of bone and cartilage [26]. Osteophyte formation in the vertebral column has been shown to be a general indicator of age [27]. Osteophytes, however, may form without overt cartilage damage, implying they may form in an otherwise healthy joint [28]. However, the presence of disc space narrowing is highly associated with osteophyte formation [29].
The process of degeneration in the spine is thought to be initiated by disc degeneration; this disc degeneration is hypothesized to result in segmental instability that increases the load on the facet joints and leads to cartilage alterations [21]. This process has been clinically debated with little research support. Recently, Suri and colleagues [30*] reported results from an ancillary project using 435 participants in the Framingham Heart Study. Using computed tomography (CT), they report that, for most individuals, there is ordered progression of spine degeneration beginning with the intervertebral disc. They also remark that increasing age, body mass index (BMI), and female sex may be related to isolated facet joint degeneration in some individuals [30].
Spine Osteoarthritis or Degeneration?
Osteoarthritis is the clinical outcome of a disease process that results in structural and functional failure of synovial joints. This process has been characterized by damage to articular cartilage, subchondral bone alteration, a synovial inflammatory response, and an overgrowth of bone and cartilage [31]. In the spine, both the presence of intervertebral disc degeneration and osteophyte formation at the same vertebral level has been used to define lumbar spine OA, otherwise known as spondylosis [32]. Intervertebral disc degeneration and osteophyte formation may not share the same pathophysiological process of degeneration or have the anatomical synovial structures necessary to collectively meet the definition of OA. This has led to challenges and discussion of spine degeneration research because there is no consensus on whether this combination of features (DSN and OST) in the spine constitutes OA or a separate phenomenon [33]. As such, a clinically relevant definition that combines spine features to accurately represent spine OA is still unknown [34] and may not be necessary. The facet joint, however, is a synovial joint with a process of degeneration that may be associated with OA in appendicular joints [6]. Our recent work, using data from the Johnston County OA Project, found a statistically significant association between radiographic knee OA and hand OA and facet joint OA, but no significant association between DSN and hip, knee, or hand OA, or between vertebral OST and hip and hand OA [35]. Findings such as these suggest the pathophysiological course of degeneration in the spine differs, depending on the radiographic feature, from that of hip, knee, and hand OA.
Prevalence: Gender and Race Differences
The presence of these radiographic spine features is quite common, as evidenced by community-based prevalence of lumbar spine DSN between 50 and 64 % and vertebral OST prevalence as high as 75–94 % [34–37]. Our recent work is the only study to quantify the prevalence of radiographic FOA in a community-based population, revealing the prevalence affecting at least one lumbar level to be 57.9 % [35**].
The prevalence of these radiographic features has been found to differ by gender and race. Most recent studies agree that OST is more prevalent [34, 35, 37] and severe [35, 37] in men whereas prevalence of DSN is greater in women [34, 35, 37]. Our recent work is the first to describe these features across a sample of African Americans (AA) and Caucasians [35]. We found the prevalence of all these radiographic features was significantly lower for AA than for Caucasians, most notably in the odds of FOA (OR = 0.45; 95 % CI 0.32, 0.62). The reason for this may be related to racial differences in occupational exposure, BMI, physical activity demands, or anatomical differences. As such, there is still much to be learned about the reasons for these race and gender differences and radiographic changes in the lumbar spine.
Associations With Low Back Pain
By far, most epidemiological studies addressing the association between LBP and spine degeneration have been cross-sectional. Most of these cross-sectional studies have been practice-based, using select or small samples that have limited generalizability. Recently, a series of population-based cross-sectional studies have specifically set out to determine the association between lumbar spine radiographic features and LBP (Table 1). Cross-sectional designs with the outcome of LBP have been criticized for their lack of uniformity in LBP questions [38]. Despite the varying definitions of LBP, and different grading and coding schemes and settings across recent studies, a modest association between DSN and LBP has been consistently found. Conversely, the associations between OST and LBP have been weaker, and most are not significant. The association between radiographic FOA and LBP has only been reported in one study without a significant association with LBP, similar to a recent finding by Kalichman and colleagues [39] that indicates an insignificant association between FOA confirmed by CT scan and LBP.
