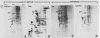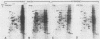Abstract
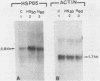
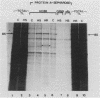
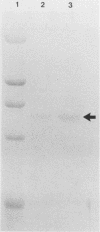
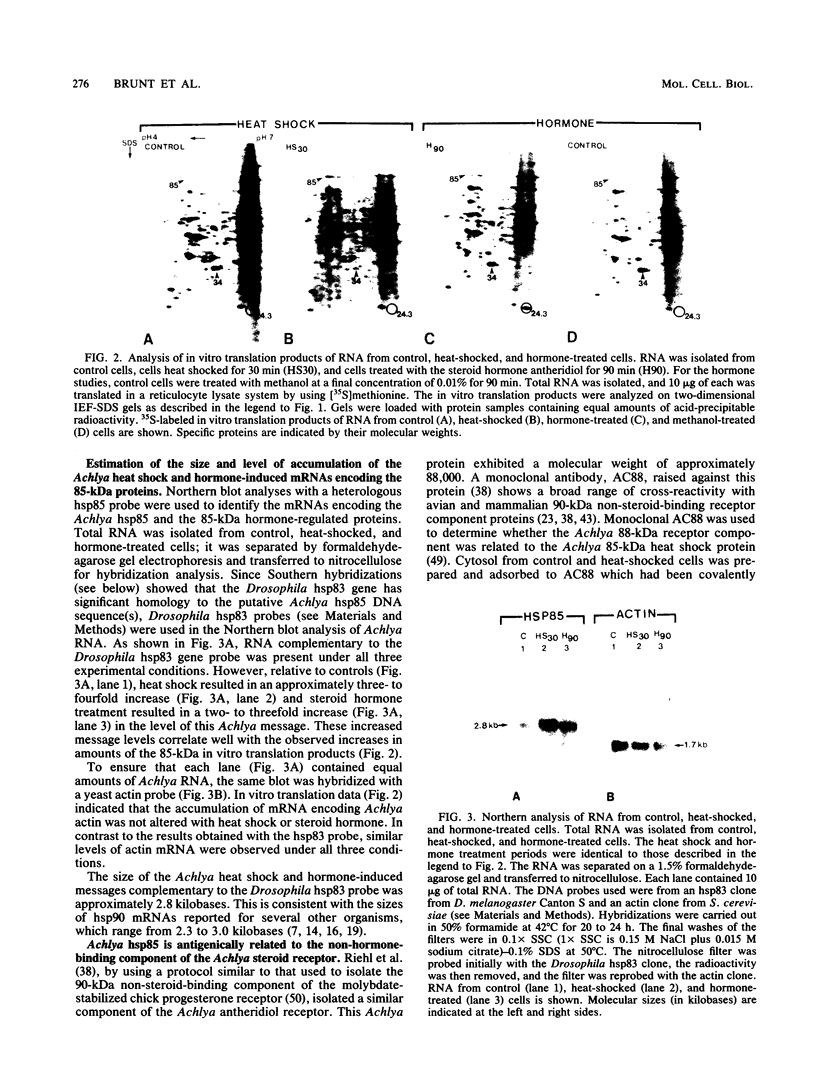
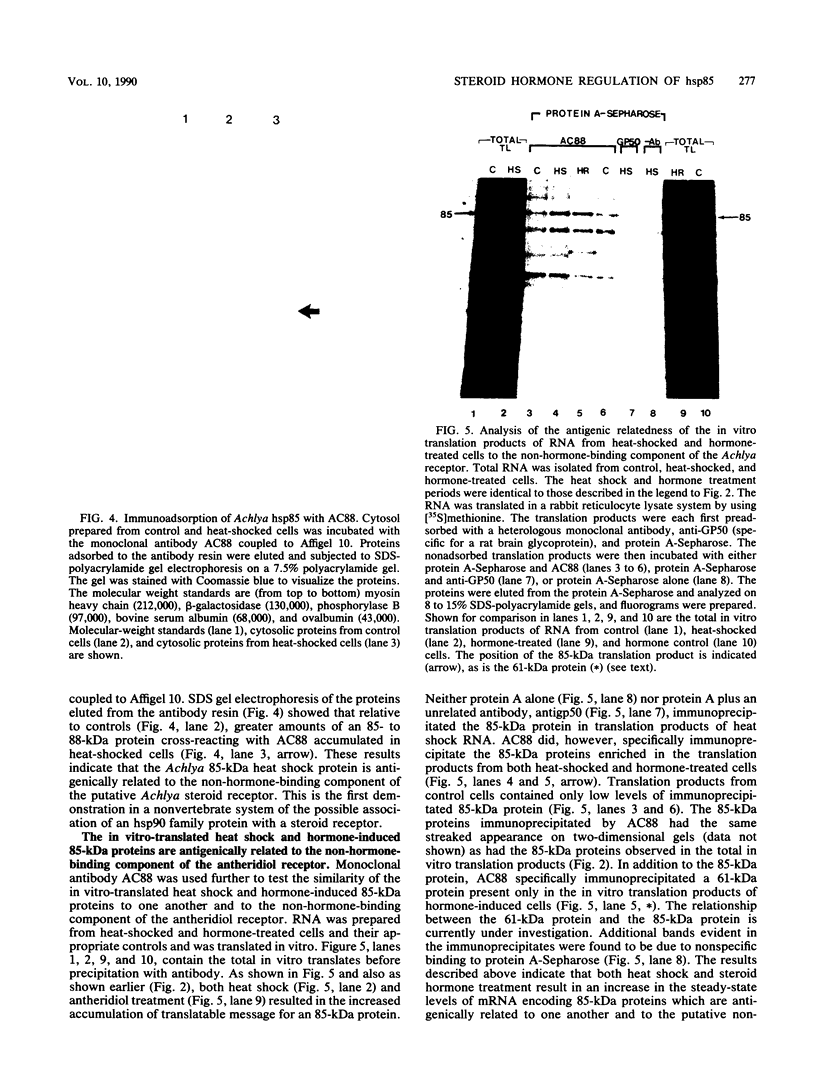
The steroid hormone antheridiol regulates sexual development in the fungus Achlya ambisexualis. Analyses of in vivo-labeled proteins from hormone-treated cells revealed that one of the characteristic antheridiol-induced proteins appeared to be very similar to the Achyla 85-kilodalton (kDa) heat shock protein. Analysis of in vitro translation products of RNA isolated from control, heat-shocked, or hormone-treated cells demonstrated an increased accumulation of mRNA encoding a similar 85-kDa protein in both the heat-shocked and hormone-treated cells. Northern (RNA) blot analyses with a Drosophila melanogaster hsp83 probe indicated that a mRNA species of approximately 2.8 kilobases was substantially enriched in both heat-shocked and hormone-treated cells. The monoclonal antibody AC88, which recognizes the non-hormone-binding component of the Achyla steroid receptor, cross-reacted with Achlya hsp85 in cytosols from heat-shocked cells. This monoclonal antibody also recognized both the hormone-induced and heat shock-induced 85-kDa in vitro translation products. Taken together, these data suggest that similar or identical 85-kDa proteins are independently regulated by the steroid hormone antheridiol and by heat shock and that this protein is part of the Achyla steroid receptor complex. Our results demonstrate that the association of hsp90 family proteins with steroid receptors observed in mammals and birds extends also to the eucaryotic microbes and suggest that this association may have evolved early in steroid-responsive systems.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale A. W. Sexual hormones of Achlya and other fungi. Science. 1969 Nov 14;166(3907):831–837. doi: 10.1126/science.166.3907.831. [DOI] [PubMed] [Google Scholar]
- Barksdale A. W. The sexual hormones of the fungus Achlya. Ann N Y Acad Sci. 1967 Aug 9;144(1):313–319. doi: 10.1111/j.1749-6632.1967.tb34026.x. [DOI] [PubMed] [Google Scholar]
- Barnier J. V., Bensaude O., Morange M., Babinet C. Mouse 89 kD heat shock protein. Two polypeptides with distinct developmental regulation. Exp Cell Res. 1987 May;170(1):186–194. doi: 10.1016/0014-4827(87)90128-5. [DOI] [PubMed] [Google Scholar]
- Berbers G. A., Kunnen R., van Bergen en Henegouwen P. M., van Wijk R. Localization and quantitation of hsp84 in mammalian cells. Exp Cell Res. 1988 Aug;177(2):257–271. doi: 10.1016/0014-4827(88)90460-0. [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Meselson M. Interspecific nucleotide sequence comparisons used to identify regulatory and structural features of the Drosophila hsp82 gene. J Mol Biol. 1986 Apr 20;188(4):499–515. doi: 10.1016/s0022-2836(86)80001-8. [DOI] [PubMed] [Google Scholar]
