Abstract
CD-68 is widely regarded as a selective marker for human monocytes and macrophages and is commonly used in human pathology studies. The purpose of this study was to investigate the expression of CD-68 in human peripheral blood mononuclear cells (PBMCs), neutrophil granulocytes (NGs) and in inflamed intestinal tissue samples for comparison. PBMCs and NGs were isolated from heparinized human blood samples. Intestinal biopsies were obtained during routine endoscopic procedures from patients with inflammatory bowel disease (IBD), e.g. ulcerative colitis and Crohn’s disease. Gene and protein expression was analyzed by real-time RT-PCR, Western blot and immunohistochemistry. Both PBMCs and NGs preparations contained cells that were positive for CD-68 and either neutrophil elastase (NE), or myeloperoxidase (MPO). CD-68+/NE-/MPO- cells were regarded as monocytes. CD-68 mRNA expression was detected in PBMCs and NGs preparations. With Western blot and by performing immunoprecipitation of cell lysate, we could clearly detect CD-68 in NGs, U-937, THP-1, Hep-G2, Jurkat cells and PBMCs. Identification of inflammatory cells in acutely inflamed colonic mucosa obtained from patients with IBD revealed a strong accumulation of CD-68+/MPO+ cells compared to normal colonic mucosa. The uptake of the marker by phagocytosis was excluded by performing a double staining with CD-163/NE and CD-163/MPO in PBMCs, NGs cultures and in inflamed colonic mucosa. These results identify CD-68+ NGs in peripheral blood and inflamed colonic mucosa. CD-68 is not only a marker for the macrophages-monocytes but also for NGs.
Keywords: CD-68, inflammatory bowel disease, monocytes, neutrophil granulocytes, peripheral blood mononuclear cells
Introduction
Cells that differ in function have distinct molecular structures in their membranes. These structures can serve as markers for particular cell types and can be recognized by specific antisera against the markers. Specific antibodies against membrane markers of human cells have a number of current and potential uses [1].
Five antibodies, Y1/82A, Y2/131, EBM11, Ki-M6 and Ki-M7, within the myeloid panel of reagents were recognized as markers of the majority of human tissue macrophages present in tissue sections [2-6]. This group of antigens was designated as CD-68, based on the results of Micklem et al. [7]. Specific CD-68 is reported to be present in a variety of tissue macrophages, including Kupffer cells, germinal center macrophages, alveolar macrophages and bone osteoclasts [8].
CD-68 (the human homologue of mouse macrosialin) is a heavily glycosylated, 110 kDa membrane protein. Although it is predominantly located in lysosomal membranes, a small fraction is also found on the cell surface [9,10].
However, in contrast to the generally accepted concept of monocyte/macrophage specificity, many authors have reported some reactivity of anti-human CD-68 antibodies with antigens present on the cell surface of various hematopoietic and non-hematopoietic cells [11-13]. Recently published reports demonstrated both at the RNA and protein level that CD-68 was not only expressed in macrophages and monocytes but also expressed by non-myeloid cell types, such as T cells, fibroblasts, endothelial and tumor cells [14,15].
Recently it has been demonstrated that the increased expression of MPO in injured rat and human liver is due to the presence of newly recruited neutrophil granulocytes (NGs) as no MPO expression was evident in Kupffer cells [16]. These reports negate the traditional view that the CD-68 antigen is specific for macrophages. If the CD-68 antigen is not expressed solely in macrophages but also by NGs, the finding of MPO in CD-68+ cells is explained. This is important especially when physiology of intestinal immune system is studied [17].
In acute tissue injury, the initial inflammatory cell influx consists predominantly of NGs, which in turn orchestrate the recruitment of monocytes and the activation of lymphocytes required for a mature inflammatory response. Inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC), are characterized by an excessive recruitment of leukocytes from the blood circulation into the inflamed gut wall. During acute flares of UC or CD there is a massive infiltration of NGs into the affected mucosa, which is manifested clinically by an increase in stool NGs. In IBD there is also a marked infiltration of macrophages into the inflamed mucosa, manifested by an increase in macrophage-derived inflammatory cytokines IL-1β, IL-6 and TNF-α during acute flares [18]. IBD are characterized by a dense infiltration of tissue by CD-68+ macrophages compared to non-inflamed colonic mucosa [19,20].
This study investigated whether CD-68 positive NGs is found in peripheral blood and inflamed colonic mucosa of IBD patients.
Methods
Antibodies
A rabbit polyclonal antibody directed against human MPO was purchased from Dako (Hamburg, Germany), a rabbit polyclonal antibody against human neutrophil elastase from Calbiochem (Germany, Cat. 481001-1ML) and a mouse monoclonal antibody directed against human CD-68 (Clone KP-1, Cat. sc-20060) from Santa Cruz (CA, USA) for Western blot analysis. Results were confirmed with a second mouse monoclonal antibody anti-human CD-68 (Clone KP-1, Cat. ab955, Abcam, Cambridge, UK). For immunohistological analysis, a monoclonal anti-human CD-68 mouse antibody (Clone PG-M1, Cat. M087601) was bought from Dako. Results were confirmed with a second anti-human CD-68 mouse monoclonal antibody (Clone KP-1, Cat. ab955, Abcam, Cambridge). A monoclonal mouse anti-human CD-11 b-c mouse antibody was purchased from DAKO (Cat. M0741), while the monoclonal mouse anti-human CD-163 antibody (5C6-FAT, Cat. BM4041) came from Novus Biologicals (Cambridge, UK).