Table 1.
Associations between low back pain and spine radiographic features
| Study (year) Ref. | Setting | Sample size (n) | DSN OR (95 % CI) | OST OR (95 % CI) | FOA OR (95 % CI) |
|---|---|---|---|---|---|
| Pye et al. (2004) [37] | Aberdeen, UK | 585 | 1.7 (1.1, 2.4) | 0.7 (0.4, 1.4) | N/A |
| Muraki et al. (2009) [36] | Japan | 2,288 |
a Men = 1.44 (0.89, 2.38) Women = 1.80 (1.38, 2.37) |
b Men = 1.15 (0.70, 1.92) Women = 0.99 (0.69, 1.42) |
N/A |
| de Schepper et al. (2010) [34] | Rotterdam, Netherlands | 2,809 | 1.4 (1.1, 1.7) | 1.2 (1.0, 1.5) | N/A |
| Goode et al. (2012) [35] | North Carolina, US | 840 | 1.37 (1.04, 1.80) | 1.11 (0.84, 1.48) | 0.84 (0.61, 1.15) |
OR, odds ratio; CI, confidence interval; N/A, not applicable; DSN, disc space narrowing; OST, osteophytes; FOA, facet joint osteoarthritis
Used Kellgren-Lawrence grading atlas:
K-L ≥ 2 and
KL ≥ 3
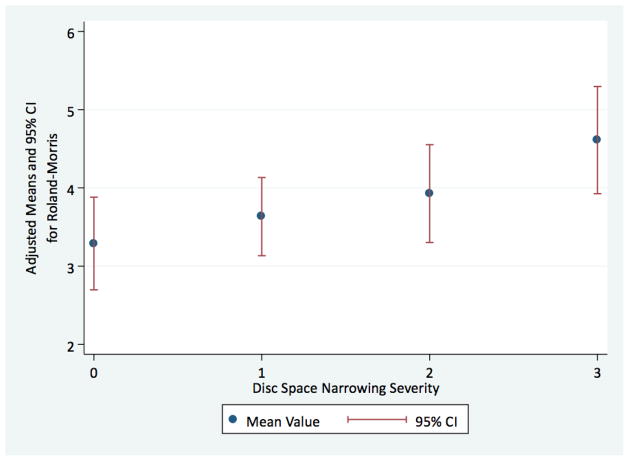
An topic that is particularly understudied is the association between spine radiographic features and physical function. de Schepper et al. [34**] found, in some instances, stronger associations between reduced physical function and disability and DSN (OR = 1.9; 95 % CI 1.4, 2.6) as measured with the Health Assessment Questionnaire. In this same cohort, Scheele et al. [40**] recently found a strong association among those with both morning stiffness and LBP with DSN (OR = 2.5; 95 % CI 1.9, 3.4). Our findings with regard to physical function are similar to those of de Schepper et al., measured by use of a condition-specific questionnaire (Roland-Morris Back Pain and Disability Questionnaire). We have found, using data from the Johnston County OA Project (n = 1,633), that after adjusting for age, BMI, and gender, mean levels of perceived disability because of LBP increase significantly (p < 0.05) across severity of DSN. (Fig. 1; unpublished data).
Fig. 1.
Adjusted mean values for the Roland–Morris Low Back Pain and Disability Questionnaire across severity of disc space narrowing. Findings from the Johnston County Osteoarthritis Project (unpublished)
Risk Factors
The vague diagnostic classifications and multidimensional, recurrent nature of LBP create challenges for longitudinal designs aimed at determining the temporal relationship between radiographic spine features and LBP. These challenges are compounded by the remaining questions related to the etiologic process of spine degeneration. Spine degeneration is a process that occurs slowly in some individuals and more rapidly in others [25]. The age at which the process of spine degeneration begins is largely unknown and may depend on the individual spine. Our recent cross-sectional findings indicate that 88 % of participants had at least mild OST with a mean age of 59.7 years, suggesting that this particular degenerative process may begin much earlier than that of DSN or FOA, for which the prevalence was 57.6 % and 57.9 %, respectively. These different factors make studying the temporal relationship between spine degeneration and LBP difficult.
