Abstract
Baclofen reduces intake of some foods but stimulates intake or has no effect on others. The reasons for these differences are not known. The present study examined effects of baclofen when composition, energy density, preference, presentation and intake of optional foods varied. Semi-solid fat emulsions and sucrose products were presented for brief periods to non-food-deprived rats. In Experiment 1, fat and sucrose composition were varied while controlling energy density. In Experiment 2A, schedule of access and the number of optional foods were varied. In Experiment 2B, the biopolymer (thickener) was examined. Baclofen reduced intake of fat and/or sugar options with different energy densities (1.28-9 kcal/g), when presented daily or intermittently, and when intakes were relatively high or low. However, the efficacy of baclofen was affected by the biopolymer used to thicken the options: baclofen had no effect when options were thickened with one biopolymer (3173), but reduced intake when options were thickened with another biopolymer (515). Baclofen failed to reduce intake of a concentrated sugar option (64% sucrose), regardless of biopolymer. Based upon these results, caution is urged when interpreting results obtained with products using different thickening agents. Systematic research is needed when designing products used in rat models of food intake.
Keywords: Behavior, binge eating, biopolymers, baclofen, emulsions, fat, sucrose, sugar
GENERAL INTRODUCTION
Baclofen is a GABA-B agonist that reduced binge frequency in both placebo-controlled and open label trials (Broft et al., 2007; Corwin et al., 2012), and also reduced the consumption of fatty food in rodent models of binge eating (Berner et al., 2009; Buda-Levin et al., 2005; Corwin & Wojnicki, 2009; Czyzyk, et al., 2010; Rao et al., 2008; Wojnicki et al., 2006; Wong et al., 2009;). While overall group effects are consistent, individual variability in the ability of baclofen to reduce binge frequency in humans has been reported (Corwin et al., 2012). Similarly, in animals, while baclofen has been reported to reduce food intake in some studies, it has been reported to have no effect on or to stimulate food intake in others (see discussion, below). The reasons for these differential effects are not known, but may involve the composition, energy density, or relative preference for the food being consumed, as well as the manner of food presentation and the amount consumed. Understanding factors that influence the efficacy of baclofen is important for basic mechanistic research, but also for targeting therapeutic use to those patients most likely to derive benefit.
Animal studies have indicated that one of the factors influencing the effects of baclofen is the composition of the food. Baclofen generally reduces intake of fatty foods, but when high carbohydrate foods (complex carbohydrates or simple sugars) are offered, baclofen either stimulates intake or has no effect (Berner et al., 2009; Buda-Levin et al., 2005; Corwin & Wojnicki, 2009; Czyzk, et al., 2010; Ebenezer, 1995; Sato et al., 2007). The available studies, however, are complicated by the energy density, baseline intake, and relative preference of the foods being offered. Baclofen reduces intake of vegetable shortening (a semi-solid fat), which is very energy dense at 9.17 kcal/g and is readily consumed (intakes are relatively high), especially when access is limited. In contrast, baclofen generally has no effect on or stimulates intake of chow, which is high in complex carbohydrates and calorically less dense at 3.3 kcal/g. However, during brief test sessions, intake of the fatty food is generally higher than that of the chow, which confounds the interpretation of results. That is, intake reductions induced by baclofen may be less specific to fat and may simply be specific to foods consumed in large amounts. Furthermore, when the fatty food and chow are simultaneously available, the fatty food is more highly preferred, i.e. baclofen may only reduce intake of preferred foods. Finally, in many studies, the chow is provided continuously, whereas the fatty food is only provided during a limited time period. Thus, while the macronutrient composition of the food appears to be important, the available research does not allow the influence of energy density, baseline intake, relative preference and availability to be ruled out as contributing factors.
In addition to the macronutrient composition of the food, some studies indicate that biopolymers used in food preparation may also influence the efficacy of baclofen. Such biopolymers are used as thickening agents in the production of human food such as bakery items, sauces and gravies, and have also been used in animal studies to produce semi-solid fat emulsions, which are lower in energy density than pure fat. When optional semi-solid fat emulsions made with a biopolymer containing 4% starch were presented for 1 hr daily (7 days/week) to non-food-deprived rats, baclofen reduced intake of 18%, 32% and 56% fat emulsions. However, when the same emulsions were presented for 1 hr intermittently (Mondays, Wednesdays, Fridays), baclofen only reduced intake of the 56% fat emulsion (Rao et al., 2008). This suggests that the manner in which the food is presented or the frequency of consumption (daily or occasionally) may influence the ability of baclofen to reduce intake. Specifically, the intake-reducing effects of baclofen may be enhanced with more frequent consumption of fatty foods, whereas effects may be attenuated when fatty foods are only consumed occasionally.
Another study, though, reported that baclofen failed to reduce intake of semi-solid 32% fat emulsions made with either one of two different biopolymers that did not contain starch even though they were presented daily (Wang, et al., 2011). However, intake of these emulsions was relatively low. When 4% starch was added to the biopolymers, emulsion intake increased and baclofen reduced intake of both emulsions, again suggesting that baseline intake may influence the efficacy of baclofen. However, even though the addition of starch increased intake of the two emulsions in the Wang et al. (2011) report, intakes were still significantly less than those reported by Rao, et al., (2008), yet baclofen reduced intake in both studies. On its face, it might appear that the presence of starch could explain the results reported by Wang et al. (2011) and Rao, et al. (2008). However, while starch was common to both studies, the results also suggest that the composition of the biopolymers themselves may be critical in determining baclofen’s effect.
The present series of studies sought to address some of this complexity by systematically comparing the effects of baclofen under conditions in which the composition, energy density, baseline intake, relative preference, and schedule of access of optional test foods are varied. For the purposes of these investigations in rats, a series of semi-solid fat emulsions and sucrose products were developed in which the fat and sugar content were similar to foods consumed by humans. These served as optional test foods that were presented for brief periods to non-food-deprived rats. The use of semi-solid fat emulsions thickened with a biopolymer blend allows for a consistent texture, but avoids phase separation that can occur with liquid oil-in-water emulsions (Wang et al., 2011; Lucas et al., 1987). The semi-solid sucrose products were also thickened with a biopolymer blend, resulting in a “pudding-like” texture that was similar to the semi-solid fat emulsions. The use of these products allows direct comparisons between fat and sucrose in foods of similar form.
Experiment 1 varied the fat and sucrose compositions while controlling the energy density of the optional test foods. Based on unpublished pilot data from this lab, all semi-solid fat emulsions and semi-solid sucrose products were made with a newly developed biopolymer that did not contain starch. The optional foods were presented for 1 h on an intermittent basis for several weeks, after which baclofen was tested for its ability to reduce intake.