Isolation and cell culture condition of human PBMCs and NGs
PBMCs and NGs were isolated from heparanized blood of healthy donors (n=3) by Ficoll density gradient centrifugation and dextran sedimentation. Residual red blood cells were hypotonically lysed and cells were washed three times with phosphate-buffered saline (PBS) pH 7.3. Cell preparations were routinely assessed for viability (>95%) by trypan blue exclusion. Cells were stimulated in vitro with phytohaemagglutinin (PHA, 5 μg/ml [Roche Molecular Biochemicals, Mannheim, Germany]) or lipopolysaccharide (LPS, 1 μg/ml [Sigma-Aldrich Chemie, Taufkirchen, Germany]) for 2, 4 and 8 hours as previously described [21].
Isolation of RNA and real-time quantitative RT-PCR
Isolation of RNA, cDNA synthesis and real-time RT-PCR were performed as previously described [22]. Real-time PCR analysis of cDNA was performed at 60°C to 95°C for 45 cycles in the Sequence Detection System of ABI Prism 7000 (Applied Biosystems, Darmstadt, Germany) following the manufacturer’s instructions and by using SYBR Green Reaction Master Mix (ABI Prism; Applied Biosystems) and the primers reported in Table 1. All primers were synthesized by Invitrogen (Groningen, Netherlands). In every sample, β-actin was taken as the housekeeping gene. Fold change expression was calculated from the threshold cycle (Ct) values. For calculation of the relative changes, gene expression measured in unstimulated cells at 0h was set at unity.
Table 1.
Human primer sequences used for RT-PCR
| Primer | Forward | Reverse |
|---|---|---|
| MPO | 5′-TCGTCAGAACCAAATTGCAG-3′ | 5′-ATGTTCAGAGCAGGCAGGTC-3′ |
| NE | 5′-GCATCTTCGAAAACGGCTAC -3′ | 5′-GACCCGTTGAGCTGGAGAAT-3′ |
| CD-68 | 5′-TCAGCTTTGGATTCATGCAG-3′ | 5′-TTGTACTCCACCGCCATGTA-3′ |
| β-actin | 5′- CTGGAACGGTGAAGGTGACA -3′ | 5′- GTCCTCGGCCACATTGTGA -3′ |
Cytospin preparation and immunofluorescence
Cytospin preparation and immunofluorescent staining of PBMCs and NGs were performed as described previously [21]. Double-staining was performed according to an established protocol [23]. The primary antibodies were incubated with the cytospins preparations overnight at 4°C. Following a short washing step in phosphate buffered saline (PBS), an incubation was carried out with Alexa-Fluor® conjugated goat anti-rabbit and anti-mouse secondary antibodies (1:200; Molecular Probes, Germany) at room temperature for 1 h and the cytospin preparations were washed three times for 5 min in PBS. Finally, the nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI), and the sections were washed and mounted.
Intestinal tissue sections (paraffin embedded)
Biopsy samples of the colon were obtained from six patients (3 men, 3 women) with CD, six patients with UC (4 men, 2 women) and three control subjects without detectable colonic disease (2 men, 1 women). The control biopsies were obtained from mucosa without any macroscopic evidence of inflammation (confirmed at the microscopic level) in patients who underwent elective colonoscopic screening for cancer. The IBD patients had clinical and endoscopical signs of acute inflammation. IBD patients had clinical and endoscopic signs of acute inflammation. All patients gave written informed consent to participate in the study in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and the ethics committee of the University Medical Center. The diagnosis of IBD was based on clinical, radiological, endoscopic and histopathologic criteria. The specific indication for the colonoscopic examination in the IBD patients was a lack of efficacy of their therapy. As a result, all biopsies were obtained from patients with active mucosa inflammation during an acute flare of the disease. The assessment of the severity of the mucosal disease was based on macroscopic and histologic findings. In the IBD group, two patients had distal colitis, two proctitis, and two had pancolitis. The duration of the IBD ranged from 6 to 22 years (mean, 9.5 years). Serum C-reactive protein (CRP) levels were determined utilizing standard clinical laboratory procedures and ranged from 2 to 28.3 mg/L (mean, 8.2 mg/L). All of the CRP levels in the control subjects were within the normal laboratory range (<2 mg/L). The number of biopsy specimens taken from a given patient ranged from 1 to 3 (mean, 1.6 specimens). Specimens for RNA analysis were immediately snap-frozen in liquid nitrogen and stored at -80°C until being used.
