Abstract
Synaptic mechanisms underlying memory reconsolidation after retrieval are largely unknown. Here we report that synapses in projections to the lateral nucleus of the amygdala implicated in auditory fear conditioning, which are potentiated by learning, enter a labile state after memory reactivation, and must be restabilized through a postsynaptic mechanism implicating the mammalian target of rapamycin kinase-dependent signaling. Fear-conditioning–induced synaptic enhancements were primarily presynaptic in origin. Reconsolidation blockade with rapamycin, inhibiting mammalian target of rapamycin kinase activity, suppressed synaptic potentiation in slices from fear-conditioned rats. Surprisingly, this reduction of synaptic efficacy was mediated by post- but not presynaptic mechanisms. These findings suggest that different plasticity rules may apply to the processes underlying the acquisition of original fear memory and postreactivational stabilization of fear-conditioning–induced synaptic enhancements mediating fear memory reconsolidation.
Newly formed memories are stabilized over several hours after their acquisition for long-term storage. This protein synthesis-dependent process, termed cellular consolidation (1), critically depends on the permanence of acquisition-induced synaptic modifications (2). Once retrieved, consolidated memory returns to an unstable state and must be restabilized/reconsolidated to persist (3–8). Reconsolidation, which is also a protein synthesis-dependent process, has been observed across many behavioral paradigms, and reported for a range of species (9–12), including humans (13). Mechanistically, reconsolidation blockade differs from extinction of conditioned fear memory, also resulting in diminished fear responses, as these behavioral processes are mediated by distinct neurochemical mechanisms (14).
To date, studies of consolidation have typically reported that the molecular and cellular changes induced by learning are prevented when this memory process is inhibited (2, 15). Thus, synaptic growth was enhanced by long-term sensitization in Aplysia californica (16), whereas blockade of consolidation of this trace with either RNA or protein synthesis inhibitors prevented the stabilization of the morphological correlates of memory changes (17). Similarly, blockade of reconsolidation has also been shown to reverse the molecular (18) and cellular (6) modifications induced by memory reactivation. Although both the memory acquisition and consolidation processes were studied previously at the level of synaptic functions (2), synaptic mechanisms of reconsolidation are largely unknown. Thus, we asked whether reconsolidation blockade reverses learning-induced synaptic plasticity, and, if so, how such modifications of synaptic mechanisms in the circuits for a learned behavior might be mediated.
In this study, we tested the hypothesis that synaptic enhancements induced by fear learning are reversed by reconsolidation blockade, using systemic injections of rapamycin that inhibits mammalian target of rapamycin (mTOR) kinase activity. mTOR kinase regulates protein synthesis at the translational level and is critical for fear memory reconsolidation (19–22). We found that fear learning-induced enhancements of synaptic efficacy were predominantly presynaptic in origin. However, although the impairment in reconsolidation reversed learning-induced synaptic enhancements, this was accomplished by changes in postsynaptic functions. These findings indicate that stabilization of fear-conditioning–associated synaptic enhancements after retrieval recruits a form of synaptic plasticity that is different from synaptic modifications induced during the acquisition of original memory, thereby revealing a distinct mechanism mediating memory reconsolidation.
Results
Fear Conditioning Is Associated with Potentiation of Synaptic Transmission in Cortical and Thalamic Inputs to the Lateral Amygdala.
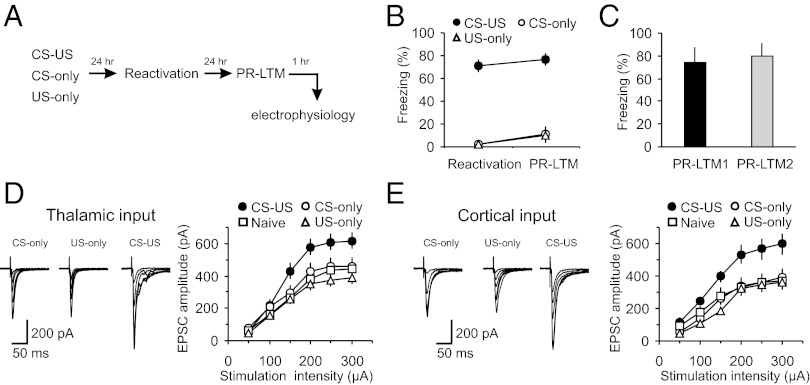
To explore synaptic mechanisms of memory reconsolidation, we trained male Sprague-Dawley rats in a classical single-trial auditory fear conditioning paradigm by pairing a tone [conditioned stimulus (CS)] with a footshock [unconditioned stimulus (US)] (23, 24). Rats in the paired (CS–US) group demonstrated more freezing than control rats (CS-only or US-only groups) in response to the CS during a long-term memory test [postreactivation long-term memory (PR-LTM)] (Fig. 1 A and B; two-way ANOVA, P < 0.001; post hoc Bonferroni’s simultaneous multiple comparisons revealed significant differences between paired and CS-only groups, P < 0.001, and paired and US-only groups, P < 0.001, but no differences between CS-only and US-only groups, P = 1.0). We found also that single CS presentations during memory reactivation did not produce fear extinction under our experimental conditions, as the amount of freezing in fear-conditioned rats at PR-LTM1 was not different from that at PR-LTM2 measured 24 h later (Fig. 1C; t test, P = 0.75 for PR-LTM1 versus PR-LTM2).
