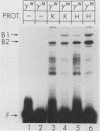
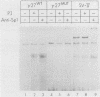
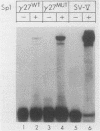
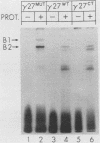
Abstract
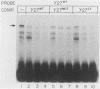
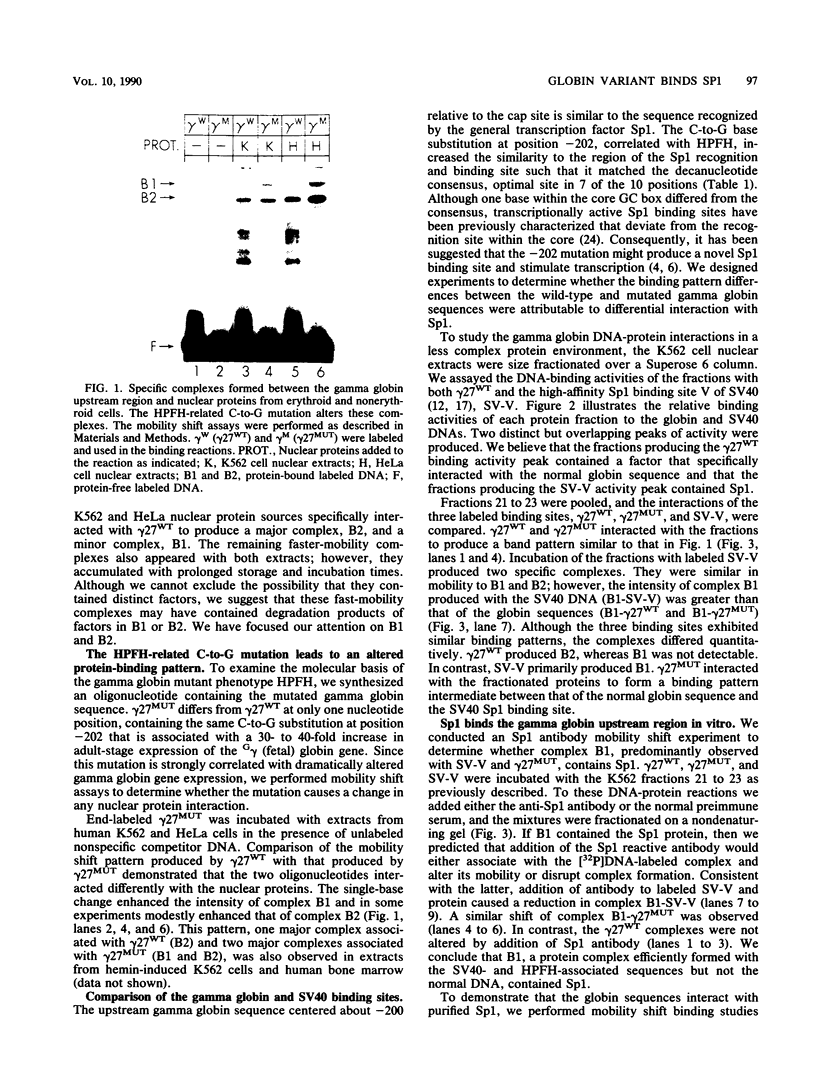
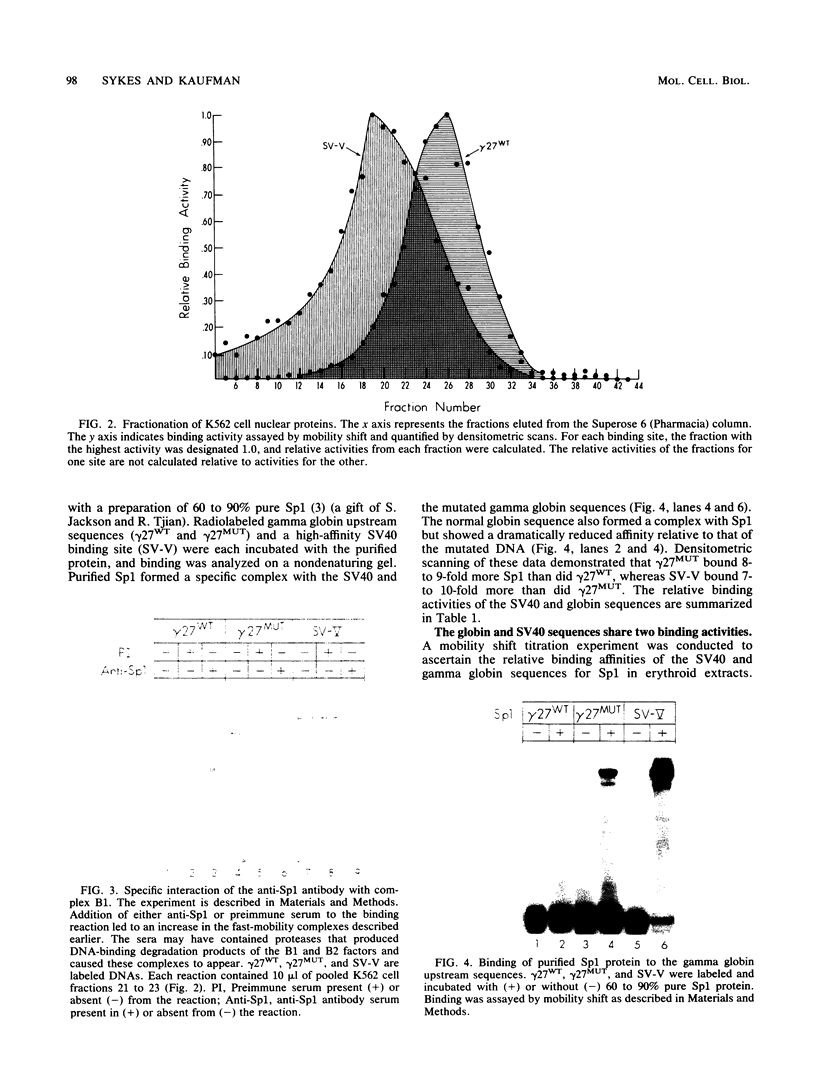
Transcription of the human fetal globin genes in erythroid cells is tightly regulated during different stages of development and differentiation. Two naturally occurring mutations 202 base pairs upstream of the duplicated gamma globin genes are associated with incorrectly regulated gamma globin gene gene expression; elevated levels of fetal globin are synthesized during adult life. A C-to-G base substitution upstream of the G gamma-globin gene is highly correlated with a dramatic increase in gene expression. It increases the similarity of the region to the consensus Sp1 recognition site. We determined that the mutated DNA had a 5- to 10-fold-higher affinity for Sp1 than did normal gamma globin gene sequence. We also observed a reduction in normal factor-binding activity. A different substitution at -202, C to T, upstream of the A gamma-globin gene was associated with a more moderate increase in fetal globin expression. This mutation decreased the similarity of the sequence to an Sp1 recognition site. We determined that it did not result in enhanced Sp1 binding but did alter normal factor binding. We suggest that these changes in nuclear protein-binding properties detected in vitro are responsible for the enhanced gamma globin gene expression found in -202 G gamma beta + patients with hereditary persistence of fetal hemoglobin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnou N. P., Karlsson S., Moulton A. D., Keller G., Nienhuis A. W. Promoter sequences required for function of the human gamma globin gene in erythroid cells. EMBO J. 1986 Jan;5(1):121–126. doi: 10.1002/j.1460-2075.1986.tb04185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnou N. P., Moulton A. D., Keller G., Karlsson S., Papayannopoulou T., Stamatoyannopoulos G., Nienhuis A. W. Cis-acting sequences that affect the expression of the human fetal gamma-globin genes. Prog Clin Biol Res. 1985;191:163–182. [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Chao M. V., Mellon P., Charnay P., Maniatis T., Axel R. The regulated expression of beta-globin genes introduced into mouse erythroleukemia cells. Cell. 1983 Feb;32(2):483–493. doi: 10.1016/0092-8674(83)90468-3. [DOI] [PubMed] [Google Scholar]