Protein extraction, western blot analysis and immunoprecipitation
Protein extraction and Western blot analysis using PBMCs and NGs, but also THP-1, U-937, Hep-G2 and Jurkatt cells were performed as previously described [21]. The immunodetection studies were performed according to the ECL Western blotting protocol of GE Healthcare (Germany). The primary rabbit polyclonal antibodies to MPO and NE were used at 1:100 dilution, whereas the mouse monoclonal anti-CD-68 was used at a 1:50 dilution. In addition, 500 μg of total proteins were immunoprecipitated from cell lysates of NGs utilizing the CD-68 antibody at 4°C overnight with rotation and then incubated with 40 μl of protein A/G-agarose (Santa Cruz Biotechnology) beads for 4 hours at 4°C with rotation. The collected beads were washed four times with lysis buffer and resuspended in sample buffer.
Immunoprecipitation of cells lysate was performed as described previously [24]. In order, NGs (unstimulated and after 12h treatment with TNF-α), U-937, THP-1, Hep-G2, Jurkatt cells and PBMCs (unstimulated and after 12h stimulation with PHA or LPS) were subjected to SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes followed by Western blot analysis for CD-68. For immunoprecipitation of cell lysates a rabbit polyclonal anti-CD68 antibody (Cat. Ab63896, Abcam), at a 1:1000 dilution and with a predicted band of 42kDa, was used.
Statistical analysis
The data were analyzed with Prism Graph Pad 5 software (San Diego, USA). All experimental errors are shown as SEM. Statistical significance was calculated using Student’s t-test, one-way analysis of variance, and Dunnett’s post hoc tests. Significance was defined at a level of P<0.05.
Results
In vitro identification of CD-68+/NE+, CD-68+/MPO+, CD-11b-c+/NE+ and CD-163+/NE+ in activated human PBMCs and NGs by immunofluorescence double staining
After 4 h of stimulation with PHA or LPS as well as in unstimulated PBMCs, 20% of the total cell populations was found to be CD-68+/NE+ (Figure 1A), and CD-68+/MPO+ (Figure 1B). Two different CD-68 antibodies (monoclonal mouse Clone PG-M1, Dako and monoclonal mouse Clone KP-1, Abcam) yielded the same result although only results with the clone PG-M1 (Dako) are reported. As the staining procedures identified positivity for the NGs markers, MPO and NE, they clearly demonstrate that a portion of the PBMCs cell population contains some NGs. In the present work, these cells are shown to have strong CD-68 expression.
Figure 1.
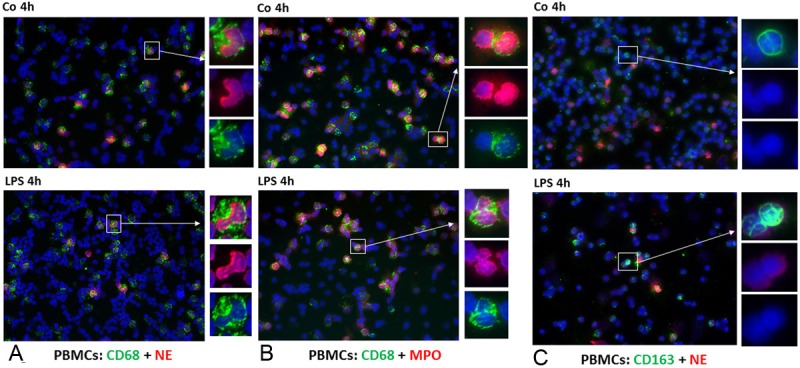
Double staining of PBMCs cytospins with monoclonal antibody directed against CD-68 and CD-163 (green), polyclonal antibody against NE and MPO (red), followed by fluorescence immunodetection. CD-68+/NE+ cells in control cultures and 4h after stimulation with LPS (Figure 2A). CD-68+/MPO+ and CD-163+/NE+ PBMCs in control cells and 4h after stimulation with LPS (Figure 2B and 2C) (Original magnification: x200. Scale bar= 100 μm).
As a control, a NG preparation was studied. About 85% and 90% of these cells stained positive for NE and MPO, respectively. Double staining with CD-68 and NE (Figure 2A) or MPO (Figure 2B) revealed that 20% of the cells were positive for both markers. As CD-11 b-c is a marker for leukocytes involved in the innate immune system, including monocytes, granulocytes, macrophages and natural killer cells, the present findings were confirmed by performing immunofluorescence staining of human NGs using antibodies against CD-11 b-c and NE (Figure 2C). As shown in Figure 2C, 50% of the NGs are positive for both markers.
Figure 2.
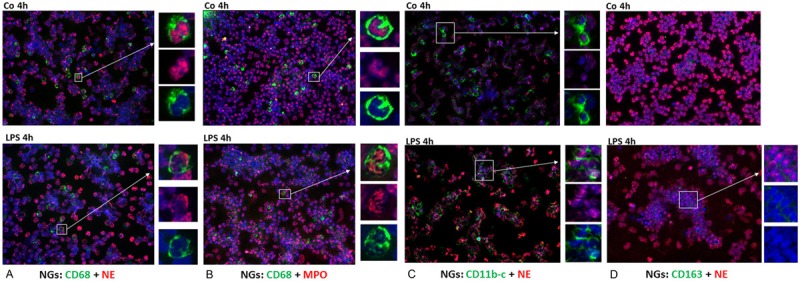
Double staining of NGs cytospins with monoclonal antibody directed against CD-68 (green) and polyclonal antibody against NE (red) followed by fluorescence immunodetection. CD-68+/NE+ cells in control cultures and 4h after stimulation with LPS (Figure 2A). CD-68+/MPO+ NGs in control cultures and 4h after stimulation with LPS (Figure 2B). Double staining of NGs cytospins with polyclonal antibody directed against NE (red) and monoclonal antibody against CD-11b-c and CD-163 (green) followed by fluorescence immunodetection. NE+/CD-11 b-c+ (Figure 2C) and NE+/CD-163+ (Figure 2D) NGs in control cultures and 4h after stimulation with LPS (Original magnification: x200. Scale bar= 100 μm).