Experiment 2A used two of the test foods from Experiment 1 that were iso-caloric, but were made with one of the biopolymers used by Wang et al., (2011). This biopolymer also did not contain starch. First, this manipulation allowed for a comparison to the newer biopolymer used in Experiment 1. Secondly, each of the two test foods was singularly provided for 1h on either a daily or intermittent schedule of access, to assess if baclofen would differentially affect intake between the daily and intermittent groups as was shown in the Rao et al., (2008) study. The macronutrient profile of the test foods was different from those that were used in the Wang et al., (2001) and Rao et al., (2008) studies. This was done in order to match energy densities, thereby allowing us to further assess if baclofen’s effects were dependent upon the macronutrient composition independent of energy density. Lastly, two additional groups were used to test preference for the two test foods. Both of the optional test foods in the two preference groups were provided for 1h on either a daily or intermittent basis, in order to assess if the effects of baclofen varied with relative preference.
Given the results of Experiment 2A, Experiment 2B used the biopolymer of Experiment 1 to make the same semi-solid test foods in order to determine the contribution of the biopolymer to the intake-reducing effects of baclofen. The rats used in Experiment 2B are the same animals that were used in Experiment 2A.
EXPERIMENT 1
INTRODUCTION
In this experiment, the effects of baclofen on the intake of several different optional test foods were evaluated when they were provided to rats for 1 h/day on an intermittent basis (Mondays, Wednesdays, and Fridays). Two of the test foods were iso-caloric at 1.28 kcal/g and were comprised of 14% fat and 32% sucrose, respectively; three additional test foods were iso-caloric at 2.56 kcal/g and were comprised of 28% fat, 64% sucrose, and 14% fat plus 32% sucrose combined (also by weight). Concentrations of fat and sucrose were based upon those in foods that people commonly consume. Some cookies and ice creams, for instance have a fat content of ~15%; chocolate candy has a fat content of ~30%; the sugar content of cookies includes concentrations of ~32%, whereas jellybeans contain ~70% sugar. In addition, the fat/sugar ratio of vanilla ice cream is 0.5 and the energy density of chocolate ice cream is 2.55 kcal/g, which are similar to those of the combined 14% fat/32% sucrose test food (USDA database, http://ndb.nal.usda.gov/ndb/foods/list). Vegetable shortening was used as a positive control for the intake-reducing effects of baclofen (Buda-Levin, et al., 2005; Corwin & Wojnicki, 2009).
METHODS
Animals
Seventy two male Sprague Dawley rats (Harlan, Indianapolis, IN), 60 days of age and weighing 259-297 g (280 ± 0.79 g) at the start of the study were individually housed in hanging stainless steel wire cages in a temperature- and humidity-controlled environment placed on a 12:12 light:dark cycle. All rats were maintained on a nutritionally complete commercially available pelleted rodent chow (Laboratory Rodent Diet 5001, PMI Feeds, Richmond IN; percent of energy as protein: 28.05%, fat: 12.14%, carbohydrate: 59.81%; 3.3 kcal/g). The chow and tap water were available ad libitum throughout all parts of the study, unless otherwise note. The Pennsylvania State University Institutional Animal Care and Use Committee approved all procedures.
After seven days of adaptation to the vivarium, body weights were recorded and all rats were provided overnight access to a semi-solid fat emulsion comprised of 40% vegetable shortening (Crisco™, J.M. Smucker Co., Orrville, OH; 9.167 kcal/g; contains no trans fats) with an energy density of 3.67 kcal/g (see complete product descriptions, below). Two days later, all rats were provided overnight access to a semi-solid sugar product comprised of 18% sucrose (Great Value, Bentonville, AR) with an energy density of 0.71 kcal/g. These fat and sugar concentrations were not used in the remainder of the study, but served only to group the rats, while also preventing subsequent intake bias due to differences in familiarity with the test foods among the various groups. Six groups of 12 rats each were then matched by body weight, as well as overnight intake of the 40% fat emulsion and 18% sucrose product [F(5,66) < 1 for all comparisons; ps NS].
Optional foods
As mentioned above, vegetable shortening was used as an optional test food for one of the six groups in order to provide a positive control for the intake-reducing effects of baclofen. In addition, five different optional test foods were prepared in which the fat and sucrose contents (w/v) systematically varied: two of these were semi-solid emulsions comprised of 14% and 28% fat, two were semi-solid products comprised of 32% and 64% sucrose, and one was a semi-solid product comprised of both fat (14%) and sucrose (32%). The 14% fat and 32% sucrose products were iso-caloric (1.28 kcal/g), as were the 28% fat, 14% fat / 32% sucrose, and 64% sucrose products (2.56 kcal/g). Two different biopolymer blends supplied by the same manufacturer (TIC Gums, Belcamp MD) were used to thicken the fat emulsions and sucrose products: TIC Pretested® Ticaloid® 515 M-T (2.83 kcal/g, abbreviated “515”) and TIC Action Gum® 10000EC (3.52 kcal/g, abbreviated “EC”). The 515 powder was a blend of ammonium alginate, calcium alginate, and gum acacia and was used as the primary thickener. The EC powder was a blend of guar gum and xanthan gum and was used in smaller amounts as an additional thickener. In order to maintain similar pudding-like consistencies across the optional foods, different amounts of the two biopolymer blends were used as shown in Table 1 (top panel).
Table 1.
| Experiment 1: 515-MT Emulsions
| |||||
|---|---|---|---|---|---|
| Emulsion Composition | 14% fat | 28% fat | 32% sucrose | 64% sucrose | 14% fat / 32% sucrose |
| Energy density (kcal/gm) | 1.28 | 2.56 | 1.28 | 2.56 | 2.56 |
| 515 MT (g/100 mL) | 2.50 | 2.00 | 2.50 | 1.50 | 2.00 |
| 10000EC (g/100 mL) | 0.30 | 0.30 | 0.40 | 0.30 | 0.40 |
|
| |||||
| Experiment 2: 3173 Emulsions
| |||||
| Emulsion Composition | 64% sucrose | 14% fat / 32% sucrose | |||
| Energy density (kcal/gm) | 2.56 | 2.56 | |||
| 3173 (g/100 mL) | 2.50 | 2.50 | |||
| 10000EC (g/100 mL) | 0.19 | 0.13 | |||
For the two semi-solid fat emulsions, the fat was heated to 85°C, mixed into a 1% w/v bovine sodium caseinate (Sigma Aldrich, St Louis, MO; 3.88 kcal/g) solution, then coarsely homogenized (Omni 5001, Omni International, Kennesaw, Ga) for 1 minute. The caseinate acts as an emulsifier and allows the temporary formation of a kinetically stable oil-in-water emulsion. Once the liquid emulsions were homogenized, each of the biopolymer powders was added to the oil-in-water emulsion and mixed in a high speed blender (Waring model 38BL52(LBC 10), Dynamics Corp. of America, New Hartford, CN) for approximately 1 minute. Each emulsion was then placed in a drying oven at 80-85°C for 2 hr, removed from the oven, and then re-mixed with a hand blender (Oster model:2614-000, Sumbeam Products, Inc., Boca Raton, Fl.). Initial mixing of the emulsions in the high speed blender allowed for the sheering of the powders to aid in their hydration. The emulsions were allowed to cool to room temperature and then refrigerated with a sheet of clear plastic wrap (Great Value, Bentonville, AR) over the emulsion to prevent the formation of a skin. The sucrose semi-solid products were also mixed into a 1% w/v bovine sodium caseinate solution and prepared as above. All foods were allowed to reach room temperature prior to their presentation to the rats. Food remaining in the jar after feeding was discarded after each session. New batches of emulsions and semi-solid products were prepared weekly and were physically stable over the course of the week with no creaming or visible phase separation.