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt S. A., Silver J. C. Cellular localization of steroid hormone-regulated proteins during sexual development in Achlya. Exp Cell Res. 1986 Aug;165(2):306–319. doi: 10.1016/0014-4827(86)90585-9. [DOI] [PubMed] [Google Scholar]
- Brunt S. A., Silver J. C. Steroid hormone-induced changes in secreted proteins in the filamentous fungus Achlya. Exp Cell Res. 1986 Mar;163(1):22–34. doi: 10.1016/0014-4827(86)90555-0. [DOI] [PubMed] [Google Scholar]
- Carbajal M. E., Duband J. L., Lettre F., Valet J. P., Tanguay R. M. Cellular localization of Drosophila 83-kilodalton heat shock protein in normal, heat-shocked, and recovering cultured cells with a specific antibody. Biochem Cell Biol. 1986 Aug;64(8):816–825. doi: 10.1139/o86-110. [DOI] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Feramisco J. R., Helfman D. M. Cloning of the chick hsp 90 cDNA in expression vector. Nucleic Acids Res. 1985 Sep 11;13(17):6035–6047. doi: 10.1093/nar/13.17.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly F. W., Finkelstein D. B. Complete sequence of the heat shock-inducible HSP90 gene of Saccharomyces cerevisiae. J Biol Chem. 1984 May 10;259(9):5745–5751. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gasc J. M., Renoir J. M., Radanyi C., Joab I., Tuohimaa P., Baulieu E. E. Progesterone receptor in the chick oviduct: an immunohistochemical study with antibodies to distinct receptor components. J Cell Biol. 1984 Oct;99(4 Pt 1):1193–1201. doi: 10.1083/jcb.99.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Sadis S., Smale G., Weber L. A. Molecular cloning of sequences encoding the human heat-shock proteins and their expression during hyperthermia. Gene. 1986;43(1-2):147–154. doi: 10.1016/0378-1119(86)90018-1. [DOI] [PubMed] [Google Scholar]
- Holmgren R., Corces V., Morimoto R., Blackman R., Meselson M. Sequence homologies in the 5' regions of four Drosophila heat-shock genes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3775–3778. doi: 10.1073/pnas.78.6.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren R., Livak K., Morimoto R., Freund R., Meselson M. Studies of cloned sequences from four Drosophila heat shock loci. Cell. 1979 Dec;18(4):1359–1370. doi: 10.1016/0092-8674(79)90246-0. [DOI] [PubMed] [Google Scholar]
- Howard K. J., Distelhorst C. W. Evidence for intracellular association of the glucocorticoid receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Mar 5;263(7):3474–3481. [PubMed] [Google Scholar]
- Joab I., Radanyi C., Renoir M., Buchou T., Catelli M. G., Binart N., Mester J., Baulieu E. E. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. 1984 Apr 26-May 2Nature. 308(5962):850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- Kasambalides E. J., Lanks K. W. Dexamethasone can modulate glucose-regulated and heat shock protein synthesis. J Cell Physiol. 1983 Jan;114(1):93–98. doi: 10.1002/jcp.1041140116. