Abstract
While much is known about adult obesity in nonhuman primates, very little is known regarding development of childhood adiposity. As small monkeys that are easy to handle and have a relatively fast life history, common marmoset monkeys (Callithrix jacchus) offer interesting opportunities to examine the question of fat versus lean mass growth in a nonhuman primate. This paper provides an overview of our understanding of early life growth in mass in marmoset monkeys, based primarily upon our past 20 years of research, culminating in our recent findings on early life obesity in this species. Common marmosets display variance in early life growth patterns that is related to both pre- and post-natal factors and the marmoset uterine environment is exquisitely designed to reflect resources available for the gestation of multiple offspring, making them an interesting model of developmental programming. We have demonstrated that obesity can be generated in very early life in captive marmosets, with excess adiposity evident by one month of age, making this species a potentially valuable model in which to study pediatric obesity and its sequelae. Birth weight is associated with adiposity in animals vulnerable to obesity. Early life exposure to higher fat diets enhances the chances of post-weaning obesity development. However, overall higher food consumption is also associated with obesity development at later ages. One unexpected finding in our studies has been the relatively high body fat percentage of neonatal (12–18%) marmosets suggesting that hypotheses regarding the uniqueness of high human neonatal adiposity merit further examination.
Keywords: pediatric obesity, adiposity, growth, nonhuman primate, marmoset
Obesity is now recognized as a preeminent human health concern. Gregg et al. (2009) reported age-adjusted estimates of obesity prevalence ranging from 12.4% to 43.7%, with a median of 28.4% in the United States in 2007. Obesity is linked to increased risk of a variety of chronic diseases including diabetes and cardiovascular disease (Eckel et al., 2005). Captive adult nonhuman primates also display obesity. Species in which spontaneous or diet-induced obesity in a captive setting have been described include macaques (Kemnitz, 1984; Bodkin et al., 1993; Wagner et al., 2006), vervet monkeys (Kavanagh et al., 2007), baboons (Comuzzie et al., 2003), squirrel monkeys (Ausman et al., 1981) and marmosets (Tardif et al., 2009; Wachtman et al., 2011). Weight gain associated with infection of adult macaques and marmosets with human adenovirus AD36 has been reported (Dhurandhar et al., 2002). Sequelae to obesity are described in most of these cases that mirror those seen in humans, including insulin resistance, diabetes, dyslipidemia, lipid accumulation in the liver and atherosclerotic changes (Hansen and Bodkin, 1993; Wagner et al., 2006; Tardif et al., 2009; Wachtman et al., 2011). Less well understood are the factors that cause obesity to occur spontaneously in some animals and not others or the reasons that variable responses to diet-induced obesity are observed.
Childhood obesity is a growing concern in the world with an average rate of 11.7% (95% CI: 8.9 – 15.3%) in developed countries and 6.1% (95% CI: 5.0 – 7.2%) in developing nations (de Onis et al., 2010). The proportion of preschool-aged (2–4 years old) children in the U.S. classified as obese is approximately 15% (CDC, 2009). Among 4 year olds the estimated prevalence of obesity in the U.S. is over 18%, ranging as high as 31.2% in American Indians (Anderson and Whitaker, 2009). Childhood obesity is associated with an increased risk of adult obesity and of early life occurrence of diseases that have been associated historically with middle- and late-age in humans, such as Type II diabetes (Gardner et al., 2009).
Animal models are important for deciphering the mechanisms underlying obesity and testing therapeutics that treat obesity and its sequelae. Such models are generally chosen to be efficient and tractable, both in terms of genetics and environment. As such, rodent models (Zhang, et al., 1994; Levin et al., 1997; Speakman et al., 2007) have provided the primary testing ground for studies of obesity. However, rodent models have limitations when applying findings to humans including phylogenetic differences in fat cell function and distribution; development and circadian rhythm of feeding behavior; and functions of some adipokines (e.g., resistin) between rodents and humans (Levin and Govek, 1998; Henrichs, 2001; Arner, 2005; Spurlock and Gabler, 2008). In terms of the study of pediatric obesity, the strikingly different patterns between mice and primates of pre- versus post-natal growth and of post-natal growth dependence upon milk versus post-weaning nutrition places some limits on the usefulness of rodent models. Therefore, nonhuman primate models of pediatric obesity may be of value to examine primate-specific aspects of pre- versus post-natal growth on obesity development and to validate findings from rodents in a model system more closely resembling humans, but offering control over genetic and environmental factors that is generally lacking in human studies.