Procedure
After matching the six groups, all rats were given overnight access to their assigned optional food to prevent neophobia during subsequent intake tests. The six group designations were as follows: 14% fat (14F), 32% sucrose (32S), 28% fat (28F), 64% sucrose (64S), 14% fat / 32% sucrose (14F/32S) and 100% fat (100F). Three days later all rats were given intermittent (Monday, Wednesday, Friday) 1-h access to their assigned optional food 2.5 h prior to the start of the dark cycle for five weeks. This access protocol has been used extensively in our laboratory and reliably produces relatively high intakes in brief periods of time without the need for food deprivation. The optional foods were provided in glass jars (2.5” diameter) clipped to the front of the home cage. Body weights were recorded weekly.
During the 6th week all rats received a saline injection to adapt them to the injection procedure. During weeks 7 and 8 the GABAB agonist (R-S)-baclofen (Tocris, Ellisville, MO) or the saline vehicle was administered intraperitoneally (0.0 (vehicle), 1.0, 1.8, 3.2 mg/kg) 30 minutes prior to the intake test; all rats received all doses, with dosing sequence assigned to each rat using a uniform Latin square. Food was not available to the rats during the 30-min pretreatment period. Injections were given on Mondays and Fridays; intake of the optional food and simultaneously available chow were both measured during the intake tests.
Statistics
SAS v9.2 (SAS Institute, Cary, NC) was used to analyze all data. One-way analysis of variance (ANOVA) was used to group the rats by overnight access to 18% sucrose, overnight access to 40% shortening, and initial body weight, and to analyze body weight prior to baclofen testing.
The effects of baclofen on 1-hr intake of each optional food and chow were determined using 2-way repeated measures ANOVA (group X dose) with baclofen dose as the repeated measure. Significant differences among doses were assessed via 1-way repeated measures ANOVA for each group, followed by Tukey’s Studentized Range (HSD) test. In one case (32S), ANOVA showed a significant effect of baclofen dose, but Tukey’s did not reveal differences among the doses. In this case, Duncan’s Multiple Range test was used for the post-hoc analyses.
RESULTS
Body weight prior to baclofen administration
There were no differences in body weights among the groups prior to baclofen testing.
Effects of baclofen on intake of the options
There was a main effect of group [F(5,66) = 25.34, p < 0.001], due to the higher intakes of the options containing sucrose (see Figure 1, top panel). Specifically, intake (in grams) of the 64S, 32S and 14F/32S options was significantly greater than that of the three options containing fat across all doses (ps < 0.05, Tukey’s HSD). There also was a main effect of dose [F(3,198) = 30.39, p < 0.0001], due to overall intake reductions induced by baclofen, but no group by dose interaction [F(15,198) = 1.38, p NS]. In spite of the lack of interaction, some interesting differences among groups emerged. Specifically, baclofen (3.2 mg/kg) significantly reduced consumption of two options containing only fat (28F, 100F), one option containing only sugar (32S), and the option containing both fat and sugar (14F/32S) [ps < 0.05]. Baclofen did not significantly reduce intake of the 14F or the 64S options, relative to intakes after saline (Figure 1, top panel).
Figure 1.
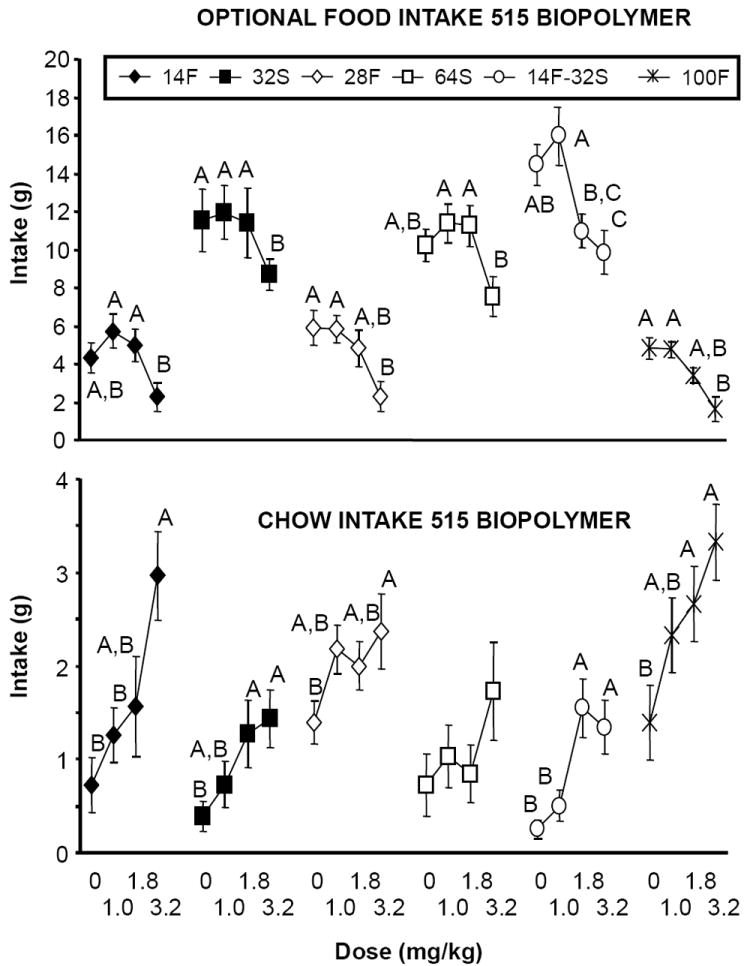
(Top panel): Effects of baclofen on 1-hr intake of different optional foods (see text for different concentrations) when prepared with the 515 biopolymer. Optional foods were provided on an intermittent schedule of access. Different letters indicate differences within groups in intake as a function of dose. (Bottom panel): Effects of baclofen on chow intake during the 1-hr test session (chow was simultaneously available with the optional food). Different letters indicate differences within groups in intake as a function of dose.
Effects of baclofen on intake of the simultaneously available chow
There was a main effect of group [F(5,66) = 8.85, p < 0.001], due to generally greater chow intakes among the groups consuming fat (see Figure 1, bottom panel). There also was a main effect of dose [F(3,198) = 21.24, p < 0.0001] due to overall increases in chow intake, but no group by dose interaction [F(15,198) = 1.21, p NS]. Baclofen significantly increased chow intake in all groups except 64S (ps < 0.05 relative to saline) (Figure 1, bottom panel). Thus, reductions in option intake reported above were not due to non-specific behavioral suppression.