To exclude the uptake of NE+ cells by phagocytosis, we stained the specific marker of the monocyte/macrophage lineage CD-163 in human PBMCs and NGs, and we clearly showed that NE was not detectable in CD-163+ cells (Figures 1C and 2D).
Identification of CD-68+/MPO+ and CD-68+/NE+ in activated human PBMCs and NG by western blot and immunoprecipitation
To determine the amount of MPO-, NE- and CD-68-protein in human PBMCs and NGs preparations, Western blot analyses were carried out. The molecular weight of MPO is 59 kDa. Figure 3A shows a constitutive expression of MPO in human NGs, which decreased after stimulation with LPS. As the next step, we performed an immunoprecipitation analysis of cells lysates from human NGs with a mouse monoclonal antibody directed against human CD-68 (clone KP1, Abcam). Figure 3B shows that KP-1 detects a band of approximately 110 kDa in stimulated and control NGs, although the expression level did not differ between control and stimulated cells. In order to confirm our results, we used U-937 and THP-1 cell lines as a positive control and we were able to detect the same 110 kDa band in both of them (Figure 3B). This is consistent with mRNA expression data (Figure 4) where we show a constitutive significant expression of CD68 mRNA transcripts in human NGs and, at a higher level, in PBMCs. Figure 3C shows MPO expression at protein level in unstimulated and stimulated PBMCs.
Figure 3.
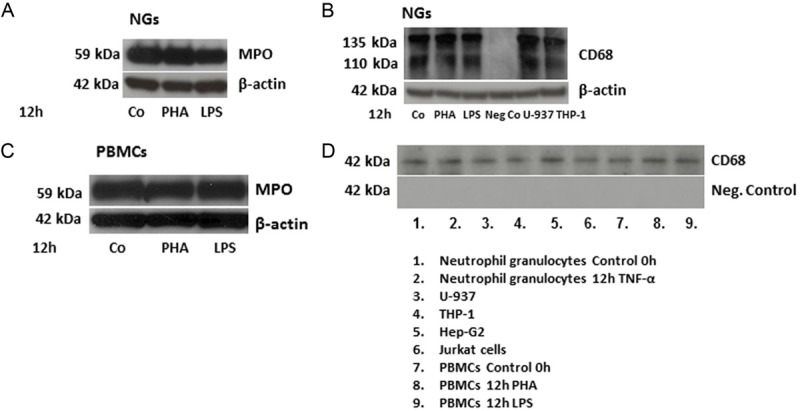
Western blot analysis of MPO and β-actin of total protein from human NGs (Figure 3A). Protein was extracted from NGs of control (Co) cells or 12 h after stimulation with PHA or with LPS. In a second set of experiments, protein from NGs cells lysate was immunoprecipitated with a monoclonal antibody anti-CD-68 (clone KP1) (Figure 3B). U-937 and THP-1 cell lines were used as positive controls, whereas NGs without additional incubation with a monoclonal antibody anti-CD-68 were used as negative control. Western blot analysis of MPO in control (Co), PHA- or LPS-stimulated PBMCs was also performed (Figure 3C). β-actin was always used as house-keeping gene. 20 μg of total protein were separated by SDS-PAGE, and blotted onto PVDF membranes. The membranes were subsequently incubated with the antibodies against MPO, NE, CD-68 and β-actin. An immunoprecipitation of cells lysate was also performed (Figure 3D). In order, NGs (unstimulated and after 12h treatment with TNF-α), U-937, THP-1, Hep-G2, Jurkatt cells and PBMCs (unstimulated and after 12h stimulation with PHA or LPS) were subjected to SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes followed by Western blot analysis for CD-68. The molecular weight of MPO is 59 kDa. The molecular weight of NE is 29 kDa, of β-actin is 42 kDa. The molecular weight of CD-68 (KP1) is between 75 and 110 kDa, as described previously. For immunoprecipitation of cell lysates a rabbit polyclonal anti-CD68 antibody with a predicted band of 42kDa was used.
Figure 4.