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai B. T., Chin N. W., Stanek A. E., Keh W., Lanks K. W. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984 Dec;4(12):2802–2810. doi: 10.1128/mcb.4.12.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Cutt J. R., Brugge J. S. Association of the transforming proteins of Rous, Fujinami, and Y73 avian sarcoma viruses with the same two cellular proteins. Mol Cell Biol. 1982 Jul;2(7):875–880. doi: 10.1128/mcb.2.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T. C., Barksdale A. W. Isolation of a sex hormone from the water mould Achlya bisexualis. Nature. 1967 Jul 15;215(5098):320–321. doi: 10.1038/215320a0. [DOI] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pekkala D., Heath B., Silver J. C. Changes in chromatin and the phosphorylation of nuclear proteins during heat shock of Achlya ambisexualis. Mol Cell Biol. 1984 Jul;4(7):1198–1205. doi: 10.1128/mcb.4.7.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkala D., Silver J. C. Heat-shock-induced changes in the phosphorylation of ribosomal and ribosome-associated proteins in the filamentous fungus Achlya ambisexualis. Exp Cell Res. 1987 Feb;168(2):325–337. doi: 10.1016/0014-4827(87)90005-x. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. Transformation of glucocorticoid and progesterone receptors to the DNA-binding state. J Cell Biochem. 1987 Sep;35(1):51–68. doi: 10.1002/jcb.240350105. [DOI] [PubMed] [Google Scholar]
- Ramachandran C., Catelli M. G., Schneider W., Shyamala G. Estrogenic regulation of uterine 90-kilodalton heat shock protein. Endocrinology. 1988 Aug;123(2):956–961. doi: 10.1210/endo-123-2-956. [DOI] [PubMed] [Google Scholar]
- Redeuilh G., Moncharmont B., Secco C., Baulieu E. E. Subunit composition of the molybdate-stabilized "8-9 S" nontransformed estradiol receptor purified from calf uterus. J Biol Chem. 1987 May 25;262(15):6969–6975. [PubMed] [Google Scholar]
- Riehl R. M., Sullivan W. P., Vroman B. T., Bauer V. J., Pearson G. R., Toft D. O. Immunological evidence that the nonhormone binding component of avian steroid receptors exists in a wide range of tissues and species. Biochemistry. 1985 Nov 5;24(23):6586–6591. doi: 10.1021/bi00344a042. [DOI] [PubMed] [Google Scholar]
- Riehl R. M., Toft D. O. Analysis of the steroid receptor of Achlya ambisexualis. J Biol Chem. 1984 Dec 25;259(24):15324–15330. doi: 10.1515/9783110885026.733. [DOI] [PubMed] [Google Scholar]
- Riehl R. M., Toft D. O., Meyer M. D., Carlson G. L., McMorris T. C. Detection of a pheromone-binding protein in the aquatic fungus Achlya ambisexualis. Exp Cell Res. 1984 Aug;153(2):544–549. doi: 10.1016/0014-4827(84)90623-2. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Housley P. R., Pratt W. B. The molybdate-stabilized glucocorticoid binding complex of L-cells contains a 98-100 kdalton steroid binding phosphoprotein and a 90 kdalton nonsteroid-binding phosphoprotein that is part of the murine heat-shock complex. J Steroid Biochem. 1986 Jan;24(1):9–18. doi: 10.1016/0022-4731(86)90025-7. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Meshinchi S., Tienrungroj W., Schlesinger M. J., Toft D. O., Pratt W. B. Relationship of the 90-kDa murine heat shock protein to the untransformed and transformed states of the L cell glucocorticoid receptor. J Biol Chem. 1987 May 25;262(15):6986–6991. [PubMed] [Google Scholar]