Fig. 1.
Fear conditioning leads to synaptic enhancements in cortical and thalamic inputs to the LA. (A) A schematic representation of the experimental design. Rats were trained in a single-trial fear conditioning paradigm and tested at 24 h (PR-LTM) after reactivation trials. (B) Percent freezing observed in fear-conditioned rats (CS–US, paired) and in rats that received CS or US only (CS–US, n = 22 rats; CS-only, n = 20 rats; US-only, n = 6 rats). There were no differences between freezing responses at reactivation and PR-LTM in the CS–US (P = 0.47), CS-only (P = 0.15), or US-only (P = 0.35) groups. (C) Percent freezing observed in CS–US rats at PR-LTM1 (a first reactivation trial) and PR-LTM2 (a second memory test performed 24 h after PR-LTM1) (n = 5 rats; paired t test, P = 0.51 for PR-LTM1 versus PR-LTM2). (D, Left) Averaged EPSCs evoked in thalamic input to the LA by presynaptic stimuli of increasing intensity in slices from naïve (10 rats), CS-only, US-only, and paired groups of rats. Traces are averages of 10 EPSCs. (D, Right) Synaptic input–output curves obtained in thalamic input to the LA (naïve, n = 26 neurons; CS-only, n = 16 neurons; US-only = 12 neurons; paired, n = 14 neurons). Peak amplitudes of the EPSCs were significantly different between naïve, CS-only, US-only, and paired groups (two-way ANOVA, P < 0.001). Post hoc Bonferroni’s simultaneous multiple comparisons revealed significant differences in the EPSC amplitudes between naïve and paired groups (P < 0.001), between CS-only and paired groups (P < 0.01), and between US-only and paired groups (P < 0.001). Thus, synaptic strength in thalamic input was enhanced in fear conditioned rats (paired group). (E) In cortical input, peak amplitudes of the EPSCs also differed significantly between naïve (n = 16), CS-only (n = 8), US-only (n = 12), and paired (n = 12) groups (two-way ANOVA, P < 0.001). EPSC amplitudes were larger in the paired group compared with either naïve (P < 0.001), CS-only (P < 0.001), or US-only group (P < 0.001; Bonferroni’s simultaneous multiple comparisons). Results are shown as means ± SEM.
We examined the effects of fear learning on synaptic transmission in the CS pathways, performing whole-cell patch-clamp recordings from visualized neurons in slices of the amygdala obtained from paired, CS-only, US-only and behaviorally naive (naïve) rats. At 48 h postconditioning, we recorded glutamatergic excitatory postsynaptic currents (EPSCs) evoked in lateral amygdala (LA) neurons under voltage-clamp conditions with stimulating electrodes placed to activate either thalamic input (internal capsule) or cortical input (external capsule) to the LA (25). These two projections deliver the auditory CS information to the LA during fear conditioning (23). Consistent with the role of synaptic enhancements in the CS pathways in retention of fear memory (26–31), we found that synaptic strength, as reflected in input–output curves, was significantly increased in both thalamic and cortical inputs to the LA in slices from paired, compared with the CS-only, US-only, and naïve control groups (Fig. 1 D and E). There were no differences in synaptic input–output curves in thalamo-LA or cortico-LA projections among the control groups, indicating that neither the CS or US alone nor exposure of rats to the training context produced detectable synaptic modifications.
Fear-Conditioning–Induced Synaptic Potentiation Is Suppressed Following Reconsolidation Blockade.
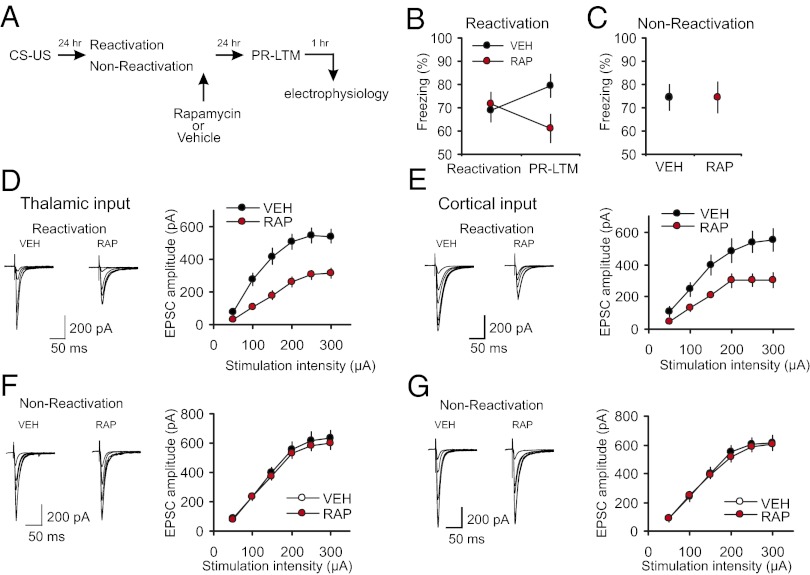
We asked whether reconsolidation blockade with rapamycin, an efficient blocker of mTOR kinase activity (22, 32), would reverse learning-induced enhancements in synaptic efficacy in thalamo-LA and cortico-LA projections. Memory reactivation entailed presentation of a single CS (24 h postconditioning), after which rats received an injection of either rapamycin (20 mg/kg, i.p.; RAP) or vehicle (VEH). It has been demonstrated previously that systemic administration of rapamycin, in doses that impair memory reconsolidation and are comparable to those used in our experiments, did not result in any unspecific alterations in behavior, including anxiety levels, foot shock sensitivity, flinch and vocalization thresholds (20). Whereas both groups showed comparable levels of conditioned freezing during reactivation, rapamycin-treated rats showed lesser freezing 24 h later (indicative of impaired PR-LTM) compared with both the vehicle group (t test, P = 0.023) and with the original fear response in same rats during the reactivation session (paired t test, P = 0.038; Fig. 2 A and B). The inhibitory action of rapamycin on conditioned freezing was not observed when reactivation session was omitted (nonreactivation control groups: rats that received rapamycin or vehicle injections without a prior memory reactivation; Fig. 2C, t test, P = 0.9 for VEH group versus RAP group). The latter finding indicates that the effect of rapamycin might be specific to its ability to suppress fear memory reconsolidation and was not due to the unspecific lasting effects on fear memory retrieval. Consistent with this notion, retrieval of conditioned fear memory was shown to be unaffected by rapamycin injected 30 min before memory reactivation (21).