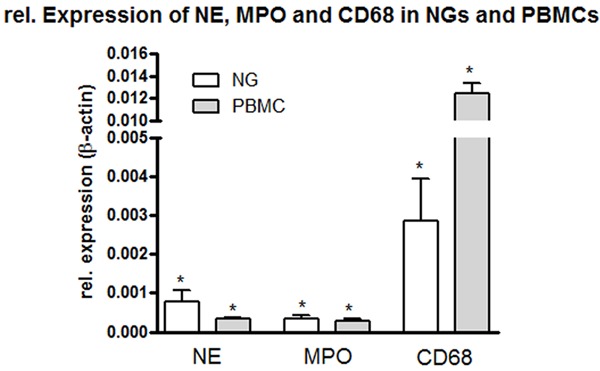
Fold change of mRNA expression of NE, MPO, CD-68 in NGs and PBMCs freshly isolated. Real time PCR was normalized by using β-actin as housekeeping gene. Results represent mean ± SEM value of three experiments (in duplicate).
CD-68 immunoprecipitation of cells lysate in different cell populations was also performed (Figure 3D) and we could clearly detect the observed band of 42 kDa in human NGs, U-937, THP-1, Hep-G2, Jurkat cells and PBMCs.
Changes of CD-68, MPO and NE gene expression in in vitro isolated human PBMCs and NGs at the level of mRNA
The gene expression of different cell markers of human PBMCs and NGs, such as MPO, NE and CD-68, using real-time PCR, was determined (Figure 4). Gene expression profiles of both cellular populations were examined. Precisely, the quantification using PCR revealed a particularly high expression of CD-68 in PBMCs but notably also, although to a lower extent, in NGs. NE was found in higher concentrations in NGs than in PBMCs. We did not find any difference in mRNA transcripts for MPO in either cell type. Interestingly, we detected at the mRNA level a higher CD-68 gene-expression than MPO. On the other hand, we performed an ELISA measurement of MPO and CD-68 in serum of freshly isolated NGs (data not shown) and found very high levels of MPO in these samples, whereas CD-68 was detected but at lower level. High levels of CD-68 in NGs at the RNA level could be due to posttranscriptional changes undergone by CD-68 that was detected at higher RNA than protein level.
Identification of CD-68+/MPO+ NGs in colon biopsy samples from patients with IBD using immunofluorescence double staining analysis
Inflamed mucosa of patients with IBD, e.g. UC (Figure 5A) or CD (Figure 5B), was compared to normal mucosa (Figure 5C). Double staining with monoclonal CD-68 and rabbit polyclonal MPO antibodies revealed a dramatically increase of inflammatory cells, which co-expressed the macrophage marker CD-68 and the granulocyte marker MPO, in the inflamed intestine compared to normal control biopsies. Double staining definitely revealed the accumulation of inflammatory cells in inflamed colonic tissue with an acute exacerbation as compared to non-inflamed tissue. Identical results were obtained using colonic tissue sections of patients with UC and CD.
Figure 5.
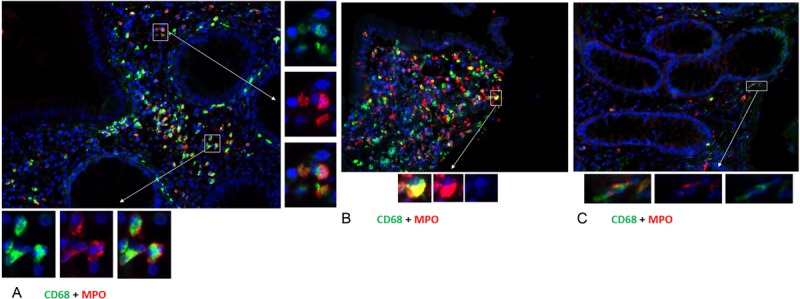
Immunofluorescence staining of paraffin samples of inflamed colonic mucosa from patients with acute exacerbation of UC (Figure 5A) or Crohn’s disease (Figure 5B) compared with non-inflamed (Figure 5C) colon mucosa. Double staining of colon mucosa was performed with a monoclonal antibody directed against CD-68 (green) and polyclonal antibody against MPO (red) followed by fluorescence immunodetection. (Original magnification: x200. Scale bar= 100 μm).
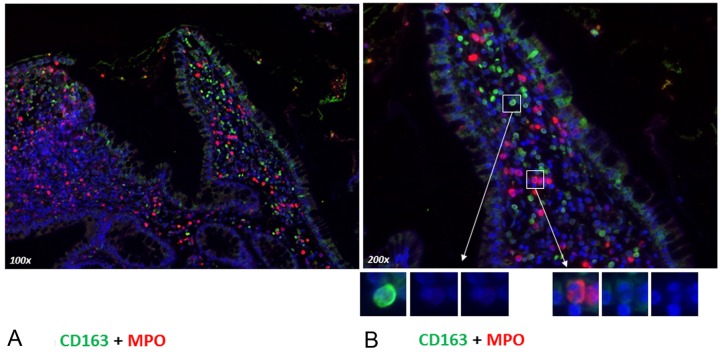
To exclude a phagocytosis of granulocytes by tissue macrophages, we performed a double staining with the macrophage-specific marker CD-163 together with MPO in colonic mucosa of patients affected by CD with an acute flare (Figure 6A and 6B). Immunofluorescence staining was performed to localize CD-163 and MPO in the intestinal mucosa of IBD patients. In the inflamed mucosa, we showed an accumulation of MPO but also of CD-163+ cells that were distributed diffusely in the colonic mucosa. By direct immunofluorescence double staining, we could clearly demonstrate that CD-163+ cells were not MPO positive (Figure 6A and 6B) excluding the uptake of one or the other marker by phagocytosis.