Two previous longitudinal studies, consisting of women only, have found few predictive risk factors for either DSN or OST [33, 41]. Notable predictors of DSN progression from these studies have been the presence of baseline DSN [41] and the presence of baseline hip or knee OA [33]. The presence of baseline DSN was not found to be a risk factor for future back pain [42]. Recently, Muraki and colleagues [32**], using data from the Research on Osteoarthritis/Osteoporosis Against Disability (ROAD), longitudinally determined the incidence of radiographic, Kellgren–Lawrence (KL) graded, lumbar spondylosis (presence of DSN and OST at the same vertebral level) and LBP in a Japanese community-based population. With a 3.3-year follow-up, the incidence of KL ≥ 2 grade lumbar spondylosis was high—50 % in men and 34.4 % in women. The presence of KL ≥ 3 grade spondylosis has been found to be a moderately significant risk factor for LBP (OR = 1.26; 95 % CI 1.03, 1.69). This risk, however, was stronger when three or more KL ≥ 3 levels were affected in both men (OR = 1.69; 95 % CI 1.03, 2.76) and women (OR = 1.77; 95 % CI 1.34, 2.34).
There is little consistency between studies of risk factors for future spine degeneration or LBP; some studies suggest baseline degeneration as a significant risk factor for future LBP whereas others do not. Ideally, such knowledge of risk factors could be used to develop primary prevention techniques for these outcomes; however, this topic is largely understudied, although we already have sufficient information to make clinical recommendations for the treatment of LBP.
Current Conservative Treatment Options
Given the effect of LBP on the US healthcare system, workplace, and individual quality of life, there is great need to better understand predictors and etiologic factors of LBP. Most research has focused on treating symptoms and functional impairment of LBP rather than on understanding the mechanisms underlying the anatomic and functional changes we currently call spine degeneration and their relationship to symptoms and functional impairment. Currently, our understanding of the treatment for LBP is, stated simply, that some activity is better than no activity [43, 44]. Exercise therapy is an activity which has long been a treatment option for LBP, with Cochrane and other reviews indicating some effectiveness for treating chronic low back pain [45]. Specifically, treatment of LBP with yoga has attracted substantial interest in recent years [46–48]. A recent randomized clinical trial by Sherman and colleagues [47**] found that outcomes for the yoga intervention group were superior to those for the self-care group, but no significant difference was found from the stretching group for chronic LBP. The functional outcomes found in the yoga and stretching group remained statistically superior to those for the self-care group after a follow-up of 26 weeks. These findings suggest it is the physical rather than psychological aspect of yoga that has moderate benefits for chronic LBP. This trial did not specifically target patients with intervertebral disc degeneration and excluded patients with severe intervertebral disc degeneration, because some patients with severe disc degeneration may not tolerate exercise therapy [48]. The use of conservative treatment to prevent spine degeneration or conservative treatment for spine degeneration as a primary technique for treating LBP has not yet been reported in the literature. Reasons for this may be the small number of longitudinal studies, inconsistencies in predictors, and weak risk factors.
Furthermore, there are few secondary prevention intervention techniques specifically aimed at treating symptomatic spine degeneration. Injection therapy has been a popular treatment technique targeting spine features for LBP, and Karppinen et al. [48] have recently summarized evidence on this therapy. Facet joint injection therapy to treat LBP has increased dramatic in recent years. Among Medicare beneficiaries, intervention for facet joint pain has increased substantially with annual growth of 60 % from 1997 to 2006 [49]. However, there is little evidence to support the use of this therapy, with recent practice guidelines recommending against the use of facet joint steroid injections [50].