However, there are substantial practical problems associated with tracking growth parameters in neonatal and pre-weaning nonhuman primates, even in a captive setting and, for this reason very little is known regarding development of early life adiposity in most monkeys and apes. There have been some studies of the effects of early nutrition via nursery rearing on body mass growth in baboons, most notably those of Lewis et al. (1983) and Coelho and colleagues (Coelho and Glassman, 1984; Coelho, 1985; Rutenberg and Coelho, 1988); however, the longitudinal data available on early life growth in mother-reared monkeys is limited. In particular, longitudinal data that track development of lean and fat mass during this period are particularly rare.
A relatively fast maturing and tractable primate would offer advantages in certain types of obesity studies. For example, evidence from animal models and human epidemiological studies suggests that prenatal condition may predispose offspring to adult obesity and associated metabolic disorders (Oiken and Gilman, 2003; Baird et al., 2005). Common marmosets (Callithrix jacchus) offer the ability to model this process in a short-lived primate. Small (350–400 grams) South American primates that are capable of producing twins or triplets every 5.5 months, marmosets are the most fertile of the anthropoid primates (Tardif et al., 2003). They begin weaning at around one month of age, have completed weaning by around 3 months of age, begin puberty at around 9 months of age, have a stable adult weight by around 17 months of age and are skeletally mature by 24 months of age (Tardif et al., 2003; Tardif and Bales, 2004). This fast growth period, combined with relative ease of handling based upon their small size, makes this species a potentially valuable model of early life adiposity development in a nonhuman primate. In addition to the benefit offered by a fast life history, the routine production of marmoset twins offers the opportunity to have more than one infant exposed to the same uterine but different post-natal family environments via cross-fostering.
This paper provides an overview of our understanding of early life growth in mass in marmoset monkeys, based primarily upon our past 20 years of research, culminating in our recent findings on early life obesity in this species. All of these findings are based upon captive studies with the majority of what is reported here based upon cross-sectional and longitudinal studies of a marmoset colony that we established in 1994 and that we continue to follow to date. We know of no information on birth weights in wild marmosets and no longitudinal data on growth in mass in young, wild animals. However, it is clear that, with average weights in the range of 350–450 grams, most captive adult marmosets exceed the weights observed in wild populations, where 320–340 grams is typical (Ford and Davis, 1992; Araujo et al., 2000; Tardif et al., 2009). Captive marmosets likely represent a modern human situation in which food is relatively abundant and required physical activity is relatively limited. Indeed, Altmann et al. (1993) documented increasing levels of fatness in wild baboons that were provided with easy access to abundant food resources. These observations suggest that nonhuman primates display the same underlying physiological processes as humans that generate increasing adiposity in the face of increasing food availability and low activity requirements.
Methods involved in collection of birth condition, including weight are described in Tardif et al. (2002). Subsequent longitudinal assessments of body mass through infancy are described in Tardif et al. (2001). Lean and fat mass were assessed via quantitative magnetic resonance imaging (QMR) using two Echo Medical Systems (Houston, TX) MRI units – one accommodating animals up to approximately 80 grams and the other accommodating animals from approximately 100 to 800 grams. Methods are further described in Tardif et al. (2009) and Power, et al (2012). Throughout the paper, masses given refer to that of individuals, unless it is specifically indicated that the reference is to the mass of an entire litter.
Birth mass as a reflection of the prenatal environment
Our population of marmosets is unique in having birth condition information - including litter size, sex, and birth mass - recorded at ~ 36 hours following birth for > 80% of the live births occurring from 1994 through 2011. We know of no other captive, mother-reared and socially housed nonhuman primate population in which birth mass is known for the majority of the population. The routine collection of information on birth condition in a non-human primate breeding population provides the opportunity to examine the relation of birth condition to maternal factors and to subsequent infant growth in an integrated manner not previously possible.
Common marmosets average around 29 grams at birth which is around 8% of adult weight. Logistical complications prevent routine lean and fat mass assessments at day 1. We have determined fat and lean mass in 2 neonatal marmosets that died on day 1 (Power, et al., 2012). The post mortem percent body fat at day 1 was 12.04% for an infant weighing 25 g and 18.36% for an infant weighing 28 g.