Figure 6.
Immunofluorescence staining of paraffin samples of inflamed colonic mucosa from patients with Crohn’s disease (Figure 6A and 6B) from an acute flare. Double staining of colon mucosa was performed with a monoclonal antibody directed against CD-163 (green) and polyclonal antibody against MPO (red) followed by fluorescence immunodetection. Original magnification: x100 (A) x200 (B). Scale bar= 100 μm.
Inflammatory cells in colonic mucosa of IBD patients
Immunohistochemical double staining with antibodies against MPO and CD-163 revealed a dense neutrophil infiltration in the colonic mucosa from patients with active CD and that the MPO positivity were mostly concentrated in the destroyed mucosa, as shown in Figure 7.
Figure 7.
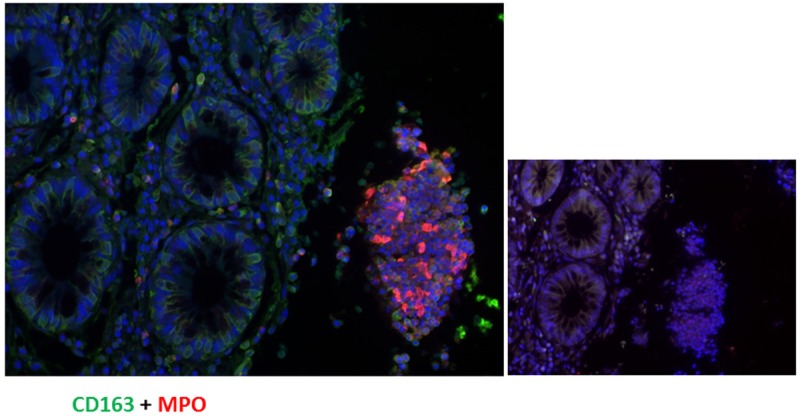
Immunofluorescence staining of paraffin samples of inflamed colonic mucosa from patients with Crohn’s disease from an acute flare. The double staining of colon mucosa was performed with a monoclonal antibody directed against CD-163 (green) and polyclonal antibody against MPO (red), showing an increased number of MPO+ cells in the destroyed epithelium coming off from the rest colon mucosa. A negative control of an inflamed colonic mucosa is also reported.
Discussion
This study identified CD-68+/NE+ and CD-68+/MPO+ cells as NGs and CD-68+/NE- as well as CD-68+/MPO- cells as macrophages-monocytes in human peripheral blood and intestinal tissue of IBD patients. Both, at the RNA and protein levels, this study identified CD-68 not only in monocytes/macrophages but also in NGs since by double staining analysis we detected cells positive for both CD-68 and NE in unstimulated and in activated PBMCs. Moreover, using the same cell populations after double staining with the antibodies directed against CD-68 and MPO, the data were confirmed. Further detection of CD-68+/CD-11 b-c+ cells confirmed these detected cells as NGs. By performing Western blot analysis and immunoprecipitation of cell lysate, we identified CD-68 in human NGs, PBMCs, but also in U-937, THP-1, Hep-G2 and Jurkat cells.
The second important finding is that the in vitro results were confirmed utilizing normal and inflamed colonic mucosal tissue from patients with IBD. Sections of colonic tissue obtained during an acute exacerbation of either UC or CD were prepared and demonstrated a strong accumulation of CD-68+/MPO+ cells while only a weak co-positivity of these two markers was present in normal colonic mucosa.
The CD-68 antigen has been identified in the cytoplasm of human macrophages and monocytes and several studies have used this anti-gene as a selective marker for this cell type [5,25]. Prior immunohistochemical studies have shown that CD-68 is expressed at very low levels in most cell types, although it is abundant in macrophages [26].
As PBMCs represent a “co-culture” of leukocytes, pure granulocyte populations were studied [27]. A strong immunoreactivity towards CD-68 antigen and CD-11 b-c in freshly isolated NGs was demonstrated and confirmed utilizing a double staining with MPO- and NE-specific antibodies. These results at the RNA and protein level, showing a strong CD-68 and CD-11 b-c mRNA expression in the NG populations, confirm the findings in blood. It needs to be noted, however, that although co-identity of CD-68 on NGs was demonstrated in this study, only a part of the NGs population was CD-68+/MPO+ or CD-68+/NE+. These results, together with the transmigration and accumulation of CD-68+/MPO+ cells in the human colonic mucosa during an acute flare of UC or CD, identify the possible involvement of CD-68+ NGs in acutely inflamed colonic mucosa.
It should also be noted that the higher mRNA expression of CD-68 than MPO detected in NGs could be due to posttranscriptional mechanisms, which regulate CD-68 protein binding. This explains why we found higher amounts of CD-68 than MPO at the RNA level as well as higher amounts of MPO at the protein level with immunohistological analysis.
