Abstract
The introduction of magnetoencephalography has made it possible to study electromagnetic signaling in deeper, paralimbic cortical structures such as the medial prefrontal/anterior cingulate (ACC) and medial parietal/posterior cingulate (PCC) cortices. Self-awareness and self-control have been attributed to these regions. To test the hypothesis that they are dysfunctional in pathological gambling with poor self-control, we studied gamblers with and without previous stimulant abuse and age- and sex-matched controls. We found that pathological gamblers were more impulsive than controls in a stop-signal task and attributed this to changes in the activity of the paralimbic network: Pathological gamblers had reduced synchronization at rest in the high gamma range (55–100 Hz) compared with controls and failed to show an increase in gamma synchronization during rest compared with the task, as observed in controls. Subgroup analysis revealed that pathological gamblers without a history of stimulant abuse had lower PCC power during the stop-signal task compared with controls and gamblers with previous stimulant abuse. Furthermore, gamblers with a history of stimulant abuse had up to four times higher power at the ACC site during rest and the task compared with controls. In conclusion, pathological gamblers had higher impulsivity and functional paralimbic abnormalities, which could not be explained by a history of stimulant abuse. In addition, previous stimulant abuse had a marked effect on the amplitude of oscillatory brain activity in the ACC and PCC, suggesting long-term deleterious effects of repeated dopaminergic drug exposure. These consequences should be investigated in more detail in longitudinal studies.
Keywords: coherence of consciousness, default mode, attention deficit hyperactivity disorder, borderline personality disorder
The introduction of magnetoencephalography (MEG) has made it possible to study neural mechanisms even in deeper parts of the cortex with a high degree of temporal resolution in combination with a decent spatial resolution. This allows investigation of one of the major networks of the brain, the paralimbic interaction between the medial prefrontal/anterior cingulate (ACC) and medial parietal/posterior cingulate (PCC) cortices (Fig. 1). This interaction has in several recent studies been associated with self-awareness (1–5).
Fig. 1.

Schematic representation of the medial cortical components of the paralimbic network of self-awareness. Schematic localization of the medial sources for MEG registration. Red, ACC; peak Talairach coordinates, 0,42,54. Blue, PCC; peak Talairach coordinates, 0,−50,28. Resolution was ∼1 cm in superior cortical structures (22).
Pathological gambling can be viewed as a default of self-control (impulsivity), which may be seen as closely related to deficient self-awareness, and is a common process across borderline personality disorder and addiction (6–13). More directly, Bechara argued that all addictions occur because of a predisposition linked to abnormal functioning of a frontal circuitry preceding any use of drugs (14). However, most studies have been limited by the difficulty of disentangling primary abnormalities from the toxic effects of drugs (8). This impediment has been overcome, for example, in a study by Xiao et al. (15). The authors demonstrated that adolescents with neuropsychological signs of frontal lobe dysfunction, but not (yet) addicted, were more likely to develop addiction than those without signs of frontal lobe dysfunction. Experimentally, electroencephalography (EEG) and magnetic resonance imaging (MRI) have been methods of choice. Focus has centered on the executive part of paralimbic regions, in particular the frontal lobe and striatum (9, 10, 13, 16–18).
Given the close interaction between self-awareness and self-control, we hypothesized that abnormalities in both the ACC and PCC regions of this paralimbic circuitry are a characteristic feature of behavioral addiction even without previous exposure to the toxic effects of drugs. To test our hypothesis, we compared pathological gamblers with age- and sex-matched controls using two measures: (i) a stop-signal task, which is a widely used measure of self-control (19), and (ii) resting. The stop-signal task consists of “go” and “nogo” trials. In go trials, the participant is instructed to press a button as soon as an “O” appears on the screen. In nogo trials, the O is followed by an “X,” and the participant is instructed to withhold his response. The task can be used to measure impulsivity with the following variables: (i) number of correct/incorrect nogo trials, (ii) critical stop-signal delay (SSD), which is the duration between the O and the X, and (iii) stop-signal reaction time (SSRT), which is the time required for the stop signal to be processed so a response can be withheld. In particular, the SSRT has been widely used as a valid measure of impulsivity in general, and in studies of patients suffering from addiction (17, 20, 21).