- Charnay P., Maniatis T. Transcriptional regulation of globin gene expression in the human erythroid cell line K562. Science. 1983 Jun 17;220(4603):1281–1283. doi: 10.1126/science.6574602. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Boehm C. D., Waber P. G., Stoeckert C. J., Jr, Weissman S. M., Forget B. G., Kazazian H. H., Jr Concordance of a point mutation 5' to the G gamma globin gene with G gamma beta +. Hereditary persistence of fetal hemoglobin in the black population. Blood. 1984 Dec;64(6):1292–1296. [PubMed] [Google Scholar]
- Collins F. S., Metherall J. E., Yamakawa M., Pan J., Weissman S. M., Forget B. G. A point mutation in the A gamma-globin gene promoter in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):325–326. doi: 10.1038/313325a0. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Stoeckert C. J., Jr, Serjeant G. R., Forget B. G., Weissman S. M. G gamma beta+ hereditary persistence of fetal hemoglobin: cosmid cloning and identification of a specific mutation 5' to the G gamma gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4894–4898. doi: 10.1073/pnas.81.15.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A., Ley T. J., Humphries R. K., Fordis M., Schechter A. N. Inducible transcription of five globin genes in K562 human leukemia cells. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5515–5519. doi: 10.1073/pnas.80.18.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Fong T. C. Erythroid-specific activation and derepression of the chick beta-globin promoter in vitro. Cell. 1989 Jun 30;57(7):1189–1200. doi: 10.1016/0092-8674(89)90056-1. [DOI] [PubMed] [Google Scholar]
- Gelinas R., Bender M., Lotshaw C., Waber P., Kazazian H., Jr, Stamatoyannopoulos G. Chinese A gamma fetal hemoglobin: C to T substitution at position-196 of the A gamma gene promoter. Blood. 1986 Jun;67(6):1777–1779. [PubMed] [Google Scholar]
- Gelinas R., Endlich B., Pfeiffer C., Yagi M., Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the A gamma-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- Gelinas R., Yagi M., Endlich B., Lotshaw C., Kazazian H. H., Jr, Stamatoyannopoulos G. Sequences of G gamma, A gamma, and beta genes of the Greek (A gamma) HPFH mutant: evidence for a distal CCAAT box mutation in the A gamma gene. Prog Clin Biol Res. 1985;191:125–139. [PubMed] [Google Scholar]