Human neonates are reported to have approximately 15% body fat (Kuzawa, 1998). It has been proposed that humans have an unusual, if not unique, early life adiposity development that may be associated with the need to protect fuel supplies for the developing brain (Kuzawa, 1998). Kuzawa (1998) proposed, based upon the published information available at that time, that the peri-natal and pre-weaning fat mass of nonhuman primates was substantially lower than that of humans, and more similar to that of other mammals of similar size. However, our findings in the marmoset suggest that neonatal marmosets may have more substantial fat than would have originally been hypothesized based upon previous published finding of 3% body fat at birth in baboons (Lewis, et al., 1983).
Marmosets typically produce two offspring at a time in the wild; however, in captivity, they often produce litters of 3 or 4 – extremely rarely, litters of 5 or 6 are reported. While litters of 1–6 may be delivered, only 1–2 offspring are generally reared. Marmosets, along with the other callitrichid primates, have an unusual placentation, in which a bi-discoid, heavily anastomosed placenta is shared by all members of a litter (Merker et al., 1988; Smith and Moore, 1988). This system offers unusual opportunities to examine the effects of intrauterine competition in different litter sizes followed by post-natal conditions that are similar across litter sizes.
We reported in 2004 (Tardif and Bales, 2004) that marmoset birth weight was inversely related to litter size, a finding that is common to many mammals that produce more than one offspring (Stearns, 1992). Subsequent analyses of placental size and structure in twin versus triplet marmosets indicates that mothers are indeed pressed to meet the demands of larger litters, such that the placental mass, and more importantly, the amount of exchange surface present per fetus is lower in triplet than in twin pregnancies (Rutherford and Tardif, 2008, 2009).
In subsequent years the litter size effect has been ameliorated as the average birth weight of triplets has increased by 6.3% while the average birth weight of twins increased by only 2.1%, such that there is a more extensive overlap of triplet and twin birth weights – see Table 1. The limited increase in twin birth weights is perhaps not unexpected, given the fact that birth weights show evidence of stabilizing selection that will place upper limits on fetal size (Jaquish et al., 1997). The increase in triplet weights, however, indicates that the marmoset uterine environment provides significant flexibility in investment up to a fetal size that does not impair delivery. The change in the relation of litter size and birth weight over time has been associated with the development of significantly higher maternal weights. The average breeding female in 2002–2011 was 9% heavier than during 1994–2001.
Table 1.
Relation of birth weights and maternal weights to litter size and time period.
| Time period | Litter Size | N | Birth weight (g) Mean +/− s.d. | N | Maternal weight (g) Mean +/− s.d. |
|---|---|---|---|---|---|
| 1994–2001 | 2 | 82 | 29.3 +/− 4.08 | 60 | 362.4 +/− 33.18 |