To address whether pathological gamblers have abnormal activity in the paralimbic circuitry, we measured MEG power in a control region (right V1) and in the ACC and PCC, two main nodes in the paralimbic circuitry of self-awareness, and the synchronization between the ACC and PCC. MEG power is a direct measure of electromagnetic activity, including oscillatory amplitude in a prespecified region [as opposed to the blood oxygen level dependent (BOLD) signal in functional MRI, which is a complex measure of vascular and metabolic events], and is complementary to the anatomical information obtained by diffusion tensor imaging (DTI). Phase locking, as used here, is a measure of synchronization of the oscillatory activity between two regions of interest (22, 23). In the present study, we did not attempt to study subcortical regions in the paralimbic circuitry, such as the thalamus or striatum, which are comparatively difficult to access by MEG due to their deep location a long distance from the MEG sensors where the estimated resolution is 50 mm (24) and, due to their modest size, the largest dimension is ∼40 mm.
We included 14 pathological gamblers and 11 age- and sex-matched controls. Pathological gamblers were further divided into two well-defined subgroups: (i) gamblers without a history of self-reported drug abuse (n = 9), and (ii) gamblers with a history of comorbid stimulant (amphetamine) abuse (but not within the last month) (n = 5). The clinical evaluation of the pathological gamblers was done using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) disorders (SCID-I), which included a special module assessing pathological gambling (25). Although tobacco was smoked by members of both the control and gambling groups, smoking was overrepresented in the pathological gambling group. This is not surprising, because these patients are more sensitive to addictive stimuli in general. To control for the effect of smoking, we used an extended group of controls: Eleven from the main analysis (comparing gamblers and controls) and 11 additional young males, who were excluded from the main analysis due to age matching (in total, 5 smokers and 17 nonsmokers, age-matched males) (SI Methods).
Results
Behavioral.
Results from the stop-signal task showed that pathological gamblers had prolonged SSRT (mean: 262.74 ms ± SE: 21.34 ms) compared with controls (212.19 ± 8.39 ms), P = 0.014 (one-tailed), thereby indicating impaired impulse control, as predicted (7, 17, 20, 26).
Subgroup analysis revealed that pathological gamblers without comorbid stimulant abuse had prolonged SSRT (268.23 ± 24.78 ms) compared with controls (212.19 ± 8.39 ms), P = 0.013 (one-tailed). Pathological gamblers with comorbid stimulant abuse also had prolonged SSRT (253.95 ± 42.46 ms) compared with controls (212.19 ± 8.39 ms), but this comparison did not reach significance (P = 0.11, one-tailed).
Pathological gamblers did not differ from controls on measures of correct/incorrect nogo trials and SSD. This is in line with recent studies of patients suffering from addiction, where prolonged SSRTs were reported in addicted individuals compared with controls whereas no differences were reported on the other variables (17, 20, 26).
Gamma Synchronization Between ACC and PCC.
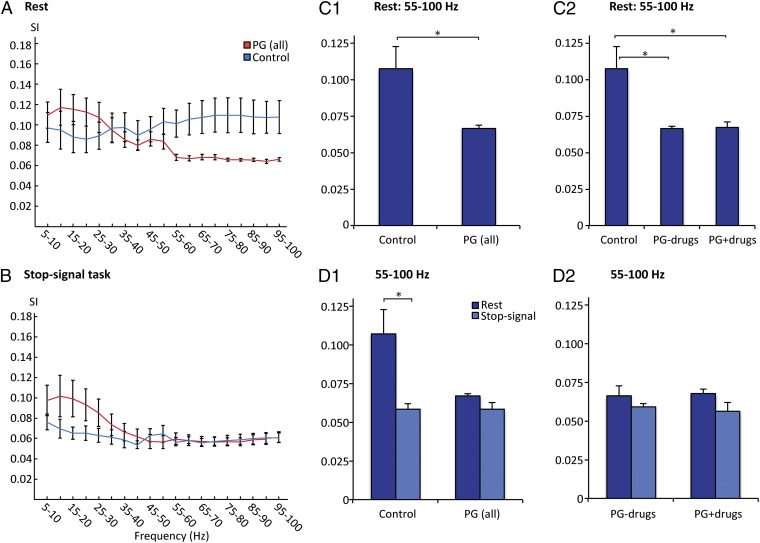
During rest, pathological gamblers had lower synchronization than controls in the 55–100 Hz (high gamma) band (P = 0.029) (Fig. 2 A and C1). Subgroup analysis revealed that this effect was independent of previous stimulant abuse: Both pathological gamblers with and without a history of comorbid stimulant abuse had lower synchronization in this band (P = 0.032, P = 0.027, respectively) (Fig. 2C2). In the 30–45 Hz (low gamma) band, pathological gamblers were not different from controls.