DISCUSSION
Baclofen significantly decreased intake of two options containing fat (28F, 100F), one option containing sugar (32S), and the 14F/32S combination. In contrast, baclofen did not significantly reduce intake of the 14F or 64S option relative to saline. However, although the results with 14F and 64S were not statistically significant, intakes tended to be reduced by the highest baclofen dose (3.2 mg/kg). Baclofen is a muscle relaxant approved for the treatment of spasticity disorders. Therefore, there is always concern that intake reductions induced by baclofen may be due to non-specific behavioral disruption that would interfere with eating. Since baclofen significantly increased consumption of chow at the same time that it was decreasing intake of the option, effects clearly were not due to sedation or the inability to chew and swallow. That said, it is also possible that stimulation of chow intake itself disrupted consumption of the option. However, this also does not appear to be the case. For example, intake of the 14F emulsion was not significantly reduced (indeed, there was a tendency for an increase at lower doses), but chow intake was significantly stimulated (see far left panels, Figure 1). Therefore, the stimulation of chow intake likely does not account for the baclofen-induced reductions in intake of other options.
The present results do not support a macronutrient explanation for the effects of baclofen, since baclofen reduced intake of options comprised of both fat and sucrose. Interestingly, when 32% sucrose and 14% fat were combined, intake was high (relative to the 14% fat alone) and baclofen significantly reduced intake. This supports a baseline explanation, but results with other options contradict the same argument. For instance, baseline intakes (in grams) of the 28F and 100F options were relatively low, but baclofen still reduced intake. Conversely, baseline intake of the 64S was relatively high, but baclofen did not reduce intake relative to saline. Baseline option energy intake also cannot account for the results obtained, as energy intake was relatively high (~28 kcal) and low (~5 kcal) for the 64S and 14F options, respectively, and significant reductions were not obtained in either case. Finally, energy density cannot account for the results, as baclofen both reduced intake and had no significant effect on intake of options with the same energy density.
Baclofen (3.2 mg/kg) significantly reduced intake of the emulsion containing 28% fat (28F) and also reduced intake of pure vegetable shortening. This dose also tended to reduce intake of the 14% fat emulsion (14F), but not significantly. Notably, lower doses did not reduce intake of the fatty options. This is similar to a previous report in which doses of 0.6, 1.0, and 1.8 mg/kg (R-S)-baclofen did not reduce intake of semisolid emulsions comprised of 18% and 32% vegetable shortening, when they were provided in the same manner as here, i.e. for 1-hr intermittently (Rao et al., 2008). It is possible that a higher dose would have reduced intake in the Rao et al. (2008) study. The ability of baclofen to decrease intake of 100% shortening confirms results reported elsewhere (Buda-Levin et al, 2005; Corwin & Wojnicki, 2009).
The fact that baclofen reduced consumption of options containing sucrose here differs from the effects of baclofen in other reports. For instance, baclofen failed to reduce intake of 32% (w/w) powdered sugar mixed into pure vegetable shortening (Wong et al., 2009), and also failed to reduce intake of liquid sucrose solutions containing 3.2%, 10% and 32% sucrose (w/v) (Berner et al., 2009; Corwin & Wojnicki, 2009). In addition, baclofen stimulated the intake of liquid (Ebenezer et al., 1995) and solid (Berner et al., 2009) foods containing both fat and sugar in other reports. In the present study, baclofen significantly reduced intake of the options containing 32% sucrose (32S, 14F/32S), and tended to reduce intake of the option containing 64% sucrose (64S). There are several differences in the options used in the previous studies and those used here, including the fat content, the form (liquid vs. solid), and the type of sugar used. Why these differences would affect the ability of baclofen to reduce intake is not clear. However, these and other reports (Wang et al., 2011) provide compelling evidence that the composition of the food can profoundly alter the effects that baclofen has on consumption. This issue is explored further in Experiment 2.
EXPERIMENT 2A
INTRODUCTION
In Experiment 1, baclofen reduced, or tended to reduce, fat and/or sucrose intake regardless of the macronutrient composition or energy density of the option. The two options that baclofen failed to significantly reduce were the 64S and the 14F. The failure to reduce the 14F was similar to a previous report in which baclofen failed to reduce intake of an 18% semi-solid fat emulsion when provided intermittently for 1 hr (Rao et al., 2008). In that report, however, baclofen did reduce intake of the emulsion when it was provided daily, suggesting that the intermittent access protocol can attenuate the efficacy of baclofen in some cases. Thus, results with the 64S and 14F in Experiment 1 of the present report may have been due to the schedule of access. That is, baclofen may not have reduced intake of these two options because they were presented intermittently.
Since the Rao et al. (2008) report already examined how schedule of access affects the ability of baclofen to reduce fat emulsion intake, we examined the effects of access schedule on intake of the 64S option in Experiment 2A here. Two access schedules were used, one in which the optional food is given to the rats intermittently, as in Experiment 1, and another in which the optional food is provided for 1 h every day. Previous research has shown that the intermittent schedule induces binge-type eating relative to the daily schedule when vegetable shortening serves as the option (Babbs et al., 2012). However, even when intermittent and daily option intakes are similar, differences between the groups have been reported in behavioral and pharmacological studies (e.g. Corwin and Wojnicki, 2009; Rao et al., 2008; Wojnicki, et al., 2010). The response of rats maintained on these access schedules to pharmacological probes, therefore, has relevance not only for preclinical assessment of potential interventions for human binge eating, but also for people who may not binge, but who consume fatty and sugary foods every day as opposed to occasionally.
Additionally, it previously has been reported that baclofen failed to reduce liquid sucrose intake at concentrations ranging from 3.2-32% (w/v) regardless of access schedule (Corwin & Wojnicki, 2009). The inclusion of the 64S semi-solid sucrose product allowed us to verify the results of Experiment 1 and to determine if baclofen would reduce intake of a higher sucrose concentration under the access conditions that were used in our previous liquid sucrose studies. The 14F/32S product was used as the positive control in Experiment 2A, since baclofen significantly reduced its intake in Experiment 1, and since it has the same energy density as the 64S product. Rats were given 1 hr access to the options either intermittently, as in Experiment 1, or daily (7days/week). Based upon the Rao et al. (2008) study, we anticipated that daily access would enhance the effects of baclofen. That is, we anticipated that baclofen would reduce emulsion intake in the daily groups, but not in the intermittent groups.