Fig. 2.
Postretrieval rapamycin impairs reconsolidation of fear memory and suppresses conditioning-induced synaptic enhancements. (A) A schematic representation of the experiments where fear-conditioned rats received a postretrieval injection of rapamycin (RAP; 20 mg/kg, i.p.) or vehicle (VEH). (B) There was no significant difference in percent freezing between VEH-treated (n = 29) and RAP-treated (n = 29) rats during memory reactivation (t test, P = 0.74). The difference in freezing between reactivation and PR-LTM tests in the VEH group did not reach the level of statistical significance (P = 0.06). A significant impairment was observed in RAP rats during the PR-LTM test (see text for details). (C) Rapamycin had no effect on conditioned freezing in “nonreactivated” control rats. Rats in nonreactivation group received rapamycin or vehicle injections at 24 h postconditioning without memory reactivation and PR-LTM was tested 24 h after the injections (RAP, n = 16 rats; VEH, n = 8 rats; t test, P = 0.9 for VEH group vs. RAP group). (D, Left) Averaged EPSCs evoked in thalamic input to the LA by stimuli of increasing intensity in slices from fear-conditioned rats which received postreactivation injections of VEH or RAP. (D, Right) Synaptic input–output curves obtained in thalamic input in slices from both groups of rats (VEH, n = 12 neurons; RAP, n = 13 neurons (two-way ANOVA, P < 0.001 for VEH group versus RAP group of conditioned rats). (E) Experiments were analogous to D, but the EPSCs were recorded in cortical input to the LA (VEH, n = 12 neurons; RAP, n = 8 neurons; two-way ANOVA, P < 0.001). (F) Rapamycin or vehicle were injected at 24 h postconditioning without memory reactivation and synaptic input–output curves were obtained in thalamic input 24 h after the injections (VEH, n = 14 neurons; RAP, n = 23 neurons; two-way ANOVA, P = 0.275). (G) Experiments were analogous to F but the EPSCs were recorded in cortical input (VEH, n = 9 neurons; RAP, n = 19 neurons; two-way ANOVA, P = 0.515). Results are shown as means ± SEM.
The observed decreases in conditioned freezing in rats which received rapamycin injections were associated with a rightward shift in the input–output curves in both thalamic and cortical inputs to the LA, compared with vehicle-injected rats, indicating a decrease in synaptic strength that had been previously increased by fear conditioning (Fig. 2 D and E). In contrast, synaptic strength remained enhanced in both auditory inputs to the LA in rapamycin-injected but nonreactivated rats (Fig. 2 F and G). Overall, these findings demonstrate the requirement for mTOR activity in maintaining the postreactivation stability of synaptic potentiation in the conditioned stimulus pathways.
Synaptic Mechanisms of Fear Learning and Reconsolidation.