Fig. 2.
Abnormal gamma synchronization (SI) in pathological gamblers. (A) During rest, pathological gamblers had lower synchronization than controls in the high gamma band (55–100 Hz). (B) During performance of the stop-signal task, synchronization was the same in the two groups. (C1) Comparing only levels of gamma synchronization during rest showed that pathological gamblers had lower levels compared with controls (P < 0.05) in the high gamma band (55–100 Hz). (C2) Subgroup analysis showed that this difference was found both for gamblers with and without a history of comorbid stimulant addiction (both P < 0.05). (D1) Directly comparing the activity during rest vs. task performance showed that controls had higher gamma synchronization at rest (P < 0.05), which is in line with previous findings (5, 27). This was not the case for pathological gamblers. (D2) Analysis of subgroups showed that this was independent of previous stimulant abuse. Control, healthy controls; PG (all), pathological gamblers with and without comorbid stimulant abuse; PG−drugs, pathological gamblers without a history of comorbid stimulant abuse; PG+drugs, pathological gamblers with a history of comorbid stimulant abuse. Error bars indicate SEM. *P < 0.05 (two-tailed).
During the stop-signal task there was no difference in synchronization between pathological gamblers and controls, nor between subgroups (Fig. 2B). In controls, we found an expected decrease in high gamma synchronization (55–100 Hz) during the task compared with rest (P = 0.016) (Fig. 2D1). This finding is consistent with our previous findings of elevated gamma synchronization during self-reflection (5), which is a characteristic feature during rest (27). There was no difference in synchronization between the two conditions in pathological gamblers, nor in any of the two subgroups of gamblers (Fig. 2 D1 and D2).
Power During Rest.
There was no effect of group (nor subgroup) on the level of power in the control region, right V1, during rest (Fig. S1). In the ACC, pathological gamblers had higher levels of power compared with controls during rest in all frequency bands (4–8 Hz theta band; 8–12 Hz alpha band; 12–30 Hz beta band; 30–45 Hz low gamma band; 55–100 Hz high gamma band; P < 0.05 in all bands; Fig. S2 A–E). This is shown across the full 4–100 Hz frequency band in Fig. 3A1. There was a highly significant trend, with power increasing linearly with complexity of addiction (controls < pathological gamblers without comorbid stimulant abuse < pathological gamblers with comorbid stimulant abuse) in all frequency bands (P < 0.001 in all bands). Subgroup analysis revealed that the effect was driven by pathological gamblers with a history of comorbid stimulant abuse. These individuals had approximately four times higher power than drug-naïve pathological gamblers and controls (for both groups; P < 0.05 in all bands; Fig. S2 A–E). This is shown across the full 4–100 Hz frequency band in Fig. 3A2. In the PCC, pathological gamblers did not differ from controls during rest across the full 4–100 Hz frequency band (Fig. 3B; Fig. S2 F–J).
Fig. 3.