Experiment 1 and previous studies have assessed the ability of baclofen to reduce intake of a single optional food (Berner et al., 2009; Buda-Levin et al., 2005; Corwin & Wojnicki, 2009). However, the relative preference rats have for one food over another can influence the effects of drugs on food intake (Taha 2010), and more than one fatty or sugary food is usually consumed in a single eating episode, such as a binge. Thus, we sought to determine if the effects of baclofen would be influenced by food preference. Therefore, in addition to the groups that only had access to a single optional food intermittently or daily, two additional groups were included in which both food options were provided simultaneously either intermittently or daily. Inclusion of the preference groups allowed us determine if relative preference contributes to the intake reducing effects of baclofen, while controlling energy density and availability. In addition, this allowed for further assessment of the contribution of macronutrient composition to the efficacy of baclofen.
As in our previous studies, chow was also available during baclofen testing. Previous studies have shown that baclofen either tends to increase or significantly increases chow intake. Hence, the simultaneous availability of chow served as a control to verify the biological activity of baclofen.
METHODS
Animals
Seventy two naive male Sprague Dawley rats (Harlan, Indianapolis, IN), 60 days of age and weighing 271-308 g (291 ± 0.81 g) at the start of the study were used. The rats were individually housed in hanging stainless steel wire cages in a temperature- and humidity-controlled environment placed on a 12:12 light:dark cycle. All rats were maintained on the same nutritionally complete rodent chow that was used in Experiment 1, with chow and tap water available ad libitum throughout, unless noted otherwise. The Pennsylvania State University Institutional Animal Care and Use Committee approved all procedures.
After seven days of adaptation to the vivarium, all rats were provided with overnight access to a 14% fat / 32% sucrose [14F/32S] emulsion. Two days later, all rats were then provided with overnight access to a 64% [64S] sucrose semi-solid product. Both of these were similar to those used in Experiment 1, except that a different biopolymer powder was used to thicken the two products (see description, below). Six groups of 12 rats each were then matched by intake of the two food options and body weight, [ps NS for all]. Four of the groups were assigned to either Daily (7 days/week) or Intermittent (Mondays, Wednesdays, and Fridays) 1 hr access to either the 14F/32S or the 64S optional foods. The groups were designated as D 14F/32S, D 64, I 14F/32S and I 64, respectively. After the assignment of each of these four groups, the above data were reanalyzed for these groups only in order to verify the matching of the groups [ps NS for all]. The remaining two groups were assigned to either Daily or Intermittent access to both of the optional foods, i.e., a preference condition. The groups were designated as DP for “Daily Preference” and IP for “Intermittent Preference”. After the assignment of these two groups, the above data were also reanalyzed to confirm that these two groups were still matched [ps NS for all]. One of the I 14F/32S rats died prior to baclofen administration.
Optional food products
The optional foods were each made with two different biopolymer blends supplied by the same manufacturer (TIC Gums, Belcamp MD) in a manner similar to that described for Experiment 1. One of these biopolymers was the same as that use in Experiment 1: TIC Action Gum® 10000EC (abbreviated “EC”), but the other was different: TIC Pretested® Aragum® 3173 (abbreviated “3173”). The 3173 powder was a blend of xanthan gum, propylene glycol alginate, and guar gum and was used as the primary emulsion thickener. The EC powder was a blend of guar gum and xanthan gum and was used as an additional thickener. Emulsions were prepared as stated above; amounts of the two biopolymer blends in each emulsion are shown in Table 1 (bottom panel).
Procedures
All six groups were provided with 1h access to their assigned optional food(s) under their respective access arrangement (Daily or Intermittent) for 6 weeks. After six weeks of emulsion access (R-S)-baclofen (Tocris, Ellisville, MO) was administered intraperitoneally (0.0, 1.0, 1.8, 3.2 mg/kg) 30 minutes prior to the intake tests. All rats received all doses, with dosing sequence assigned to each rat using a uniform Latin square. Injections were given on Mondays and Fridays.
Statistics
Data were analyzed using ANOVA as described above in Experiment 1.
RESULTS
Body weight prior to baclofen administration
There were no differences in body weights among the groups prior to baclofen administration [F(3,46) = 1.84, p < 0.1548].
Effects of baclofen on intake of the 3173 options and chow
Baclofen had no effect on intake of the optional foods relative to vehicle in any of the groups (Figure 2, top panel; ps > 0.05). Despite the lack of effect on consumption of the optional foods, baclofen significantly increased chow intake in the I 14/32 group, and in the IP group indicating that the drug was biologically active (Figure 2, bottom panel; ps < 0.05).
Figure 2.
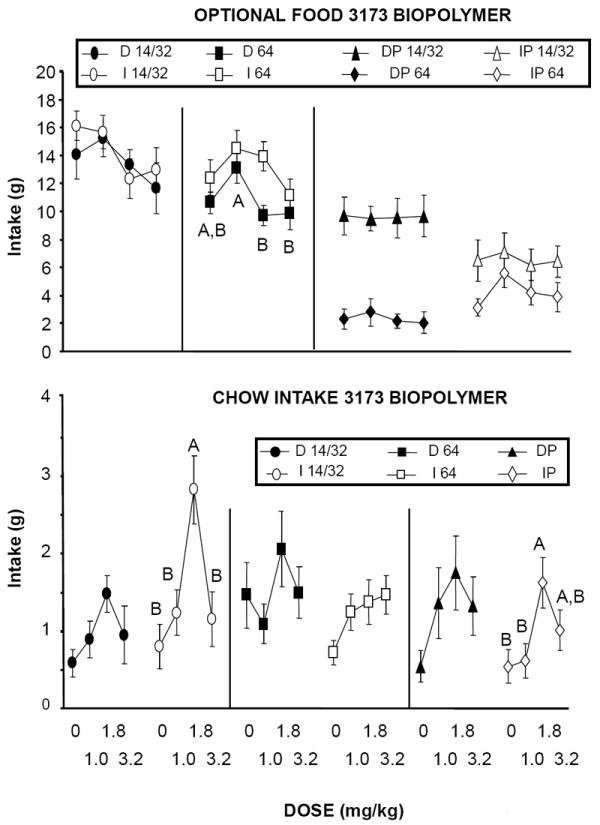
(Top panel): Effects of baclofen on 1-hr intake of different optional foods (see text for concentrations) when prepared with the 3173 biopolymer. Optional foods were provided either on an intermittent (I), or daily (D) schedule of access alone (D 14/32, I 14/32, D 64, I 64), or simultaneously (IP, DP). Different letters indicate differences within groups in intake as a function of dose. (Bottom panel): Effects of baclofen on chow intake during the 1-hr test session (chow was simultaneously available with the optional food). Different letters indicate differences within groups in intake as a function of dose.
Rats in both the DP and IP groups preferred the 14F/32S option to the 64S option. 2-way ANOVA revealed a main effect of group [F(1,22 = 8.18, p 0.0091] due to a greater overall preference for the 14F/32S option in the DP group than in the IP group. There was no effect of baclofen dose on the preference ratio [F(3,66) = 1.64, p NS], and no group by dose interaction [F(3,66) = 0.35, p NS].