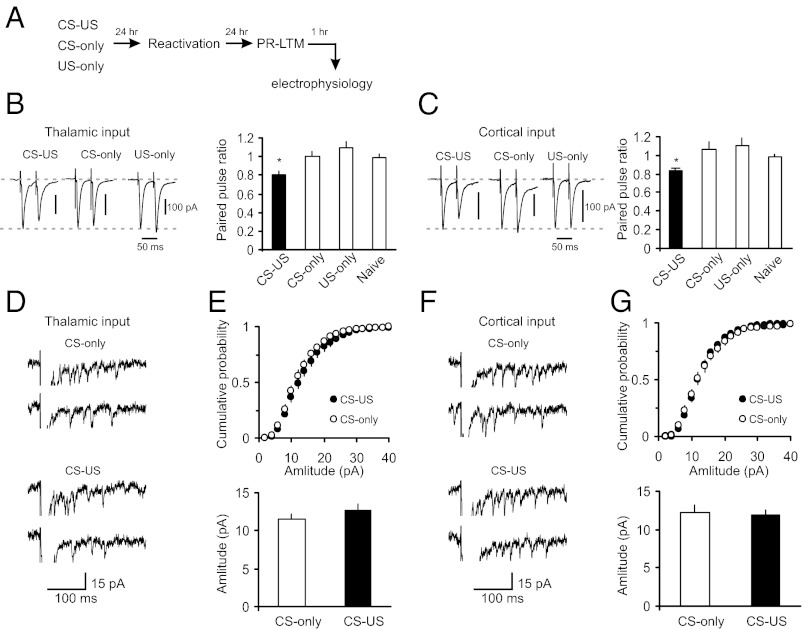
What are the loci (pre- versus postsynaptic) of synaptic enhancements after learning, compared with those involved in synaptic modifications after reconsolidation blockade? Efficacy of synaptic transmission is determined by the probability of neurotransmitter (glutamate) release (Pr) and/or postsynaptic responsiveness to glutamate contained in single synaptic vesicles (quantal amplitude), as well as by the number of effective synapses (33). We therefore estimated Pr and quantal amplitude in both thalamic and cortical inputs to the LA following fear conditioning and postreactivation rapamycin treatment. In agreement with previous findings (26, 28), we found that the increase in synaptic strength in fear-conditioned rats (as shown in Fig. 1 D and E) was accompanied by a decrease in the magnitude of paired-pulse ratio (PPR) recorded at a 50-ms interstimulus interval in both studied pathways, compared with control rats (Fig. 3 A–C). Because the magnitude of PPR varies inversely with the basal Pr (ref. 34; but see ref. 35), the observed increases in synaptic efficacy in the CS pathways of conditioned rats appear at least in part be due to the higher Pr. To estimate postsynaptic responsiveness, we recorded asynchronous single-quanta synaptic events evoked by stimulation of either thalamic or cortical inputs in the external medium where strontium (Sr2+) was substituted for Ca2+ (25). Asynchronous EPSCs may be observed for hundreds of milliseconds following the presynaptic stimulation pulse, thus permitting analysis of quantal responses in specific projections to the target area (36). Surprisingly, the acquisition of conditioned fear memory did not lead to detectable changes in the amplitude of single-quantum EPSCs in either thalamic (Fig. 3 D and E) or cortical inputs compared with the CS-only group (Fig. 3 F and G), which suggests a lack of postsynaptic modifications under present conditions (37).
Fig. 3.
Fear-conditioning–induced synaptic strengthening in inputs to the LA is primarily presynaptically mediated. (A) A schematic representation of the experimental design. Rats were trained in a single-trial fear conditioning paradigm and tested at 24 h (PR-LTM) after reactivation trials. (B, Left) Examples of EPSCs evoked in thalamic input to the LA with paired presynaptic stimuli in slices from CS-only, US-only, and fear-conditioned (CS–US) rats. The interstimulus interval was 50 ms. Traces are averages of 10 paired EPSCs. (B, Right) Summary plot of the paired-pulse stimulation experiments. Paired pulse ratio (PPR) was calculated as the ratio of the second EPSC amplitude to the first EPSC amplitude. CS-only group of rats, n = 10 neurons; US-only group, n = 12 neurons; naïve group, n = 17 neurons; CS–US group, n = 9 neurons. The magnitude of PPR in the paired group of rats (CS–US) was significantly decreased compared with naïve, CS-only, or US-only rats (one-way ANOVA, F3,44 = 4.02, P = 0.013. There was no difference in PPR values between naïve and CS-only (P = 0.45) or US-only groups (P = 0.203). All electrophysiological recordings for Fig. 3 were performed at 48 h post-CS–US pairing or single CS or US presentations (24 h postreactivation). (C) Experiments were analogous to B, but the EPSCs were recorded in cortical input to the LA. CS-only group, n = 8 neurons; US-only group, n = 9 neurons; naïve group, n = 18 neurons; paired group, n = 7 neurons. The magnitude of PPR in the paired group was significantly decreased compared with naïve, CS-only, or US-only rats (one-way ANOVA, F3,38 = 3.37, P = 0.028). There was no difference between naïve and CS-only rats (P = 0.1) or US-only rats (P = 0.1). (D) Traces of the asynchronous quantal EPSCs evoked by stimulation of thalamic input (VH= −70 mV) in slices from the CS-only and paired rats. In these experiments, Sr2+ was substituted for extracellular Ca2+. (E, Upper) Cumulative amplitude histograms of asynchronous quantal events recorded in thalamic input to the LA in slices from the CS-only and paired groups. (E, Lower) Summary plot of asynchronous EPSCs data (mean amplitude; CS-only, n = 9 neurons; paired, n = 10 neurons; t test, P = 0.34). (F and G) Experiments were analogous to D and E, but the asynchronous EPSCs were recorded in cortical input to the LA (CS-only, n = 5 neurons; paired, n = 7 neurons; t test, P = 0.73). Error bars indicate SEM.