Future Research Directions
Biological Markers, Genetics and Pain Pathways
What future research areas are ahead for understanding of spine degeneration and LBP? The topic of spine degeneration seems to follow the research trends of OA in the knee and hip. A recent review of the literature indicates several serum and urine biomarkers may be useful in understanding the etiology of knee and hip OA [51]. Gruber and Hanley [52] indicated that biological markers for intervertebral disc degeneration that could be used to correlate patients’ status regarding pain and symptoms would be especially meaningful. Minimum work has been performed on this topic, but a few small studies of select samples have found associations between DSN and C-terminal cross-linked telopeptide (CTX-II), a biomarker of type II collagen degradation [53, 54]. Our recent work led to an interesting result regarding DSN, cartilage oligomeric matrix protein (COMP), and LBP [55**]. Among those with LBP (n = 265), there was a strong positive association between COMP and DSN (OR = 1.82; 95 % CI 1.02, 3.27) that was not observed in those without LBP (OR = 0.65; 95 % CI 0.35, 1.20). Therefore, it is possible that elevated COMP levels reflect the ongoing process of degeneration in the intervertebral disc, as evidenced by DSN, and results in associated symptoms that have been reported for this degenerative process.
Another topic of increasing interest is understanding the possible involvement of genetics in spine degeneration and LBP. Disc degeneration may be partially explained by genetic factors [5] and several review articles have already summarized these genetic factors extensively [56–60]. A relationship between LBP and intervertebral disc degeneration has been established, and several studies have identified genetic risk factors for lumbar disc degeneration [60]. However, most of these studies are cross-sectional with small sample sizes, and longitudinal, population-based studies are needed to confirm these findings.
Understanding the involvement of pain pathways in OA is beginning to become clearer as studies are discovering more about central pain sensitization in knee OA [61, 62]. Our recent work measuring pressure–pain threshold tolerance among participants with knee and/or hip OA or knee and/or hip symptoms indicates that those with higher pain thresholds are significantly less likely to report knee or hip symptoms, and plain film radiographs were not significantly associated with pressure–pain threshold in either joint [63]. This is also true for other conditions with musculoskeletal components, for example fibromyalgia [64, 65]. Use of the pressure–pain threshold to improve our understanding of central and peripheral pain sensitization is not new to the study of LBP [66, 67]. However, its use with radiographic findings of the lumbar spine has not been studied at the population level and may improve our understanding of nervous system pathways in this complex relationship between spine degeneration and LBP.
Conclusions
The radiographic features of spine degeneration are common and are associated with LBP and reduced physical function. Therefore, facet joint OA and degeneration of the spine resulting in DSN and OST formation should not be overlooked in the discussion of OA and population estimates of prevalence and disease burden. This is particularly true of the facet joint, which may follow an OA-related degenerative process similar to that of appendicular synovial joints. However, recent population-based studies have been unable to link FOA with LBP and the multidimensional nature of LBP may cloud these associations. In recent years population-based studies have consistently demonstrated that DSN is associated with LBP, and recent longitudinal evidence suggests it may be a risk factor for LBP. Current treatment has not focused on primary or secondary prevention of LBP resulting from intervertebral disc degeneration, but evidence suggests that exercise therapy in general, including stretching and yoga, are good treatments for LBP. The different associations of LBP, physical function, and radiographic features in the spine are indicative of the importance of individual assessment of radiographic features and measured outcomes in future studies. Given the multidimensional nature of LBP, determining the association between spine degeneration and both LBP and reduced physical function may prove more worthwhile and clinically applicable in understanding the burden of spine degeneration than self-reported LBP alone. Finally, the role of genetics, biological markers, and understanding pain pathways in spine degeneration and LBP may be crucial to understanding the complex relationship between these two conditions.
Acknowledgments
Dr Goode is supported by the NIH Loan Repayment Program, the National Institute of Arthritis Musculoskeletal and Skin Diseases (1-L30-AR057661-01), and by the Agency for Health Care Research and Quality (AHRQ) K-12 Comparative Effectiveness Career Development Award grant number HS19479-01. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or AHRQ.