Abnormal ACC and PCC power (measured in ampere-meter squared) in pathological gamblers. (A1) Pathological gamblers had higher levels of ACC power than healthy controls (HC) during rest (P < 0.05). (A2) Subgroup analysis revealed that the effect was driven by pathological gamblers with a history of comorbid stimulant abuse. These individuals had approximately four times higher power than drug-naïve pathological gamblers and controls (P < 0.05 and P < 0.05, respectively). This was seen in all frequency bands, and is shown here for all bands combined (4–100 Hz). (B 1 and 2) In the PCC, pathological gamblers did not differ from controls during rest (B1), nor did the subgroups (B2) in the analysis shown here with all bands combined (4–100 Hz). However, in the 4–8 Hz (theta) and 55–100 Hz (high gamma) bands, pathological gamblers with comorbid stimulant abuse had higher power than pathological gamblers without (P < 0.05 and P < 0.05, respectively) (not shown here, but see Fig. S2). (C1) Pathological gamblers had higher ACC power than controls during the stop-signal task (P < 0.01). (C2) This effect was mainly driven by pathological gamblers with a history of comorbid amphetamine abuse, who had approximately three times higher ACC power than controls (P < 0.05). (D1) In the PCC, pathological gamblers did not differ from controls during the stop-signal task. (D2) However, subgroup analysis showed that pathological gamblers without a history of drug abuse had lower levels of power than pathological gamblers with a history of stimulant abuse and controls during task performance (P < 0.05 and P < 0.05, respectively). This was seen in all frequency bands, and is illustrated here for all bands combined (4–100 Hz). Error bars indicate SEM. *P < 0.05, **P < 0.01 (two-tailed). Abbreviations are as in Fig. 2.
Power During Stop-Signal Task.
There was no effect of group (nor subgroup) on the level of power in the control region, right V1, during the stop-signal task (Fig. S1). In the ACC, pathological gamblers had higher levels of power during the stop-signal task compared with controls in all frequency bands (P < 0.05 in all bands; Fig. S3 A–E). This is shown across the full 4–100 Hz frequency band in Fig. 3C1. Similar to the findings during rest, there was a highly significant linear trend (controls < pathological gamblers without comorbid stimulant abuse < pathological gamblers with comorbid stimulant abuse) in all frequency bands (P < 0.001 in all bands). Again, subgroup analysis revealed that this effect was primarily driven by pathological gamblers with comorbid stimulant abuse, who had higher ACC power than controls in all frequency bands (P < 0.05 or P < 0.01; Fig. S3 A–E). This is shown across the full 4–100 Hz frequency band in Fig. 3C2.
In the PCC, pathological gamblers did not differ from controls during the stop-signal task (Fig. 3D1). Subgroup analysis revealed that pathological gamblers without comorbid stimulant abuse had lower power than controls (P < 0.05 or P < 0.01 across bands) and pathological gamblers with a history of comorbid stimulant abuse (P < 0.05 in all bands). This is shown across the full 4–100 Hz frequency band in Fig. 3D2. For details on separate bands, see Fig. S3 F–J.
Discussion
The main finding of the present study was that behavioral addiction is linked to abnormal activity in, and communication between, nodal regions of the paralimbic network of self-awareness, the ACC and PCC, which are effective in different aspects of self-awareness processing (28, 29). Pathological gamblers had lower synchronization between the ACC and PCC at rest in the high gamma band compared with controls, and failed to show an increase in gamma synchronization during rest compared with the task (as observed in controls). These findings could not be attributed to previous drug abuse or smoking habits. Furthermore, pathological gamblers without previous drug abuse had lower PCC power than controls and gamblers with previous stimulant abuse during the stop-signal task. In contrast, a history of stimulant abuse in gamblers caused a marked increase in power across regions and frequencies both at rest and during the stop-signal task.
Addiction is a well-known example of impaired self-control, confirmed here with a stop-signal task that showed prolonged SSRTs in pathological gamblers compared with controls. This is in line with previous findings in drug-addicted individuals (17, 20, 21, 26); for example, Monterosso et al. observed prolonged SSRTs in chronic methamphetamine users compared with controls (26). Another study combined behavioral with electromagnetic data in addiction. It showed low self-control with subjective impulsivity and longer stopping reaction times to interrupt a go signal linked to low EEG theta amplitudes during the task (7). In contrast to EEG, MEG, as used here, is better-suited for detection of electromagnetic signals in paralimbic regions. Power changes in oscillations are thought to be responsible for common disorders of self-regulation such as attention deficit hyperactivity disorder (ADHD), autism, and schizophrenia (30). The causality of gamma oscillations for cognition has been shown directly by electromagnetic manipulation in animal experiments (31). Gamma oscillations are tightly interacting with lower frequencies in the theta range (4–8 Hz) (32). Traditionally, theta oscillations have been attributed to longer-range interregional coupling, whereas the faster gamma coupling has been linked to shorter-range intraregional time coupling (33). However, more recent work indicates that gamma oscillations may participate in longer-range coupling as well (30, 34, 35). It has been shown that cross-frequency interaction occurs in the way that amplitudes of gamma oscillations are modulated by theta oscillations (32). Our finding of decreased amplitudes in the theta range of the PCC region is therefore relevant for the pathophysiology of pathological gambling.