DISCUSSION
Baclofen failed to decrease intake of both the 14F/32S and the 64S options in all groups. The effect of baclofen on intake of the 64S option replicates what was reported in Experiment 1. That is, there was a tendency for low doses of baclofen to stimulate intake and for the highest dose to reduce intake when 64S was the only option available (Figure 2, top middle panel). However, like Experiment 1, none of these effects achieved statistical significance relative to saline. When a choice between options was provided, baclofen also had no significant effect. The results from this Experiment indicate that providing a high sugar option on a daily or intermittent basis or in a choice situation does not enhance baclofen’s efficacy.
In contrast to the 64S results, the effects of baclofen on intake of the 14F/32S option were different from those obtained in Experiment 1. Specifically, baclofen significantly reduced intake of the 14F/32S option in Experiment 1, but had no effect in Experiment 2A. This was not due to the manner in which it was presented, as a group with intermittent access was included in both experiments. Furthermore, allowing rats to choose between the two options did not alter the outcome: baclofen was ineffective when the 14F/32S was highly preferred (DP) or only slightly preferred (IP), relative to the 64S.
One factor that differed between Experiments 1 and 2A was the biopolymer used to make the optional foods. In Experiment 1, 515 was used, whereas in Experiment 2A, 3173 was used. In a previous report we showed that baclofen reduced intake of a 32% vegetable shortening emulsion made with 3173 that was provided daily for 1 hr (Wang et al., 2011). It is possible that the sucrose in the present options contributed to the reduced efficacy of baclofen, as another study showed that increasing concentrations of sucrose mixed into vegetable shortening attenuated the intake reducing effects of baclofen (Wong et al., 2009). Even so, that does not explain why baclofen reduced the intake of 14F/32S in Experiment 1, but not in Experiment 2A.
Wang et al. (2011) reported that biopolymers can alter the effects of baclofen on intake. In that report, fat emulsions made with several different biopolymer blends were tested. A key finding was that the efficacy of baclofen was enhanced when starch was a component of the blend. Although neither the 515 nor the 3173 contain starch in the present studies, differences between the two biopolymers may have contributed to the differential effects of baclofen. Therefore, Experiment 2B was undertaken to determine if the biopolymer accounted for the negative results obtained in Experiment 2A.
EXPERIMENT 2B
METHODS
The same rats that were used in Experiment 2A were used in Experiment 2B. Following baclofen administration in Experiment 2A, all emulsions were prepared with the 515 powder, as described in Experiment 1. All rats received their respective emulsions for two weeks. Saline and baclofen (3.2 mg/kg) were then administered intraperitoneally in a counter-balanced manner 30 minutes prior to the intake tests. Data were analyzed as described in the previous experiments, except that paired t-tests were used to compare intakes when products were made with the 3173 biopolymer vs. the 515 biopolymer, and to compare effect of baclofen (3.2 mg/kg) on intake to that of saline.
RESULTS
Body weight prior to baclofen administration
There were no differences in body weights among the groups prior to baclofen administration.
Intake of the 515 options compared to intake of the 3173 options
Paired t-tests comparing intakes during the saline test sessions showed that when the 14F/32S product was made with the 515 biopolymer, intakes were significantly greater than when the 14F/32S product was made with the 3173 biopolymer in all groups whether analyzed as energy normalized to body weight (Heusner, 1985) or gram intake [ps < 0.05] (Figure 3). In contrast, when the 64S product was made with the 3173 biopolymer, intakes were no different for the D64 and I64 groups than when it was made with the 515 biopolymer whether analyzed as energy normalized to body weight or gram intake [ps > 0.05]. Further, in the groups given a choice between options (DP and IP groups), intakes of the 64S product were significantly lower when made with the 515 biopolymer than when made with the 3173 biopolymer whether analyzed as energy normalized to body weight or gram intake[ps < 0.05]. Thus, the preference in the DP and IP groups was shifted away from the 64S toward the 14F/32S when the options were made with 515, due to the simultaneous stimulation of 14F/32S and reduction of 64S (preference ratio t-tests comparing DP and IP groups: ps < 0.05). Intake of the simultaneously available chow was unaffected by changing the biopolymer to 515 (data not shown).
Figure 3.
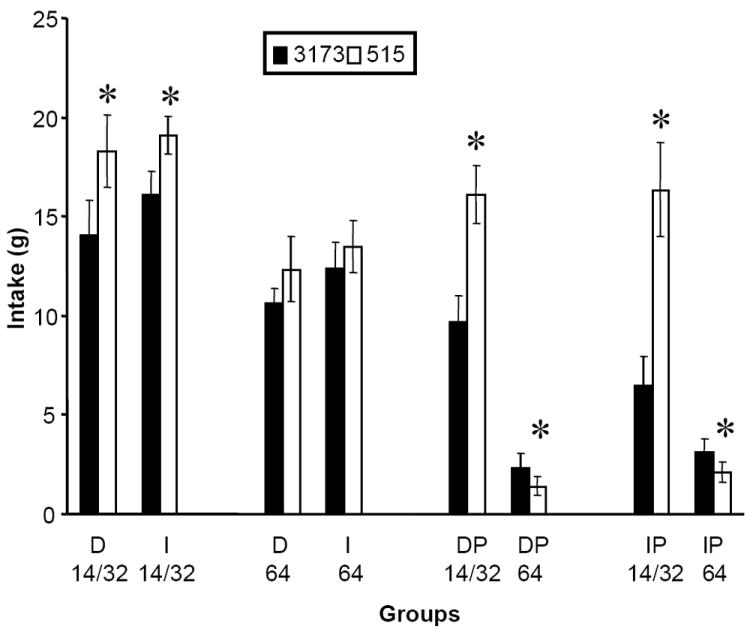
Intakes of different optional foods made with the 3173 and 515 biopolymers during the saline test sessions. Group designations are as described for Figure 2. Asterisks indicate significant differences in intakes within groups between each of the biopolymers (p < 0.05)
Effects of baclofen on intake of 515 options and chow
Paired t-tests comparing baclofen (3.2 mg/kg) to saline showed that baclofen decreased intake of the 14F/32S product in the D 14/32, I 14/32 and IP 14/32 groups (Figure 4, top panel; ps < 0.05). In contrast, baclofen had no effect on consumption of the 64% sucrose semi-solid product in any of the groups. Unlike the results obtained in Experiment 2A, preference ratio was significantly altered by baclofen in Experiment 2B, primarily due to the reduction in 14F/32S intake [main effect of dose: F(1,22) = 5.30, p 0.0312]. Thus, baclofen reduced preference for the 14F/32S option relative to the 64S. Similar to the results obtained in Experiment 2A, 2-way ANOVA revealed a main effect of group [F(1,22 = 4.63, p 0.0426], due to a greater overall preference for the 14F/32S option in the DP group than in the IP group. There was no group by dose interaction.
Figure 4.