- Gidoni D., Kadonaga J. T., Barrera-Saldaña H., Takahashi K., Chambon P., Tjian R. Bidirectional SV40 transcription mediated by tandem Sp1 binding interactions. Science. 1985 Nov 1;230(4725):511–517. doi: 10.1126/science.2996137. [DOI] [PubMed] [Google Scholar]
- Giglioni B., Casini C., Mantovani R., Merli S., Comi P., Ottolenghi S., Saglio G., Camaschella C., Mazza U. A molecular study of a family with Greek hereditary persistence of fetal hemoglobin and beta-thalassemia. EMBO J. 1984 Nov;3(11):2641–2645. doi: 10.1002/j.1460-2075.1984.tb02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H. DNA sequence variation associated with elevated fetal G gamma globin production. Blood. 1985 Oct;66(4):783–787. [PubMed] [Google Scholar]
- Gilman J. G., Kutlar F., Johnson M. E., Huisman T. H. A G to A nucleotide substitution 161 base pairs 5' of the G gamma globin gene cap site (-161) in a high G gamma non-anemic person. Prog Clin Biol Res. 1987;251:383–390. [PubMed] [Google Scholar]
- Gilman J. G., Mishima N., Wen X. J., Kutlar F., Huisman T. H. Upstream promoter mutation associated with a modest elevation of fetal hemoglobin expression in human adults. Blood. 1988 Jul;72(1):78–81. [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Lin H. J., Anagnou N. P., Rutherford T. R., Shimada T., Nienhuis A. W. Activation of the human beta-globin promoter in K562 cells by DNA sequences 5' to the fetal gamma- or embryonic zeta-globin genes. J Clin Invest. 1987 Aug;80(2):374–380. doi: 10.1172/JCI113082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingrel J. B., Weimer J., Menon A. Binding of nuclear factors to an upstream region of the human A gamma globin gene. Prog Clin Biol Res. 1987;251:201–210. [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Giglioni B., Comi P., Cappellini N., Nicolis S., Ottolenghi S. A protein factor binding to an octamer motif in the gamma-globin promoter disappears upon induction of differentiation and hemoglobin synthesis in K562 cells. Nucleic Acids Res. 1987 Nov 25;15(22):9349–9364. doi: 10.1093/nar/15.22.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Nicolis S., Taramelli R., Malgaretti N., Mantovani R., Comi P., Giglioni B., Longinotti M., Dore F., Oggiano L. Sardinian G gamma-HPFH: a T----C substitution in a conserved "octamer" sequence in the G gamma-globin promoter. Blood. 1988 Mar;71(3):815–817. [PubMed] [Google Scholar]