The authors would like to thank Holly R. Thompson, BA, for her careful editing of the manuscript.
Footnotes
Disclosure
Dr Goode has received grant support from the Agency for Health Care Research and Quality. Dr Carey has served as a consultant for Blue Cross and the Blue Shield Association. Dr Jordan has received grant support from Johnson and Johnson, has received honoraria from LEK Consulting and Marston Consulting, holds stock options in Algynomics, and has had travel and/or accommodation expenses covered and/or reimbursed by the Osteoarthritis Research Society International and the Arthritis Foundation.
Contributor Information
Adam P. Goode, Email: adam.goode@duke.edu.
Timothy S. Carey, Email: timothy.carey@unc.edu.
Joanne M. Jordan, Email: joanne_jordan@med.unc.edu.
References
Paper of particular interest, published recently, have been highlighted as:
*Of importance
**Of particular importance
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33(11):2271–2279. [PubMed] [Google Scholar]
- 3.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy DJ, Fredericson M. Introduction. Pm R. 2012;4(5 Suppl):S1–2. doi: 10.1016/j.pmrj.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Battie MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine (Phila Pa 1976) 2004;29(23):2679–2690. doi: 10.1097/01.brs.0000146457.83240.eb. [DOI] [PubMed] [Google Scholar]
- 6.Borenstein D. Does osteoarthritis of the lumbar spine cause chronic low back pain? Curr Rheumatol Rep. 2004;6(1):14–19. doi: 10.1007/s11926-004-0079-z. [DOI] [PubMed] [Google Scholar]
- 7.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2):353–371. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 9.Guo HR, Tanaka S, Halperin WE, Cameron LL. Back pain prevalence in US industry and estimates of lost workdays. Am J Public Health. 1999;89(7):1029–1035. doi: 10.2105/ajph.89.7.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC. Back pain exacerbations and lost productive time costs in United States workers. Spine. 2006;31(26):3052–3060. doi: 10.1097/01.brs.0000249521.61813.aa. [DOI] [PubMed] [Google Scholar]
- 11.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. Jama. 2003;290(18):2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 12.Weiner DK, Haggerty CL, Kritchevsky SB, Harris T, Simonsick EM, Nevitt M, et al. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain Med. 2003;4(4):311–320. doi: 10.1111/j.1526-4637.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 13.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine (Phila Pa 1976) 1995;20(1):11–19. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137(7):586–597. doi: 10.7326/0003-4819-137-7-200210010-00010. [DOI] [PubMed] [Google Scholar]
- 15.Chou R, Qaseem A, Snow V, Casey D, Cross JT, Jr, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Bindman R, Miglioretti DL, Larson EB. Rising use of diagnostic medical imaging in a large integrated health system. Health Aff (Millwood) 2008;27(6):1491–1502. doi: 10.1377/hlthaff.27.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey TS, Freburger JK, Holmes GM, Castel L, Darter J, Agans R, et al. A long way to go: practice patterns and evidence in chronic low back pain care. Spine (Phila Pa 1976) 2009;34(7):718–724. doi: 10.1097/BRS.0b013e31819792b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22(4):427–434. doi: 10.1097/00007632-199702150-00015. [DOI] [PubMed] [Google Scholar]
- 20.Varlotta GP, Lefkowitz TR, Schweitzer M, Errico TJ, Spivak J, Bendo JA, et al. The lumbar facet joint: a review of current knowledge: part 1: anatomy, biomechanics, and grading. Skeletal Radiol. 2011;40(1):13–23. doi: 10.1007/s00256-010-0983-4. [DOI] [PubMed] [Google Scholar]
- 21.Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum. 2007;37(2):69–80. doi: 10.1016/j.semarthrit.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Videman T, Battie MC, Gill K, Manninen H, Gibbons LE, Fisher LD. Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine. 1995;20(8):928–935. doi: 10.1097/00007632-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Urban JPG, Roberts S, Ralphs JR. The Nucleus of the Intervertebral Disc from Development to Degeneration. Amer Zool. 2000;40(1):53–61. [Google Scholar]