The focus of the present study has been regions in a previously described paralimbic network effective in self-processing: the ACC and PCC. The contribution of the anterior region to self-processing is predominantly self-evaluation (28). The PCC region is also of interest, because our transcranial magnetic stimulation studies have shown that the contribution of the posterior region is to supply episodic memory retrieval for extended self-awareness (29). Interference with extended self-awareness would be expected to impair the ability to maintain focus during an extended period during the stop-signal task.
The crux of the matter is whether functional abnormalities in relevant regions exist in addiction independent of potential toxic effects of drug exposure. Importantly, we found low gamma synchronization in pathological gambling in the paralimbic circuitry of self-processing independent of stimulant addiction. Gamma synchronization in this circuitry is characteristic for self-reference, linking conscious experiences as their common tag. Deficient gamma synchronization at rest could therefore be a sign of decreased self-reflection, the major mental activity during stimulus penuria at rest (5, 27), although we did not attempt to measure the degree of self-reflection in the present study. In a recent paper, structural abnormalities were demonstrated in the amygdala, putamen, and postcentral gyrus in drug-naïve, first-degree relatives of cocaine abusers (17). This suggests that executory and primary sensory functions could also be affected in individuals genetically susceptible to addiction. It is also an indication of striatal dopaminergic influence on self-awareness (4). The five participants with pathological gambling plus periodic amphetamine/“speed” abuse were found to have markedly increased amplitudes of brain oscillations (i.e., power) compared with drug-naïve gamblers in both the ACC and PCC regions throughout the frequency spectrum. Modulation of oscillatory power has recently been demonstrated to be a consequence of repeated amphetamine injections in rats (36), and seems to be due to increased sensitivity of dopamine D1 and D2 receptors. This idea is reinforced by animal experiments indicating premorbid dopamine D2 abnormalities in drug dependency (37). Even prenatal methamphetamine has been found to increase drug-seeking behavior after birth (38). A similar mechanism may be responsible for increased oscillatory power after amphetamine/speed addiction seen in the present study of human addiction. Also, the high sensitivity of paralimbic cortical activity to dopaminergic changes stresses the importance of striatal influences on brain resource allocation and, hence, impulse control (4).
Most pathological gamblers smoked, whereas most controls did not. One might ask whether the reduced gamma synchrony at rest and other abnormalities in gamblers could be attributed to smoking as a confounding variable. This is highly unlikely a priori, because nicotine addiction has been demonstrated to be linked to increased gamma synchronization (39), rather than decreased as we see here, and because power spectra are generally unaffected by smoking habits (40, 41). In a separate study on an extended nongambling control group, we compared power at the ACC, PCC, and right V1 sites and gamma synchronization between the ACC and PCC and found no difference between smokers and nonsmokers. Similarly, smokers and nonsmokers had elevated gamma synchronization at rest compared with the stop-signal task, and did not differ on our behavioral measure of impulsivity, SSRT (for details, see SI Methods). The results of the present study can therefore not be explained by smoking.
In conclusion, we found impaired self-control and abnormal activity in the paralimbic circuitry both at rest and during a stop-signal task that could not be explained by a history of stimulant abuse. In addition, a marked effect of such abuse on power was identified. The consequences of such mechanisms for addiction should be investigated in more detail in longitudinal studies. Consistent with animal experiments on repeated exposure to amphetamine, the present study suggests that both behavioral and stimulant addiction changes the circuitry of self-awareness and self-control and thereby exposes the individual to further addiction. Future longitudinal studies and complementary studies with DTI will be useful (42, 43).