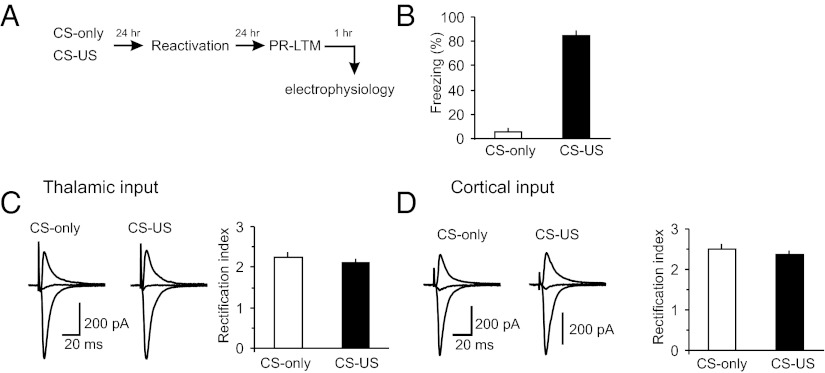
To explore further the possibility of postsynaptic modifications in the CS pathways during the single-trial fear-conditioning, we recorded AMPA receptor (AMPAR) EPSCs in both cortical and thalamic inputs to the LA in slices from the CS–US and CS-only groups at holding potentials of −70 mV or +40 mV. In these experiments, the intrapipette recording solution contained spermine (200 μM), a naturally occurring polyamine. We then calculated the rectification index for AMPAR EPSCs at cortico-LA and thalamo-LA synapses in slices from both behavioral groups, dividing the amplitude of AMPAR EPSC at −70 mV by the EPSC amplitude at +40 mV (as in ref. 30). Modifications in this index are indicative of changes in the AMPA receptor subunit composition. Specifically, the GluR1 subunit trafficking to synapses is normally expected to increase the rectification index (30). In our experiments, the values of rectification index, calculated at PR-LTM test, were not different between the CS-only and CS–US groups (Fig. 4 A–D). The observed lack of changes in rectification index, at times when consolidated fear memory was assayed, indicates that fear memory consolidation under conditions of the single-trial fear conditioning did not implicate increased GluR1 trafficking at activated synapses.
Fig. 4.
Rectification index for AMPAR EPSCs in inputs to the LA is not affected by single-trial fear conditioning. (A) A schematic representation of the experimental design. (B) Percent freezing observed in fear-conditioned rats (CS–US group) and CS-only rats at PR-LTM test (CS–US, n = 5 rats; CS-only, n = 6 rats; P < 0.001 between the groups). (C, Left) Averaged AMPAR EPSCs (15 traces) recorded in thalamic input to the LA at holding potentials of −70 mV, 0 mV, and +40 mV in slices from CS–US or CS-only rats. The AMPAR EPSCs were recorded in the presence of the NMDAR antagonist D-AP5 (50 μM). Intrapipette recording solution contained spermine (200 μM). The intensity of presynaptic stimulation was adjusted to produce the EPSCs of approximately same amplitude in both behavioral groups at a holding potential of −70 mV. (C, Right) the rectification index values at the thalamo-LA synapses in slices from CS–US and CS-only groups (CS–US group, n = 19 neurons from five rats; CS-only group, n = 23 neurons from six rats; P = 0.44 between two groups). (D) Experiments were analogous to C but the EPSCs were recorded in cortical input to the LA (CS–US group, n = 16 neurons from five rats; CS-only group, n = 22 neurons from six rats; P = 0.4 between two groups). Error bars indicate SEM.
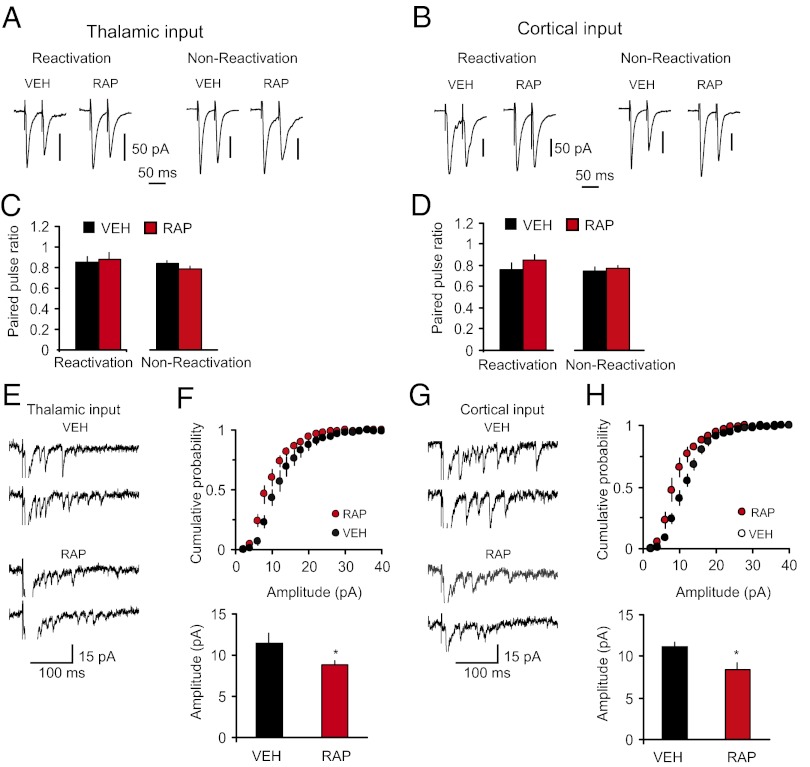
In contrast, we did not observe changes in the PPR magnitude in rats that received postretrieval injections of rapamycin, compared with the vehicle group (Fig. 5 A–D). Moreover, the magnitude of postretrieval PPR in rapamycin-treated rats did not differ from that in the paired group that did not receive the rapamycin treatment (as shown in Fig. 3 B and C; t test, P = 0.39 and P = 0.37 between groups in thalamic and cortical inputs, respectively), suggesting that presynaptic enhancements associated with fear conditioning were retained following reconsolidation blockade. Confirming that rapamycin had no direct effects on synaptic plasticity associated with the acquisition of conditioned fear memory, the magnitude of PPR in nonreactivated rats was also unaffected by rapamycin (Fig. 5 A–D). However, the amplitude of single-quantum thalamo-LA or cortico-LA EPSCs was significantly decreased in slices from rats with an impairment in reconsolidation, compared with the vehicle-injected rats (Fig. 5 E–H). Notably, postretrieval reconsolidation itself had no effect on the quantal amplitude. Thus, we compared the quantal amplitude values in thalamic and cortical inputs in the VEH group in Fig. 5 F and H, where fear memory was reactivated, with the quantal amplitude in the CS-only group in Fig. 3 E and G, respectively, where no reconsolidation was present as fear memory was not formed. The amplitude of unitary EPSCs did not differ between the groups (thalamic input: CS-only group in Fig. 3E versus VEH group in Fig. 5F, t test, P = 0.98; cortical input: CS-only group in Fig. 3G versus VEH group in Fig. 5H, t test, P = 0.36). Taken together, our results suggest that mTOR-dependent reconsolidation of fear memory and stabilization of conditioning-produced synaptic enhancements in CS pathways may implicate the mechanisms of postsynaptic plasticity, preventing decreases in the postsynaptic responsiveness to glutamate.