- 24.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492(1):29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menkes CJ, Lane NE. Are osteophytes good or bad? Osteoarthritis and cartilage. 2004;12 (Suppl A):S53–54. doi: 10.1016/j.joca.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Snodgrass JJ. Sex differences and aging of the vertebral column. J Forensic Sci. 2004;49(3):458–463. [PubMed] [Google Scholar]
- 28.Alonge TO, Oni OO. An investigation of the frequency of co-existence of osteophytes and circumscribed full thickness articular surface defects in the knee joint. Afr J Med Med Sci. 2000;29(2):151–153. [PubMed] [Google Scholar]
- 29.Boegard T, Rudling O, Petersson IF, Jonsson K. Correlation between radiographically diagnosed osteophytes and magnetic resonance detected cartilage defects in the tibiofemoral joint. Annals of the rheumatic diseases. 1998;57(7):401–407. doi: 10.1136/ard.57.7.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Suri P, Miyakoshi A, Hunter DJ, Jarvik JG, Rainville J, Guermazi A, et al. Does lumbar spinal degeneration begin with the anterior structures? A study of the observed epidemiology in a community-based population. BMC Musculoskelet Disord. 2011;12:202. doi: 10.1186/1471-2474-12-202. This was an ancillary study using data from the Framingham Heart Study to determine if the anterior structures of the spine degenerate in sequence to the posterior structures of the spine. For most participants, anterior structure degeneration occurred before posterior; however, there were cases of posterior structure degeneration in isolation of anterior structures, suggesting that facet joint OA may occur without intervertebral disc degeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuki G. Osteoarthritis: a problem of joint failure. Z Rheumatol. 1999;58(3):142–147. doi: 10.1007/s003930050164. [DOI] [PubMed] [Google Scholar]
- 32**.Muraki S, Akune T, Oka H, Ishimoto Y, Nagata K, Yoshida M, et al. Incidence and risk factors for radiographic lumbar spondylosis and lower back pain in Japanese men and women: the ROAD study. Osteoarthritis and cartilage. 2012;20(7):712–718. doi: 10.1016/j.joca.2012.03.009. This is one of few longitudinal studies aimed at determining the incidence of lumbar spondylosis changes and risk factors for progression. Findings from this study indicate an overall high proportion of Kellgren Lawrence (K-L) graded lumbar spondylosis. The incidence of K-L 2 was greater among men, as was the progression of lumbar spondylosis. Severe K-L grade at baseline was significantly associated with the incidence of low back pain. [DOI] [PubMed] [Google Scholar]
- 33.Hassett G, Hart DJ, Manek NJ, Doyle DV, Spector TD. Risk factors for progression of lumbar spine disc degeneration: the Chingford Study. Arthritis Rheum. 2003;48(11):3112–3117. doi: 10.1002/art.11321. [DOI] [PubMed] [Google Scholar]
- 34**.de Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976) 2010;35(5):531–536. doi: 10.1097/BRS.0b013e3181aa5b33. This is the largest population-based study describing the prevalence and associations with low back pain of individual radiographic features of disc space narrowing and vertebral osteophytes. Their findings indicate a significant association between disc space narrowing and vertebral osteophytes and low back pain. However, the associations with vertebral osteophytes were small and have not been replicated in other studies. This is also the first study to determine the relationship between spine radiographic features and physical function with some associations being stronger than with low back pain alone. [DOI] [PubMed] [Google Scholar]
- 35**.Goode A, Marshall SW, Renner JB, Carey TS, Kraus VB, Irwin DE, Sturmer T, Jordan JM. Lumbar spine radiographic features and demographic, clinical and concomitant knee, hip and hand OA: The Johnston County Osteoarthritis Project [in press] Arthritis Care Res. 2012 doi: 10.1002/acr.21720. This is the only community-based study in the US of spine radiographic features and their relationship between hip, knee, and hand OA and low back symptoms. Only disc space narrowing was significantly associated with low back symptoms. Associations differed by appendicular joint OA with significant associations between facet joint OA and knee and hand OA, but no associations between disc space narrowing and knee or hand OA. No radiographic feature in the spine was associated with hip OA. This was also the first study to determine that associations between spine radiographic features differed by race. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muraki S, Oka H, Akune T, Mabuchi A, En-Yo Y, Yoshida M, et al. Prevalence of radiographic lumbar spondylosis and its association with low back pain in elderly subjects of population-based cohorts: the ROAD study. Ann Rheum Dis. 2009;68(9):1401–1406. doi: 10.1136/ard.2007.087296. [DOI] [PubMed] [Google Scholar]