(Top panel): Effects of baclofen on 1-hr intake of different optional foods (see text for different concentrations) when prepared with the 515 biopolymer after a history of consuming the same optional foods made with the 3173 biopolymer. Group designations are as described for Figure 2. Asterisks indicate significant differences in intakes within groups after baclofen (3.2 mg/kg) or saline administration (p < 0.05). (Bottom panel): Effects of baclofen on chow intake during the 1-hr test session (chow was simultaneously available with the optional food). Asterisks indicate significant differences in intakes within groups after baclofen (3.2 mg/kg) or saline administration (p < 0.05).
Baclofen (3.2 mg/kg) significantly increased 1 hr chow intake in the D 14/32, DP and IP groups (Figure 4, bottom panel; ps < 0.05).
DISCUSSION
When the options were made with the 515 biopolymer instead of the 3173 biopolymer, consumption of and preference for the 14F/32S product increased. In contrast, intake of the 64S product decreased in the rats given a choice, and there was no change in consumption of the 64S product when it was the only option available. The reason intake of the 64S did not increase is not clear, but may have been due to the large osmotic load produced by that product. No sign of illness was noted in the rats consuming 64S, but additional consumption may not have been possible without producing adverse effects.
In addition, baclofen decreased intake of the 14F/32S product, but not the 64S product. It appears that baclofen does not reduce consumption of a highly concentrated sucrose product regardless of access conditions or which biopolymer is used to thicken the product. In contrast, the 515 biopolymer appears to enhance the ability of baclofen to reduce consumption of an option containing fat. Baclofen reduced intake of the 14F/32S product when it was made with 515 in Experiment 2B, which replicated results obtained in Experiment 1. Baclofen was equally effective when the 14F/32S was presented daily and when presented intermittently, as the only option. When rats were allowed to choose between the two options, baclofen reduced preference for the fatty 14F/32S option, and significantly reduced intake of the 14F/32S in the intermittent (IP) group.
Significant reductions were not just an artifact of the statistical analysis used. When results of Experiment 2A were subjected to paired t-test analysis (3.2 mg/kg vs. saline), results from that experiment were still not significant (data not shown). In short, the biopolymer that was used to make the optional foods affected the ability of baclofen to reduce the intake of a fatty food.
Baclofen either significantly stimulated chow intake or had no effect, again demonstrating that reductions in option intake were not due to sedation. Furthermore, the intake reducing effects of baclofen were minimally affected by the manner in which the options were presented, if at all.
GENERAL DISCUSSION
The studies reported here were designed to address some of the questions regarding the effects of baclofen on food intake. Several different semi-solid optional food products were developed in order to determine if energy density, food composition (fat, sugar), relative preference, manner of presentation, and baseline intake affected baclofen’s efficacy. Baclofen reduced intake of optional foods that had different energy densities (1.28-9 kcal/g), that contained both fat and sugar, that were presented either daily or intermittently, and that induced relatively high and low intakes. Furthermore, relative preference did not systematically influence results obtained with baclofen. However, the efficacy of baclofen was affected by the biopolymer used to thicken the options. Specifically, baclofen had no significant effect when the options were made with the 3173 biopolymer, but reduced intake of options made with the 515 biopolymer.
The results of the present study confirm a previous report (Wang, et. el., 2011) showing that the constituents that comprise a biopolymer can alter the effects of baclofen on intake. In the Wang et al., (2011) study, baclofen failed to decrease intake of a 32% fat emulsion made with a different biopolymer than those used here. However, when 4% starch was added to the emulsion, baclofen decreased emulsion intake. This confirmed the results of another study (Rao, et al., 2008) in which baclofen reduced intake of emulsions made with a biopolymer containing 4% starch. In the present study, none of the biopolymers contained starch. Regardless, the constituents of the biopolymer itself altered baclofen’s ability to decrease option intake.
The biopolymers used in the present research (10000 EC, 515 M-T, Aragum 3173) are comprised primarily of nondigestible polysaccharides (gums) and are used in foods containing fat and sucrose manufactured for human consumption, many of which are foods upon which people binge. For instance, 515 is used in bakery products, icings, co-extruded doughs, glazes, and fruit fillings. Aragum 3173 is used in bakery emulsions. Action Gum 10000 EC is used in sauces, beverages, relishes, bakery products, gravies, soups, prepared meals, and frozen foods. These biopolymers are also useful for the development of model test foods containing fat and sucrose for basic ingestive behavior research in rats. While these biopolymers are purported to be tasteless to humans, it is possible that they are detectable to rats in a manner similar to that of digestible polysaccharides such as starches and glucose (Ramirez, 1991a,b,c,d, 1992, 1993; Sclafani, 1987). For instance, propylene glycol, which is a component of the 3173 biopolymer, elicits electrophysiological and behavioral responses similar to those elicited by bitter (quinine) and sweet (sucrose) tastants in rats (Sako and Yamamoto, 1999). The bitter component may have attenuated intake of the products made with 3173 relative to the intake of products made with 515. In addition, the different gums that comprised 3173 and 515 likely produced different orosensory effects (e.g. “mouthfeel”) that may have contributed to the intake differences.
The factors that account for the influence of the two biopolymers on baclofen efficacy is unknown. Baclofen has been reported to have similar effects on solid and liquid food intake in other studies (Ebenezer 1995). Thus, subtle rheological differences between products made with different biopolymers probably did not contribute significantly to the differential effects of baclofen reported here. Other factors such as flavor or taste alterations, however, may have contributed. For instance, available evidence strongly suggests that GABA, acting at GABA-B receptors, functions as an inhibitory neurotransmitter within the rat taste bud and may serve to modulate bitter and sweet taste (Cao et al., 2009). Furthermore, at doses comparable to those used here, baclofen has been reported to disrupt the ability of rats to discriminate different concentrations of solutions sweetened with saccharin (Wilson et al., 2011). Thus, differences in taste receptor dynamics induced by the biopolymers, possibly due to the presence of propylene glycol in the 3173, may account for the differential effect of baclofen on their intake.
The use of biopolymers to make different semi-solid fat emulsions for research purposes overcomes the difficulties of phase separation found in liquid oil-in-water emulsions. Despite this advantage, it appears that the use of different biopolymers (thickeners) presents a unique problem itself. Specifically, the ingredients that comprise the biopolymers not only affect the amount of optional food consumed, but also alter the effects of the GABA-B agonist baclofen on this intake. Another difficulty in using biopolymers is that each has its own thickening properties and the ability to accommodate fat and sucrose. This laboratory has tested other biopolymers from the same manufacturer in pilot studies. While rats will consume the emulsions and semi-solid products, each biopolymer was found each to have unique properties. For example, the Ticalose CMC 6000 (cellulose gum) biopolymer will not accommodate fat concentrations of 56% and above, while the Aragum 3173 and Ticaloid 515 M-T will. Both Ticagel Bind-KX (Konjac, xanthan gum) and Tacagel Konjac High Viscosity (Konjac) biopolymers impart an odor to the emulsion and we therefore have not thoroughly tested them. The Ticaloid 1155 FF (gum acacia, guar gum, and xanthan gum) biopolymer and the Ticaloid 210 S (gum acacia, xanthan gum) biopolymer do not thicken for the purposes of this research under certain fat and sucrose concentrations. In light of the above results and those of Rao et al, (2008) and Wang et al., (2011), caution should be taken in interpreting results of studies when products made with different biopolymers are used. Systematic research is needed when designing emulsion and semi-solid products for use in rat models of food intake. Further, definitive statements regarding why baclofen reduces intake of some foods while having no effect on or stimulating intake of others still cannot be made.