Fig. 5.
Postretrieval stabilization of conditioning-induced potentiation in inputs to the LA implicates postsynaptic mechanisms. (A, Left) Reactivation, examples of EPSCs evoked in thalamic input to the LA with paired stimuli in slices from fear-conditioned rats that received one injection of either rapamycin (RAP; 20 mg/kg, i.p.) or vehicle (VEH) immediately after the fear memory reactivation (memory was retrieved at 24 h postconditioning). Recordings were performed 24 h after the memory reactivation. (A, Right) Nonreactivation, examples of EPSCs recorded in slices from rats that received rapamycin or vehicle injections at 24 h postconditioning without memory reactivation. Recordings were performed 24 h after the injections. (B) Analogous to A, but the EPSCs were recorded in cortical input. (C) Summary plot of PPR data in thalamic input (reactivation: VEH, n = 19 neurons; RAP, n = 21 neurons; t test, P = 0.79; nonreactivation: VEH, n = 17 neurons; RAP, n = 24 neurons; t test, P = 0.19). (D) Summary plot of PPR data in cortical input (reactivation: VEH, n = 11 neurons; RAP, n = 13 neurons; t test, P = 0.31; nonreactivation: VEH, n = 10 neurons; RAP, n = 19 neurons; t test, P = 0.63). (E) Traces of the asynchronous quantal EPSCs evoked by stimulation of thalamic input in slices from VEH or RAP groups. (F, Upper) Cumulative amplitude histograms of asynchronous quantal events recorded in thalamic input to the LA in slices from VEH or RAP rats. (F, Lower) Summary plot of asynchronous EPSCs data (mean amplitude; VEH, n = 5 neurons; RAP, n = 7 neurons; t test, *P = 0.048). (G and H) The experiments were analogous to E and F, but the asynchronous EPSCs were recorded in cortical input to the LA (VEH, n = 5 neurons; RAP, n = 6 neurons; t test, *P = 0.026). Error bars indicate SEM.
Discussion
Our findings demonstrate that retrieval of fear memory converts learning-induced synaptic modifications to a labile state. Although retrieval, presumably, triggers the mechanisms of extinction learning in addition to reconsolidation of the original fear memory, augmentation of extinction following rapamycin treatment is an unlikely explanation for our results because extinction is blocked by inhibition of protein synthesis, not promoted by it (38). The cellular processes that maintain increased synaptic strength in the CS pathways after a memory recall require mTOR kinase activity. If mTOR signaling-dependent reconsolidation is blocked, synaptic strength returns to the default (preconditioning) level. Reconsolidation likely resulted from a form of synaptic plasticity that is mechanistically distinct from that involved in the acquisition of conditioned fear memory. Specifically, the decreases in synaptic strength, which we observed following the disruption of reconsolidation by rapamycin, appear due to modifications in postsynaptic processes, rather than reversal of presynaptic enhancements produced by initial fear learning. In our experiments, a single CS–US pairing was associated with increased Pr in auditory inputs to the LA. It is possible that multiple CS–US pairings would recruit postsynaptic mechanisms during the memory acquisition (as in ref. 30). The finding that the fear learning-induced enhancements in presynaptic function were retained following reconsolidation blockade, whereas postsynaptic restabilization of synaptic transmission was required to sustain its potentiation, indicates the potential role for both pre- and postsynaptic plasticity in maintaining conditioned fear memory after retrieval. The observed dissociation of the mechanisms used to enhance synaptic efficacy during learning and those affected by reconsolidation implies that reconsolidation might be not a unitary process from the cellular and molecular prospective.