- 37.Pye SR, Reid DM, Smith R, Adams JE, Nelson K, Silman AJ, et al. Radiographic features of lumbar disc degeneration and self-reported back pain. J Rheumatol. 2004;31(4):753–758. [PubMed] [Google Scholar]
- 38.Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 2008;33(1):95–103. doi: 10.1097/BRS.0b013e31815e7f94. [DOI] [PubMed] [Google Scholar]
- 39.Kalichman L, Li L, Kim DH, Guermazi A, Berkin V, O’Donnell CJ, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine. 2008;33(23):2560–2565. doi: 10.1097/BRS.0b013e318184ef95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Scheele J, de Schepper EI, van Meurs JB, Hofman A, Koes BW, Luijsterburg PA, et al. Association between spinal morning stiffness and lumbar disc degeneration: the Rotterdam Study. Osteoarthritis and cartilage. 2012 doi: 10.1016/j.joca.2012.05.011. This study is the first to describe the involvement of morning stiffness in combination with low back pain among those with spine degeneration. The associations between self reported morning stiffness in conjunction with low back symptoms were stronger than self reported low back pain alone. These findings are of particular importance because self-reported low back pain has only had modest associations with spine degeneration. [DOI] [PubMed] [Google Scholar]
- 41.Symmons DP, van Hemert AM, Vandenbroucke JP, Valkenburg HA. A longitudinal study of back pain and radiological changes in the lumbar spines of middle aged women. II. Radiographic findings. Ann Rheum Dis. 1991;50(3):162–166. doi: 10.1136/ard.50.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Symmons DP, van Hemert AM, Vandenbroucke JP, Valkenburg HA. A longitudinal study of back pain and radiological changes in the lumbar spines of middle aged women. I. Clinical findings. Annals of the rheumatic diseases. 1991;50(3):158–161. doi: 10.1136/ard.50.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigos SJ, Holland J, Holland C, Webster JS, Battie M, Malmgren JA. High-quality controlled trials on preventing episodes of back problems: systematic literature review in working-age adults. Spine J. 2009;9(2):147–168. doi: 10.1016/j.spinee.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Buchbinder R, Pransky G, Hayden J. Recent advances in the evaluation and management of nonspecific low back pain and related disorders. Best Pract Res Clin Rheumatol. 2010;24(2):147–153. doi: 10.1016/j.berh.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Hayden JA, van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335. doi: 10.1002/14651858.CD000335.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 47**.Sherman KJ, Cherkin DC, Wellman RD, Cook AJ, Hawkes RJ, Delaney K, et al. A randomized trial comparing yoga, stretching, and a self-care book for chronic low back pain. Arch Intern Med. 2011;171(22):2019–2026. doi: 10.1001/archinternmed.2011.524. This randomized clinical trial examined stretching, yoga, and self-care books for primary care patients. Although not specific to spine degeneration, these findings indicate that yoga was more effective than self-care books but no more effective than stretching classes for the treatment of chronic low back pain. Both types of intervention resulted in improved symptoms and increased physical function with benefits lasting for several months. These results support several other reports that some exercise is better than no exercise for low back pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karppinen J, Shen FH, Luk KD, Andersson GB, Cheung KM, Samartzis D. Management of degenerative disk disease and chronic low back pain. Orthop Clin North Am. 2011;42(4):513–528. viii. doi: 10.1016/j.ocl.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Manchikanti L, Singh V, Pampati V, Smith HS, Hirsch JA. Analysis of growth of interventional techniques in managing chronic pain in the Medicare population: a 10-year evaluation from 1997 to 2006. Pain Physician. 2009;12(1):9–34. [PubMed] [Google Scholar]