| 1994–2001 | 3 | 55 | 26.8 +/− 2.71 | 29 | 382.6 +/− 29.01 |
| 2002–2011 | 2 | 67 | 29.9 +/− 4.68 | 54 | 390.5 +/− 55.41 |
| 2001–2011 | 3 | 122 | 28.5 +/− 4.50 | 84 | 409.8 +/− 43.95 |
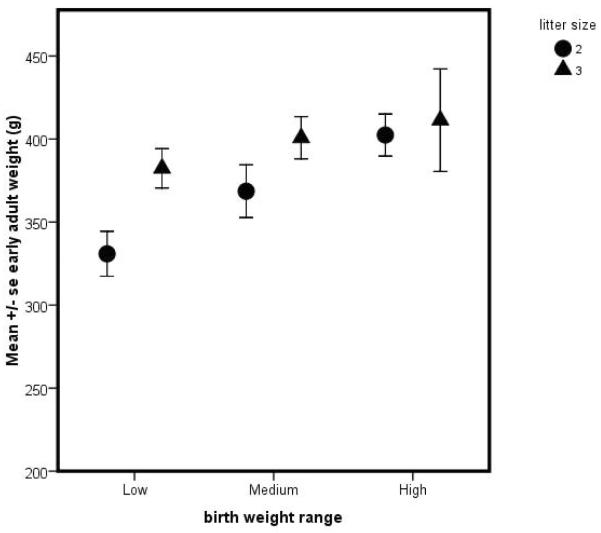
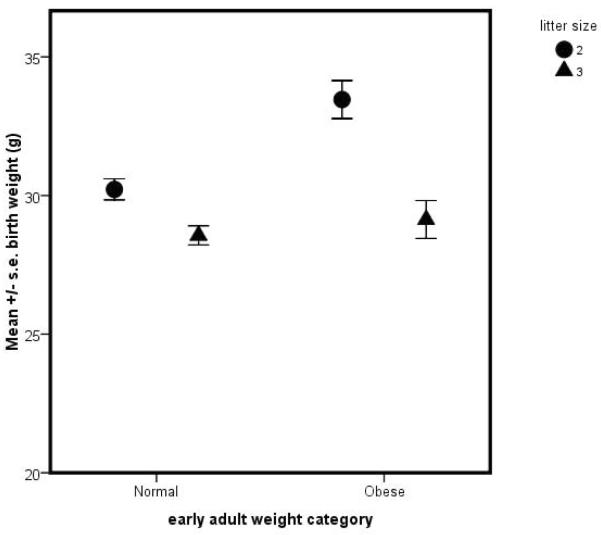
Marmosets display an interesting association between litter size, birth weight and adult size. Birth weight is positively associated with adult weight in animals born as twins –i.e., small birth weight twins became small adults while large birth weight twins became large adults. However, that relationship is not observed in triplets – see Figure 1a. While twins and triplets were both observed to become obese as defined by weight (i.e., exceeding the 90th percentile of early adult weight – see Tardif, et al., 2009), only twins who were large at birth became obese while small birth-weight triplets became obese (Fig. 1b). These findings suggest that marmosets may make an interesting species in which to explore aspects of developmental programming. The basic tenet of developmental programming is that resource access in early life – usually defined as the prenatal environment – may predispose an individual to altered physiological function such that adult risk for various chronic diseases and for obesity may be linked to a prenatal environment with altered resource access. Both exceptionally low resource access and exceptionally high resource access during the developmental period may be associated with future disease risk. Because marmosets produce variable litter sizes in captivity and litter mates directly compete for prenatal resources, they represent an ideal natural experiment in the role of differential intrauterine competition.
Figure 1.
(a) Relation of birth weight to mean (+/− s.e.) early adult weight for subjects born into twin (●) versus triplet (▲) litters; (b) relation of mean birth weight to early adult weight categories of lean and obese for twins versus triplets.
Post-natal growth in mass and its relation to litter size and maternal condition
In 2004, we reported on body mass growth in a captive population of 89 marmosets for which birth weights were available. For 68 of these subjects, sufficient longitudinal data were available to estimate growth rates (g/day) during the first year of life. This population displayed an early, linear growth rate, over the first five months of life that averaged 1.15 grams/day – a rate almost identical to the average of 1.17 grams per day reported by Kirkwood for four marmoset colonies (1985). There was substantially more variation in later growth trajectories, up through two years of age. We proceeded to examine the relation of maternal condition, litter size and birth condition to the variation in both early and late growth rates. Figure 2 illustrates the findings from this study, with the arrows indicating positive relationships between variables as determined by generalized linear models – see Tardif and Bales (2004) for additional details on these analyses.
Figure 2.
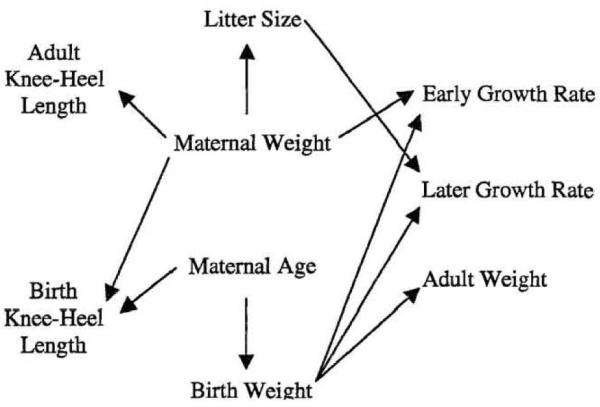
Relations among maternal factors, birth factors and growth (from Tardif and Bales, Am J Primatol 2004).