These results, however, do not exclude a possibility that there might be different rules determining whether pre- and postsynaptic mechanisms are recruited during reconsolidation. One scenario is that the presynaptic mechanisms do not undergo reconsolidation and retained as a molecular and cellular legacy of prior learning. Alternatively, the presynaptic mechanisms may undergo reconsolidation, but the molecular pathways mediating presynaptic reconsolidation do not require mTOR activity. Another possibility might be that the postretrieval rapamycin administration might uncover or trigger a certain postsynaptic process reducing unitary events amplitude through the mechanisms not related to memory reconsolidation. The latter possibility is, however, unlikely as the effects of rapamycin were specifically linked to reactivation of consolidated fear memory. It would be interesting to examine in future studies whether postreactivation infusions of compounds (when they become available) that block specifically presynaptic mechanisms of memory consolidation could also suppress reconsolidation. Moreover, certain experimental characteristics, including the intensity of training procedures or memory age, could also determine whether and how consolidation and reconsolidation occur (7). Thus, although presynaptic mechanisms did not undergo reconsolidation under present experimental conditions, it might be possible that, under other conditions (e.g., with a stronger training protocol), the presynaptic mechanisms could become susceptible to reconsolidation.
Although postretrieval rapamycin virtually completely reversed the postconditioning enhancement in thalamo-LA and cortico-LA EPSCs produced by fear conditioning, it produced only a partial reduction in learned freezing. This divergence between electrophysiological and behavioral results suggests first, that there might be other mechanisms besides synaptic enhancement in CS pathways to the LA that underlie fear learning, and second that these other mechanisms do not require mTOR activity for maintaining their stability, thus warranting future investigation.
Further experiments will be required to identify other molecular components, both upstream and downstream, implicated in the mTOR-dependent control of fear memory reconsolidation at synaptic level and differentiate between the above-described hypotheses. Regardless, our findings suggest that targeting the mechanisms underlying postretrieval stabilization of synaptic plasticity could potentially be used to alleviate symptoms of anxiety disorders in which conditioned fear plays a role, such as posttraumatic stress disorder (PTSD) (39).
Experimental Procedures
Behavior: Single-Trial Fear Conditioning.
All animal procedures were approved by McLean Hospital's Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (350–375 g) were housed for at least one week before the experiment. Before behavioral training, rats were assigned randomly to one of four groups: paired (CS–US), CS-only, US-only, and naïve. On the training day, rats from the paired group were placed into a conditioning chamber, housed within a sound-attenuating cabinet (Med Associates), for 2 min before the onset of the CS. The CS was a tone (5 kHz, 75 dB) that lasted for 30 s. The last 2 s of the CS were paired with a continuous foot shock (0.6 mA, the US). After additional 30 s in the chamber, the rat was returned to its home cage. Memory was reactivated 24 h after training. Rats were then tested 24 h later for PR-LTM. For all tests, rats were placed into a different context and, 2 min later, exposed to the tone CS (5 kHz, 75 dB) for 60 s. Thirty sec after the termination of the tone, they were removed from the chamber and returned to their home cage. Freezing scores were calculated as the percentage of the total CS duration that the rat remained immobile (frozen) other than breathing. The identical training protocol has been used previously to demonstrate that auditory fear conditioning can undergo reconsolidation after retrieval that was blocked by BLA infusions of anisomycin (3). Rats in the CS-only group were trained and tested similarly to those of the paired CS–US group except that the foot shock US was omitted during training. Rats in the behaviorally naïve group were handled but not exposed to either training or testing chambers. Rats in the US-only group were placed into the chamber where they received a continuous foot shock (0.6 mA, 2 s) without any delay and then immediately removed from the chamber. Under these training conditions, the contribution of contextual fear learning was minimized. Responses of US-only rats to the tone CS (5 kHz, 75 dB) for 60 s were tested 24 h later in a different context and retested the next day (24 h later). Immediately after the conclusion of the PR-LTM session, rats were killed and brain slices containing the amygdala were prepared for electrophysiological recordings. In the experiments testing the effects of mTOR blockade on fear memory reconsolidation, rapamycin (20 mg/kg; LC Laboratories) or vehicle [70% DMSO (700 mg/mL)] was injected i.p. immediately after the fear memory reactivation. Freezing responses were evaluated 24 h later, and rats were used for electrophysiological recordings immediately after that. For statistical analysis, we used either a Student t test or two-way ANOVA with post hoc Bonferroni’s simultaneous multiple comparisons or one-way ANOVA (P < 0.05 was considered significant). The comparisons between slices from different experimental groups of rats were performed blind.
Electrophysiological Recordings.