The results published previously by Gottfried et al. [14] using an antibody KP1, that has its strongest reactivity independent of the specific cell type examined, were confirmed. The article by Kunisch et al. [13] discussing a cross reactivity of allegedly macrophage-specific anti-CD-68 antibodies with fibroblasts and activated endothelial cells demonstrates amply that these antibodies should not be used for the identification of macrophages only.
Recently, MPO and NE have been shown to be expressed by NGs in normal and inflamed hepatic tissue but not in resident Kupffer cells [16]. These results support the present data and indicate that NGs that transmigrate during an inflammatory response in human colonic mucosa are strongly positive for MPO as well as for CD-68, suggesting that the increased expression of CD-68 in inflamed human colonic tissue is due to the recruitment of MPO-positive NGs that are CD-68+.
Moreover, by direct immunofluorescence double staining of human PBMCs and NGs, we could clearly demonstrate that CD-163+ cells were not MPO+ excluding the uptake of this marker by phagocytosis. The same results were confirmed in specimens obtained from patients with an acute flare of CD, by performing a double staining with antibodies against CD-163 and MPO, where we found that CD-163+ and MPO+ cells are two different cell populations excluding the uptake of one or the other marker by phagocytosis.
The present findings need to be considered when immunohistological studies on cryostat or paraffin material from human inflammatory disorders are undertaken, as it is conceivable that a potentially erroneous impression of macrophage-derived damage is present [28-30].
The recognition of an increase in newly recruited CD-68+ cells at the site of tissue injury, together with the expression of both CD-68 and CD-11 b-c in pure granulocyte populations, opens a new window on the understanding of inflammatory responses and underscores the important role of these cells during the multistep process of tissue injury and repair.
In conclusion, this study identifies CD-68 as a marker for NGs and macrophages-monocytes in peripheral blood and acutely inflamed colonic mucosa. The observation that different types of non-macrophage-like cells express the “macrophage” marker CD-68 in several diseases clearly means that these “macrophage-like” cells have to be more thoroughly identified using other cell type-specific markers and the appropriate technique and fixation.
Acknowledgments
The authors are greatly indebted to Mrs. E. Neumann and Mrs S. Heyroth for their expert technical assistance.
References
- 1.Ionescu RM, Vlasak J, Price C, Kirchmeier M. Contribution of variable domains to the stability of humanized IgG1 monoclonal antibodies. J Pharm Sci. 2008;97:1414–1426. doi: 10.1002/jps.21104. [DOI] [PubMed] [Google Scholar]
- 2.Davey FR, Cordell JL, Erber WN, Pulford KA, Gatter KC, Mason DY. Monoclonal antibody (Y1/82A) with specificity towards peripheral blood monocytes and tissue macrophages. J Clin Pathol. 1988;41:753–758. doi: 10.1136/jcp.41.7.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly PM, Bliss E, Morton JA, Burns J, Mc-Gee JO. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988;41:510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreipe H, Radzun HJ, Parwaresch MR, Haislip A, Hansmann ML. Ki-M7 monoclonal antibody specific for myelomonocytic cell lineage and macrophages in human. J Histochem Cytochem. 1987;35:1117–1126. doi: 10.1177/35.10.3476670. [DOI] [PubMed] [Google Scholar]
- 5.Parwaresch MR, Radzun HJ, Kreipe H, Hansmann ML, Barth J. Monocyte/macrophage-reactive monoclonal antibody Ki-M6 recognizes an intracytoplasmic antigen. Am J Pathol. 1986;125:141–151. [PMC free article] [PubMed] [Google Scholar]
- 6.Pulford KA, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, Mason DY. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989;42:414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micklem K, Rigney E, Cordell J, Simmons D, Stross P, Turley H, Seed B, Mason D. A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol. 1989;73:6–11. doi: 10.1111/j.1365-2141.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Ii C, Takahashi H, Imai Y, Tanaka J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J Neurosci Res. 2007;85:994–1009. doi: 10.1002/jnr.21198. [DOI] [PubMed] [Google Scholar]
- 9.Strobl H, Scheinecker C, Csmarits B, Majdic O, Knapp W. Flow cytometric analysis of intracellular CD68 molecule expression in normal and malignant haemopoiesis. Br J Haematol. 1995;90:774–782. doi: 10.1111/j.1365-2141.1995.tb05195.x. [DOI] [PubMed] [Google Scholar]
- 10.Umino T, Skold CM, Pirruccello SJ, Spurzem JR, Rennard SI. Two-colour flow-cytometric analysis of pulmonary alveolar macrophages from smokers. Eur Respir J. 1999;13:894–899. doi: 10.1034/j.1399-3003.1999.13d33.x. [DOI] [PubMed] [Google Scholar]