RESEARCH HIGHLIGHTS.
Semi-solid test foods with fat and sugar matched for energy density were used.
Effects of baclofen were determined under a variety of conditions.
Unexpectedly, the biopolymer used to thicken the foods altered intake and baclofen efficacy.
Acknowledgments
The authors express their sincere thanks and appreciation to Tim Andon at TIC Gums for his continued advice, support and generous donations of the biopolymers. Support for this study by MH67943 (RLC). The views expressed are those of the authors of this paper and not that of The San Antonio Uniformed Services Health Education Consortium (SAUSHEC), the United States Army, the United States Department of Defense or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babbs RK, Wojnicki FH, Corwin RL. Assessing binge eating. An analysis of data previously collected in bingeing rats. Appetite. 2012;59:478–82. doi: 10.1016/j.appet.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner LA, Miriam E, Bocarsly ME, Hoebel BG, Avena NM. Baclofen suppresses binge eating of pure fat but not a sugar rich or sweet-fat diet. Behav Pharmacol. 2009;20(7):631–34. doi: 10.1097/FBP.0b013e328331ba47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broft AI, Spanos A, Corwin RL, Mayer L, Steinglass J, Devlin MJ, Attia E, Walsh BT. Baclofen for binge eating : an open-label Trial. Int J Eat Disorder. 2007;40:687–91. doi: 10.1002/eat.20434. [DOI] [PubMed] [Google Scholar]
- Buda-Levin A, Wojnicki FHE, Corwin RLW. Baclofen reduces fat intake under binge-type conditions. Physiol Behav. 2005;86:176–84. doi: 10.1016/j.physbeh.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhao FL, Kolli T, Hivley R, Herness S. GABA expression in the mammalian taste bud functions as a route of inhibitory cell-to-cell communication. Proc Natl Acad Sci USA. 2009;106:4006–11. doi: 10.1073/pnas.0808672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Boan J, Peters KF, Ulbrecht JS. Baclofen reduces binge eating in a double-blind, placebo-controlled, crossover study. Behav Pharmacol. 2012;23(5-6):616–25. doi: 10.1097/FBP.0b013e328357bd62. [DOI] [PubMed] [Google Scholar]
- Corwin RLW, Wojnicki FHE. Baclofen, raclopride and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behavioral Pharmacology. 2009;20(5–6):537–548. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- Czyzyk TA, Sahr AE, Statnick MA. A model of binge-like eating behavior in mice that does not require food deprivation or stress. Obesity (Silver Spring) 2010;18:1710–17. doi: 10.1038/oby.2010.46. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS. Intraperitoneal administration of baclofen increases consumption of both solid and liquid diets in rats. Euro J Pharmacol. 1995;273:183–85. doi: 10.1016/0014-2999(94)00707-e. [DOI] [PubMed] [Google Scholar]
- Heusner AA. Body size and energy metabolism. Ann Rev Nutr. 1985;5:267–93. doi: 10.1146/annurev.nu.05.070185.001411. [DOI] [PubMed] [Google Scholar]
- Lucas F, Ackroff K, Sclafani A. Dietary fat-induced hyperphagia in rats as a function of fat type and physical form. Physiol Behav. 1987;45:937L–46. doi: 10.1016/0031-9384(89)90218-7. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Relative preference for starch and sugar in rats. Physiol Behav. 1993;54:1195–1200. doi: 10.1016/0031-9384(93)90348-j. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Is starch flavor Unitary? Evidence from studies of cooked starch. Physiol Behav. 1992;52:535–40. doi: 10.1016/0031-9384(92)90343-z. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Chemoreception for an insoluble nonvolital substance: starch taste? American Journal of Physiology-Regulatory Integrative Comparative Physiology. 1991a;260(29):R192–R199. doi: 10.1152/ajpregu.1991.260.1.R192. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Does starch taste like polycose. Physiol Behav. 1991b;50:389–92. doi: 10.1016/0031-9384(91)90083-z. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Thresholds for starch and Polycose are lower than for sucrose in rats. Physiol Behav. 1991c;50:699–703. doi: 10.1016/0031-9384(91)90005-9. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Starch flavor: apparent discrimination between amlyopectin and amylose by rats. Physiol Behav. 1991d;50:1181–86. doi: 10.1016/0031-9384(91)90580-h. [DOI] [PubMed] [Google Scholar]
- Rao RE, Wojnicki FHE, Coupland J, Ghosh S, Corwin RLW. Baclofen, raclopride, and naltrexone differentially reduce solid fat emulsion intake under limited access conditions. Pharmacol Biochem Behav. 2008;89(4):581–590. doi: 10.1016/j.pbb.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako N, Yamamoto T. Electrophysiological and behavioral studies on taste effectiveness of alcohols in rats. Am J Physiol. 1999;276:R388–96. doi: 10.1152/ajpregu.1999.276.2.R388. [DOI] [PubMed] [Google Scholar]
- Sato I, Arima H, Ozaki N, Ozaki N, Watanabe M, Goto M, et al. Peripherally administered baclofen reduced food intake and body weight in db/db as well as diet-induced obese mice. FEBS Lett. 2007;581:4857–64. doi: 10.1016/j.febslet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Carbohydrate taste, appetite, and obesity: an overview. Neuroscience and Biobehavioral Reviews. 1987;11:131–53. [PubMed] [Google Scholar]
- Taha SA. Preference or fat? Revisiting opioid effects on food intake. Physiol Behav. 2010;100:429–37. doi: 10.1016/j.physbeh.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GN, Biesan OR, Remus JL, Mickley GA. Baclofen alter gustatory discrimination capabilities and induces a conditioned taste aversion (CTA) BMC Res Notes. 2011;4:527. doi: 10.1186/1756-0500-4-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FHE, Babbs RK, Corwin RLW. Reinforcing efficacy of fat, as assessed by progressive ratio responding, depends upon availability not amount consumed. Physiol Behav. 2010;100:316–21. doi: 10.1016/j.physbeh.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FHE, Roberts DCS, Corwin RLW. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacol, Biochem Behav. 2006;84:197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wilt DC, Wojnicki FHE, Babbs RK, Coupland JN, Corwin RLW. Fat emulsion composition alters intake and the effects of baclofen. Appetite. 2011;57:628–34. doi: 10.1016/j.appet.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KJ, Wojnicki FHE, Corwin RLW. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol Biochem Behav. 2009;92(3):528–536. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