- Sanchez E. R., Toft D. O., Schlesinger M. J., Pratt W. B. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985 Oct 15;260(23):12398–12401. [PubMed] [Google Scholar]
- Schuh S., Yonemoto W., Brugge J., Bauer V. J., Riehl R. M., Sullivan W. P., Toft D. O. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985 Nov 15;260(26):14292–14296. [PubMed] [Google Scholar]
- Shyamala G., Gauthier Y., Moore S. K., Catelli M. G., Ullrich S. J. Estrogenic regulation of murine uterine 90-kilodalton heat shock protein gene expression. Mol Cell Biol. 1989 Aug;9(8):3567–3570. doi: 10.1128/mcb.9.8.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J. C., Andrews D. R., Pekkala D. Effect of heat shock on synthesis and phosphorylation of nuclear and cytoplasmic proteins in the fungus Achlya. Can J Biochem Cell Biol. 1983 Jun;61(6):447–455. doi: 10.1139/o83-060. [DOI] [PubMed] [Google Scholar]
- Silver J. C. Basic nuclear proteins in the aquatic fungus Achlya ambisexualis. Biochim Biophys Acta. 1979 Oct 25;564(3):507–516. doi: 10.1016/0005-2787(79)90040-6. [DOI] [PubMed] [Google Scholar]
- Silver J. C., Horgen P. A. Hormonal regulation of presumptive mRNA in the fungus Achlya ambisexualis. Nature. 1974 May 17;249(454):252–254. doi: 10.1038/249252a0. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Vroman B. T., Bauer V. J., Puri R. K., Riehl R. M., Pearson G. R., Toft D. O. Isolation of steroid receptor binding protein from chicken oviduct and production of monoclonal antibodies. Biochemistry. 1985 Jul 16;24(15):4214–4222. doi: 10.1021/bi00336a060. [DOI] [PubMed] [Google Scholar]
- Thomas S. R., Lengyel J. A. Ecdysteroid-regulated heat-shock gene expression during Drosophila melanogaster development. Dev Biol. 1986 Jun;115(2):434–438. doi: 10.1016/0012-1606(86)90263-0. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Alterations in RNA and protein synthesis associated with steroid hormone-induced sexual morphogenesis in the water mold Achlya. Dev Biol. 1976 Jul 15;51(2):202–214. doi: 10.1016/0012-1606(76)90138-x. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]
- Wieben E. D. Regulation of the synthesis of lactate dehydrogenase-X during spermatogenesis in the mouse. J Cell Biol. 1981 Mar;88(3):492–498. doi: 10.1083/jcb.88.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Catelli M. G., Joab I., Moncharmont B. Association of the heat shock protein hsp90 with steroid hormone receptors and tyrosine kinase oncogene products. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1298–1307. doi: 10.1016/s0006-291x(86)80424-7. [DOI] [PubMed] [Google Scholar]