Methods
Study Groups.
Eleven healthy controls were recruited through a pool of individuals who had participated in a previous study at our site, and 14 pathological gamblers were recruited through the Aarhus and Odense Center for Ludomani (Centre for Pathological Gambling), which is a large treatment facility for pathological gamblers in Denmark. All participants were right-handed [assessed using the Edinburgh Test of Handedness (44)] males, with the exception of one who was left-handed among the pathological gamblers. Healthy controls were included if they did not suffer from any neurological or psychiatric problems and did not receive medication affecting the brain (assessed using the SCID) (25). Pathological gamblers were included if they were currently suffering from, or had a recent history of, pathological gambling, possibly with a history of comorbid stimulant addiction (assessed using the SCID). All participants were without other current or past psychiatric and neurological illnesses, with the exception of one pathological gambler with a history of a single episode of depression. All pathological gamblers were severely socially and economically distressed and impaired by gambling, three with a criminal record and two with a gambling debt of more than 200,000 Danish kroner ($34,500). The mean age of pathological gamblers and controls was 33.9 y (SD ± 6.0) and 32.6 y (± 3.8), respectively. An independent t test showed no age difference between groups, t(23) = −0.598, P = 0.556.
The group of pathological gamblers could be divided into two well-defined subgroups: Nine pathological gamblers were without a history of comorbid stimulant addiction [mean age 35.3 y (± 5.9)], and five pathological gamblers had a history of periodically comorbid amphetamine/speed addiction (but not within the last month) [mean age 31.3 y (± 5.9)]. Planned contrasts (one-way independent ANOVA) showed no difference in age between controls and pathological gamblers with comorbid stimulant addiction, t(22) = −0.498, P = 0.624; controls and pathological gamblers without a history of comorbid stimulant addiction, t(22) = 1.185, P = 0.249; nor pathological gamblers with and without a history of comorbid stimulant addiction, t(22) = −1.436, P = 0.165. One pathological gambler without a history of drug abuse was excluded from the MEG analysis due to problems with coregistration, but is included in the behavioral analysis. Although the subgroups were small, they allowed us to obtain surprisingly clear results on (i) the effect of gambling without stimulant addiction vs. controls, and (ii) the effect of adding stimulant addiction to gambling. Smoking was heavily overrepresented (n = 12) among the 14 gamblers, and underrepresented (n = 3) in the control group of 11. We therefore analyzed the possible significance of smoking for oscillations in the paralimbic circuitry and on impulsivity separately. For this purpose, we used an extended number of age-matched controls (5 smokers vs. 17 nonsmokers), consisting of the 11 original controls and 11 additional controls who were excluded from the main analysis to ensure age matching with the group of pathological gamblers.
The study was approved by the Center of Functionally Integrative Neuroscience research board and the local ethics committee (De Videnskabsetiske Komitéer for Region Midtjylland), and written consent was obtained before participation.
Procedure and Behavioral Task.
The experiment was carried out at the MEG Unit, MINDLab Core Experimental Facility, Aarhus University. The experiment consisted of (i) an introduction to the stop-signal task including a brief training session in the shielded room, (ii) resting-state recordings with eyes closed (5 min), and (iii) the main experimental session with the stop-signal task (15 min).
The stop-signal task was based on recent work by Li et al. (19) (for details, see the introduction and SI Methods). The task consisted of 75% go trials and 25% nogo trials. We used a staircase procedure, in which the delay between O and X varies according to the participant’s previous response on a nogo trial. Clearly, it is easier for the participant to withhold his response if the stop-signal (X) appears early after the go signal (O), and correspondingly difficult if the delay is increased. The task starts with an SSD of 200 ms, and if the participant incorrectly presses the button in a nogo trial, the delay is decreased by 30 ms in the next nogo trial, and vice versa if the participant correctly withholds a response.
MEG Analysis.