- 11.Kempf W, Adams V, Wey N, Moos R, Schmid M, Avitabile E, Campadelli-Fiume G. CD68+ cells of monocyte/macrophage lineage in the environment of AIDS-associated and classic-sporadic Kaposi sarcoma are singly or doubly infected with human herpesviruses 7 and 6B. Proc Natl Acad Sci U S A. 1997;94:7600–7605. doi: 10.1073/pnas.94.14.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakkinen T, Karkola K, Yla-Herttuala S. Macrophages, smooth muscle cells, endothelial cells, and T-cells express CD40 and CD40L in fatty streaks and more advanced human atherosclerotic lesions. Colocalization with epitopes of oxidized low-density lipoprotein, scavenger receptor, and CD16 (Fc gammaRIII) Virchows Arch. 2000;437:396–405. doi: 10.1007/s004280000239. [DOI] [PubMed] [Google Scholar]
- 13.Kunisch E, Fuhrmann R, Roth A, Winter R, Lungershausen W, Kinne RW. Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry. Ann Rheum Dis. 2004;63:774–784. doi: 10.1136/ard.2003.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottfried E, Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Muller A, Kastenberger M, Brockhoff G, Andreesen R, Kreutz M. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008;67:453–463. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 15.Hameed A, Hruban RH, Gage W, Pettis G, Fox WM III. Immunohistochemical expression of CD68 antigen in human peripheral blood T cells. Hum Pathol. 1994;25:872–876. doi: 10.1016/0046-8177(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 16.Amanzada A, Malik IA, Nischwitz M, Sultan S, Naz N, Ramadori G. Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver. Histochem Cell Biol. 2011;135:305–315. doi: 10.1007/s00418-011-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazaki K, Sakuma K, Kawamura YI, Izawa M, Ohmori K, Mitsuki M, Yamaji T, Hashimoto Y, Suzuki A, Saito Y, Dohi T, Kannagi R. Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors siglec-7 and -9. J Immunol. 2012;188:4690–4700. doi: 10.4049/jimmunol.1100605. [DOI] [PubMed] [Google Scholar]
- 18.Woywodt A, Ludwig D, Neustock P, Kruse A, Schwarting K, Jantschek G, Kirchner H, Stange EF. Mucosal cytokine expression, cellular markers and adhesion molecules in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:267–276. doi: 10.1097/00042737-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Bataille F, Klebl F, Rummele P, Schroeder J, Farkas S, Wild PJ, Furst A, Hofstadter F, Scholmerich J, Herfarth H, Rogler G. Morphological characterisation of Crohn’s disease fistulae. Gut. 2004;53:1314–1321. doi: 10.1136/gut.2003.038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perminow G, Reikvam DH, Lyckander LG, Brandtzaeg P, Vatn MH, Carlsen HS. Increased number and activation of colonic macrophages in pediatric patients with untreated Crohn’s disease. Inflamm Bowel Dis. 2009;15:1368–1378. doi: 10.1002/ibd.20916. [DOI] [PubMed] [Google Scholar]
- 21.Moriconi F, Malik IA, Amanzada A, Blaschke M, Raddatz D, Khan S, Ramadori G. The anti-TNF-alpha antibody infliximab indirectly regulates PECAM-1 gene expression in two models of in vitro blood cell activation. Lab Invest. 2012;92:166–177. doi: 10.1038/labinvest.2011.160. [DOI] [PubMed] [Google Scholar]
- 22.Moriconi F, Raddatz D, Ho NA, Yeruva S, Dudas J, Ramadori G. Quantitative gene expression of cytokines in peripheral blood leukocytes stimulated in vitro: modulation by the anti-tumor nerosis factor-alpha antibody infliximab and comparison with the mucosal cytokine expression in patients with ulcerative colitis. Transl Res. 2007;150:223–232. doi: 10.1016/j.trsl.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Malik IA, Moriconi F, Sheikh N, Naz N, Khan S, Dudas J, Mansuroglu T, Hess CF, Rave-Frank M, Christiansen H, Ramadori G. Single-dose gamma-irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am J Pathol. 2010;176:1801–1815. doi: 10.2353/ajpath.2010.090505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahar-Gohad P, Sultan H, Esteban Y, Stabile A, Ko JL. RACK1 identified as the PCBP1-interacting protein with a novel functional role on the regulation of human MOR gene expression. J Neurochem. 2013;124:466–477. doi: 10.1111/jnc.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gough PJ, Gordon S, Greaves DR. The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class A scavenger receptor in macrophages in vitro and in vivo. Immunology. 2001;103:351–361. doi: 10.1046/j.1365-2567.2001.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warnke RA, Pulford KA, Pallesen G, Ralfkiaer E, Brown DC, Gatter KC, Mason DY. Diagnosis of myelomonocytic and macrophage neoplasms in routinely processed tissue biopsies with monoclonal antibody KP1. Am J Pathol. 1989;135:1089–1095. [PMC free article] [PubMed] [Google Scholar]
- 27.Hodge GL, Flower R, Han P. Optimal storage conditions for preserving granulocyte viability as monitored by Annexin V binding in whole blood. J Immunol Methods. 1999;225:27–38. doi: 10.1016/s0022-1759(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 28.Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, Wagner B, Barnardo A, Pomplun S, Auzinger G, Bernal W, Heaton N, Vergani D, Thursz MR, Wendon J. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–746. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- 29.Tousson E, Beltagy DM, Gazia MA, Al-Behbehani B. Expressions of P53 and CD68 in mouse liver with Schistosoma mansoni infection and the protective role of silymarin. Toxicol Ind Health. 2012 doi: 10.1177/0748233712442733. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Miura K, Yang L, van RN, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]