- 50.Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine. 2009;34(10):1078–1093. doi: 10.1097/BRS.0b013e3181a103b1. [DOI] [PubMed] [Google Scholar]
- 51.van Spil WE, DeGroot J, Lems WF, Oostveen JC, Lafeber FP. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis and cartilage. 2010;18(5):605–612. doi: 10.1016/j.joca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Gruber HE, Hanley EN. Do we need biomarkers for disc degeneration? Biomark Insights. 2007;1:131–133. [PMC free article] [PubMed] [Google Scholar]
- 53.Garnero P, Sornay-Rendu E, Arlot M, Christiansen C, Delmas PD. Association between spine disc degeneration and type II collagen degradation in postmenopausal women: the OFELY study. Arthritis Rheum. 2004;50(10):3137–3144. doi: 10.1002/art.20493. [DOI] [PubMed] [Google Scholar]
- 54.Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellio Le Graverand MP, et al. Urinary CTX-II levels are associated with radiographic subtypes of osteoarthritis in hip, knee, hand, and facet joints in subject with familial osteoarthritis at multiple sites: the GARP study. Ann Rheum Dis. 2006;65(3):360–365. doi: 10.1136/ard.2005.040642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Goode A, Marshall SW, Kraus VB, Renner JB, Sturmer T, Carey TS, Irwin DE, Jordan JM. Association between serum and urine biomarkers and individual radiographic features in the spine: The Johnston County Osteoarthritis Project [in press] Osteoarthritis Cartilage. 2012 doi: 10.1016/j.joca.2012.08.003. This study examined a broad range of urine and serum biomarkers and their associations with either disc space narrowing or vertebral osteophytes. Associations between biomarkers and disc space narrowing were stronger than association of biomarkers with vertebral osteophytes. This is the only study to compare a wide range of biomarkers among those with and without low back symptoms. Interestingly, serum COMP was significantly associated with disc space narrowing among those with low back symptoms but not among those without low back symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Sun Z, Liu J, Guo X. Advances in susceptibility genetics of intervertebral degenerative disc disease. Int J Biol Sci. 2008;4(5):283–290. doi: 10.7150/ijbs.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalichman L, Hunter DJ. The genetics of intervertebral disc degeneration. Associated genes. Joint Bone Spine. 2008;75(4):388–396. doi: 10.1016/j.jbspin.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Chan D, Song Y, Sham P, Cheung KM. Genetics of disc degeneration. European spine journal. 2006;15 (Suppl 3):S317–325. doi: 10.1007/s00586-006-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ala-Kokko L. Genetic risk factors for lumbar disc disease. Ann Med. 2002;34(1):42–47. doi: 10.1080/078538902317338634. [DOI] [PubMed] [Google Scholar]
- 60.Kao PY, Chan D, Samartzis D, Sham PC, Song YQ. Genetics of lumbar disk degeneration: technology, study designs, and risk factors. Orthop Clin North Am. 2011;42(4):479–486. vii. doi: 10.1016/j.ocl.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis and Rheumatism. 2008;59(10):1424–1431. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 63.Goode A, Shi XA, Renner JR, Gracely R, Maleki-Fischban M, Jordan JM. Association Between Pain Threshold, Symptoms and Radiographic Knee and Hip Osteoarthritis: The Johnston County Osteoarthritis Project [accepted abstract] Arthritis Rheum. 2012 [Google Scholar]
- 64.Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48(10):2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 65.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 66.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50(2):613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 67.O’Neill S, Kjaer P, Graven-Nielsen T, Manniche C, Arendt-Nielsen L. Low pressure pain thresholds are associated with, but does not predispose for, low back pain. European spine journal. 2011;20(12):2120–2125. doi: 10.1007/s00586-011-1796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]