The neurophysiological data were recorded in a magnetically shielded room using an Elekta Neuromag TRIUX MEG system with 204 planar gradiometers and 102 magnetometers. It was sampled with 1,000 Hz after analog filtering of 0.1--330 Hz. The data were coregistered to individual MR images by registering three landmark points recognizable on the MR in addition to 50+ points marking the head shape. Using five head coils, a continuous measure of the head position in the scanner was obtained. Horizontal and vertical eye movements were recorded bipolarly using surface electrodes. Using Elekta's MaxFilter software (Version 2.1), the head position was transformed to the one initially recorded. Electromagnetic sources external to the participant’s head were suppressed based on Maxwell's equations and, in addition, sources in the boundary region were projected out using a temporal extension of the Signal Space Separation method. A log of the head movement was inspected and the participant was excluded in cases of excessive movement. The results of the MaxFiltering were visually inspected, and artifact-inducing channels were excluded in an iterative manner.
In the remaining analysis, only planar gradiometers were used. Using principal component analysis, the dimension of the data was reduced to 64 components and independent component analysis (ICA) was used to project out electrooculography (eye movement), electrocardiography (heartbeat) artifacts, and flux jumps on sensors. The data were epoched into 4-s segments, independent of the task. Segments with a variance higher than the mean + 2 SDs for the participant were rejected.
For all participants, a structural MR scan was recorded with a Siemens 3T MR scanner with a resolution of 1 × 1 × 1 mm. Using FieldTrip (F. C. Donders Centre for Cognitive Neuroimaging; www.ru.nl/fcdonders/fieldtrip), the head shape was extracted and linearly normalized to an Montreal Neurological Institute (MNI) template to generate a single-shell forward model. This was corrected using the previously found ICA weights to account for projecting out mostly frontal components. With a spatial filter, the data were projected into the brain (24, 45), and time series were extracted from the three prespecified regions of interest (ROIs) for each participant (ACC, PCC, and right V1). It is estimated that this method has a spatial accuracy of ∼10 mm for the cortical regions (22).
Using FieldTrip, multitapers were applied to the individual time series to extract spectral information from each ROI. This was done using 400-ms windows time-shifted by 20 ms and a frequency resolution of 2 Hz. The extracted Fourier coefficients were used to calculate both the power for each ROI as well as the synchronization index (SI) between each pair of regions, where the SI was calculated by summing up the complex phase differences between two given regions, calculating the absolute value, and normalizing to the number of epochs (46). Thus, an SI value of 1 indicates complete phase locking and a value of 0 indicates no phase locking at all.
Statistical Analysis.
All statistical analyses were conducted using IBM SPSS Statistics version 20 for Mac OS X. One-way independent ANOVA was used to test for differences in synchronization and power between groups, and a paired-samples t test was used to test whether there was a difference between synchronization during rest and during the stop-signal task. In the analysis of the behavioral data, we used the Mann–Whitney U test to test for differences between groups on the dependent variables.
Supplementary Material
Acknowledgments
We thank Christopher Bailey for excellent scientific and technical support in implementing the MEG recordings and Henriette Vuust for her expert help with the figures. We are grateful for the support of the MINDLab Investment Capital for University Research Fund and the TrygFonden Charitable Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302374110/-/DCSupplemental.