Slices of the amygdale (both left and right, 250–300 μm) were prepared from behaviorally trained and naïve rats (as described above) with a vibratome. Slices were continuously superfused in solution containing 119 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.0 mM MgSO4, 1.25 mM NaH2PO4, 26.0 mM NaHCO3, 10 mM glucose, and 0.05 mM picrotoxin and equilibrated with 95% O2 and 5% CO2 (pH 7.3–7.4) at room temperature (22–24 °C). Whole-cell recordings of compound EPSCs were obtained from pyramidal neurons in the lateral nucleus of the amygdala (LA) under visual guidance (DIC/infrared optics) with an EPC-10 amplifier and Pulse v8.67 software (HEKA Elektronik). Evoked synaptic responses were triggered by field stimulation of the internal capsule (thalamic input) or the external capsule (cortical input) at 0.05 Hz with a fine-tipped (∼2 mm) glass stimulation pipette. The recording patch electrodes (3–6 MΩ resistance) contained 120 mM Cs-methane-sulfonate, 5 mM NaCl, 1 mM MgCl2, 10 mM BAPTA, 10 mM Hepes, 2 mM MgATP, and 0.1 mM NaGTP (adjusted to pH 7.2 with CsOH). A high concentration of the Ca2+ chelator BAPTA was included in the pipette solution to prevent the induction of synaptic plasticity in the studied pathways in slices which is not related to the modifications induced by behavioral training. Currents were filtered at 1 kHz and digitized at 5 kHz. The AMPAR EPSC amplitude was measured at a holding potential of −70 mV as the difference between the mean current during a prestimulus baseline and the mean current over a 1- to 2-ms window at the response peak. The evoked asynchronous EPSCs were recorded in both thalamic and cortical inputs to the LA in the Sr2+-containing external solution and analyzed using Mini Analysis Program (Synaptosoft).
Acknowledgments
This study was supported by National Institutes of Health Grants MH083011 (to V.Y.B) and MH090464 (to V.Y.B.), and US Army Medical Research Acquisition Activity Grant W81XWH-08-2-0126 (to R.K.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.McGaugh JL. Memory—a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 3.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 4.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36(3):521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 5.Dudai Y. Reconsolidation: The advantage of being refocused. Curr Opin Neurobiol. 2006;16(2):174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Doyère V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10(4):414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- 7.Nader K, Hardt O. A single standard for memory: The case for reconsolidation. Nat Rev Neurosci. 2009;10(3):224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 8.Rao-Ruiz P, et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14(10):1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- 9.Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23(12):5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47(6):873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Sangha S, Scheibenstock A, Lukowiak K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. J Neurosci. 2003;23(22):8034–8040. doi: 10.1523/JNEUROSCI.23-22-08034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SH, Ostlund SB, Nader K, Balleine BW. Consolidation and reconsolidation of incentive learning in the amygdala. J Neurosci. 2005;25(4):830–835. doi: 10.1523/JNEUROSCI.4716-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425(6958):616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24(20):4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93(24):13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey CH, Chen M. Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc Natl Acad Sci USA. 1988;85(23):9356–9359. doi: 10.1073/pnas.85.23.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey CH, Montarolo P, Chen M, Kandel ER, Schacher S. Inhibitors of protein and RNA synthesis block structural changes that accompany long-term heterosynaptic plasticity in Aplysia. Neuron. 1992;9(4):749–758. doi: 10.1016/0896-6273(92)90037-e. [DOI] [PubMed] [Google Scholar]
- 18.Rose JK, Rankin CH. Blocking memory reconsolidation reverses memory-associated changes in glutamate receptor expression. J Neurosci. 2006;26(45):11582–11587. doi: 10.1523/JNEUROSCI.2049-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26(50):12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90(1):28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gafford GM, Parsons RG, Helmstetter FJ. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience. 2011;182:98–104. doi: 10.1016/j.neuroscience.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoica L, et al. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci USA. 2011;108(9):3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 24.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 25.Shin RM, Tsvetkov E, Bolshakov VY. Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways. Neuron. 2006;52(5):883–896. doi: 10.1016/j.neuron.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390(6660):607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 27.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390(6660):604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 28.Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34(2):289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 29.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308(5718):83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 30.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330(6007):1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho JH, et al. Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in amygdala. Nat Neurosci. 2012;15(1):113–122. doi: 10.1038/nn.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casadio A, et al. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99(2):221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- 33.Regehr WG, Stevens CF. In: Synapses. Cowan WM, Sudhof TC, Stevens CF, editors. Baltimore: John Hopkins Univ Press; 2000. pp. 135–175. [Google Scholar]
- 34.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 35.Wang JH, Kelly PT. Attenuation of paired-pulse facilitation associated with synaptic potentiation mediated by postsynaptic mechanisms. J Neurophysiol. 1997;78(5):2707–2716. doi: 10.1152/jn.1997.78.5.2707. [DOI] [PubMed] [Google Scholar]
- 36.Oliet SH, Malenka RC, Nicoll RA. Bidirectional control of quantal size by synaptic activity in the hippocampus. Science. 1996;271(5253):1294–1297. doi: 10.1126/science.271.5253.1294. [DOI] [PubMed] [Google Scholar]
- 37.Enoki R, Hu YL, Hamilton D, Fine A. Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: Optical quantal analysis. Neuron. 2009;62(2):242–253. doi: 10.1016/j.neuron.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci USA. 2001;98(21):12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitman RK. Will reconsolidation blockade offer a novel treatment for posttraumatic stress disorder? Front Behav Neurosci. 2011;5:11. doi: 10.3389/fnbeh.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]