- Rixon M. W., Gelinas R. E. A fetal globin gene mutation in A gamma nondeletion hereditary persistence of fetal hemoglobin increases promoter strength in a nonerythroid cell. Mol Cell Biol. 1988 Feb;8(2):713–721. doi: 10.1128/mcb.8.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford T., Nienhuis A. W. Human globin gene promoter sequences are sufficient for specific expression of a hybrid gene transfected into tissue culture cells. Mol Cell Biol. 1987 Jan;7(1):398–402. doi: 10.1128/mcb.7.1.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Stoeckert C. J., Jr, Metherall J. E., Yamakawa M., Eisenstadt J. M., Weissman S. M., Forget B. G. Expression of the affected A gamma globin gene associated with Greek nondeletion hereditary persistence of fetal hemoglobin. Mol Cell Biol. 1987 Aug;7(8):2999–3003. doi: 10.1128/mcb.7.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga G., Barberis A., Schaffner G., Busslinger M. The -117 mutation in Greek HPFH affects the binding of three nuclear factors to the CCAAT region of the gamma-globin gene. EMBO J. 1988 Oct;7(10):3099–3107. doi: 10.1002/j.1460-2075.1988.tb03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey S., Delgrosso K., Malladi P., Schwartz E. A single-base change at position -175 in the 5'-flanking region of the G gamma-globin gene from a black with G gamma-beta+ HPFH. Blood. 1988 Mar;71(3):807–810. [PubMed] [Google Scholar]
- Tate V. E., Wood W. G., Weatherall D. J. The British form of hereditary persistence of fetal hemoglobin results from a single base mutation adjacent to an S1 hypersensitive site 5' to the A gamma globin gene. Blood. 1986 Dec;68(6):1389–1393. [PubMed] [Google Scholar]
- Trudel M., Magram J., Bruckner L., Costantini F. Upstream G gamma-globin and downstream beta-globin sequences required for stage-specific expression in transgenic mice. Mol Cell Biol. 1987 Nov;7(11):4024–4029. doi: 10.1128/mcb.7.11.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Waber P. G., Bender M. A., Gelinas R. E., Kattamis C., Karaklis A., Sofroniadou K., Stamatoyannopoulos G., Collins F. S., Forget B. G., Kazazian H. H., Jr Concordance of a point mutation 5' to the A gamma-globin gene with A gamma beta + hereditary persistence of fetal hemoglobin in Greeks. Blood. 1986 Feb;67(2):551–554. [PubMed] [Google Scholar]
- Wright S., Rosenthal A., Flavell R., Grosveld F. DNA sequences required for regulated expression of beta-globin genes in murine erythroleukemia cells. Cell. 1984 Aug;38(1):265–273. doi: 10.1016/0092-8674(84)90548-8. [DOI] [PubMed] [Google Scholar]