References
- 1.Lou HC, Luber B, Stanford A, Lisanby SH. Self-specific processing in the default network: A single-pulse TMS study. Exp Brain Res. 2010;207(1-2):27–38. doi: 10.1007/s00221-010-2425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doering S, et al. Personality functioning and the cortical midline structures—An exploratory fMRI study. PLoS One. 2012;7(11):e49956. doi: 10.1371/journal.pone.0049956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Lou HC, Joensson M, Kringelbach ML. Yoga lessons for consciousness research: A paralimbic network balancing brain resource allocation. Front Psychol. 2011;2:366. doi: 10.3389/fpsyg.2011.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou HC, Gross J, Biermann-Ruben K, Kjaer TW, Schnitzler A. Coherence in consciousness: Paralimbic gamma synchrony of self-reference links conscious experiences. Hum Brain Mapp. 2010;31(2):185–192. doi: 10.1002/hbm.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornovalova MA, Lejuez CW, Daughters SB, Rosenthal MZ, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clin Psychol Rev. 2005;25(6):790–812. doi: 10.1016/j.cpr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Lansbergen MM, Schutter DJ, Kenemans JL. Subjective impulsivity and baseline EEG in relation to stopping performance. Brain Res. 2007;1148:161–169. doi: 10.1016/j.brainres.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Changeux JP, Lou HC. Emergent pharmacology of conscious experience: New perspectives in substance addiction. FASEB J. 2011;25(7):2098–2108. doi: 10.1096/fj.11-0702ufm. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 11. Brevers D, et al. (2013) Impaired self-awareness in pathological gamblers. J Gambl Stud 29(1):119–129. [DOI] [PubMed]
- 12.van Holst RJ, van Holstein M, van den Brink W, Veltman DJ, Goudriaan AE. Response inhibition during cue reactivity in problem gamblers: An fMRI study. PLoS One. 2012;7(3):e30909. doi: 10.1371/journal.pone.0030909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, et al. Affective decision-making predictive of Chinese adolescent drinking behaviors. J Int Neuropsychol Soc. 2009;15(4):547–557. doi: 10.1017/S1355617709090808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fjell AM, et al. Pediatric Imaging, Neurocognition, and Genetics Study Multimodal imaging of the self-regulating developing brain. Proc Natl Acad Sci USA. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ersche KD, et al. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein RZ, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: Neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26(1):186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85(3):205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Li CS, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32(3):581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross J, Timmermann L, Kujala J, Salmelin R, Schnitzler A. Properties of MEG tomographic maps obtained with spatial filtering. Neuroimage. 2003;19(4):1329–1336. doi: 10.1016/s1053-8119(03)00101-0. [DOI] [PubMed] [Google Scholar]
- 23.Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6(4):285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- 24.Gross J, et al. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98(2):694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-N/P) New York: N Y State Psychiatr Inst; 2002. Version 2.0. [Google Scholar]
- 26.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Schneider F, et al. The resting brain and our self: Self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience. 2008;157(1):120–131. doi: 10.1016/j.neuroscience.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Luber B, Lou HC, Keenan JP, Lisanby SH. Self-enhancement processing in the default network: A single-pulse TMS study. Exp Brain Res. 2012;223(2):177–187. doi: 10.1007/s00221-012-3249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou HC, et al. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci USA. 2004;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: Toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75(6):963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsáki G. Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. J Neurosci. 2012;32(2):423–435. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97(4):1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 35.Vicente R, Gollo LL, Mirasso CR, Fischer I, Pipa G. Dynamical relaying can yield zero time lag neuronal synchrony despite long conduction delays. Proc Natl Acad Sci USA. 2008;105(44):17157–17162. doi: 10.1073/pnas.0809353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapish CC, Chiang J, Wang JZ, Phillips AG. Oscillatory power and synchrony in the rat forebrain are altered by a sensitizing regime of D-amphetamine. Neuroscience. 2012;203:108–121. doi: 10.1016/j.neuroscience.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 37.Dalley JW, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slamberová R, Hrubá L, Matějovská I, Bernášková K, Rokyta R. Increased seizure susceptibility induced by prenatal methamphetamine exposure in adult female rats is not affected by early postnatal cross-fostering. Epilepsy Behav. 2011;20(1):6–11. doi: 10.1016/j.yebeh.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Phillips JM, Ehrlichman RS, Siegel SJ. Mecamylamine blocks nicotine-induced enhancement of the P20 auditory event-related potential and evoked gamma. Neuroscience. 2007;144(4):1314–1323. doi: 10.1016/j.neuroscience.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knott VJ, Harr A. Assessing the topographic EEG changes associated with aging and acute/long-term effects of smoking. Neuropsychobiology. 1996;33(4):210–222. doi: 10.1159/000119279. [DOI] [PubMed] [Google Scholar]
- 41.Teneggi V, et al. EEG power spectra and auditory P300 during free smoking and enforced smoking abstinence. Pharmacol Biochem Behav. 2004;77(1):103–109. doi: 10.1016/j.pbb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Tang YY, et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA. 2010;107(35):15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang YY, Lu Q, Fan M, Yang Y, Posner MI. Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci USA. 2012;109(26):10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 45.Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- 46.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.