Early (pre- and immediately post-weaning) growth rates are related to maternal size with larger mothers supporting a higher pre-weaning growth rate (Tardif et al., 2001; Tardif and Bales, 2004). This finding is not surprising, given that the early growth period encompasses that period during which the infant's growth is being supported entirely by resources from the mother. In captive marmosets, milk composition is affected by maternal condition with larger mothers or mothers in positive energy balance during lactation producing more energy dense milk (Tardif, et al., 2001; Power et al., 2002). Maternal effects are strongest when mothers were nursing two infants, rather than one. Human epidemiological studies report variable relations between maternal BMI and early infant growth, with Regnault et al. (2011) and Ong et al. (2008) reporting no relationship between maternal BMI and infant growth during the first three months of life, while Knight et al. (2007) report a positive correlation between maternal BMI and infant growth during that time period. One factor that clearly differs in the human and marmoset studies is the transition of infants to non-maternal milk supplies. Both formula-feeding of human infants and early transition to solid foods are associated with higher growth velocities in infancy, though the relations of these variables to other potentially important variables, such as socioeconomic status can be extremely complex and difficult to decipher (Regnault et al., 2011; Wijlaars et al., 2011; May et al., 2012). While all the marmosets in our studies were breast-fed, we did find a positive relation between earlier occurrence of first observed solid food consumption and being classified as obese as juveniles – i.e., those animals that later became obese were observed eating solid food earlier (Tardif, unpublished observation; Ross et al., in press)
Later growth rates – from 5 to 24 months – are significantly more variable than the early growth rate. Later growth rates are related to an interaction between birth weight and litter size. There is a positive association between birth weight and later growth rate in twins but not in triplets, resulting in the pattern described by Figure 1. Therefore, as a group, the highest rates of body mass gain during this period are in triplets that had a low birth weight. This pattern suggests that some prenatal factors may be driving development of larger final body mass through differences in post-weaning growth. That this effect is prenatal is suggested by the fact that this difference is seen between birth litter sizes though the rearing litter size of triplets did not differ from that of twins – i.e., it was either one or two.
Growth rates in relation to obesity development
Many captive marmoset populations show a secular trend of increase in adult body mass. At what point do these mass increases indicate obesity? In humans, obesity has been variously defined relative to weight, BMI and, less often, in relation to relative body fat. Another aspect of defining obesity has been identifying the point at which weight/fat increases result in metabolic or cardiovascular dysfunction. Using such criteria, similar to those in human studies, we documented that marmosets with relative body fat in the upper quintile (> 80th percentile of relative fat or > 14% body fat) had significantly higher fasting blood glucose and HbA1C as well as higher circulating triglycerides and very low-density lipoprotein (VLDL), suggesting that the increasing weight observed in the population reflects increasing obesity (Tardif et al., 2009). As cited previously, similar patterns of spontaneous adult obesity associated with metabolic and cardiovascular dysfunction have been reported for many species of nonhuman primates held in captivity. Generally, the extent to which these adult obese phenotypes are present during the period preceding adulthood is unknown.
To begin to address this question, we initially examined the ages at which animals classified as obese in early adulthood could be differentiated from normal weight individuals (Tardif et al., 2009). Because we did not have body composition information for this population, we used a more conservative definition of obesity – including only those individuals who exceeded the 90th percentile of body weight as obese. For a population of 49 individuals for whom sufficient longitudinal weight information was available, obese by weight individuals were significantly different from normal weight individuals at birth as well as at 2–6 months of age (Table 2). Approximately half of the % difference in weight between obese and normal individuals was already present at 6 months of age. However, the degree to which these early life differences reflect different fat versus lean gains was unknown.
Table 2.
Total, lean and fat mass averages throughout infancy and adolescence for marmosets classified as obese versus normal in two different studies. Values in bold represent significant differences between obese and normal within a study.
| Source | Tardif, et al., 2009 | Power, et al., 2012 | |||
|---|---|---|---|---|---|
| Definition of obesity | Obese defined as > 90 percentile of body mass at 17 months of age | Obese defined as > 14% relatively body fat at 12 months of age | |||
| Age (mo) | Measurement | Obese N=13 | Normal N=36 | Obese N=16 | Normal N=15 |
| 0 | Mass (g) | 31.6 | 29.9 | 29.5 | 29.7 |
| 1 | Mass (g) | 61.8 | 62.5 | ||
| Lean mass (g) | 48.8 | 49.6 | |||
| Fat mass (g) | 9.7 | 8.6 | |||
| % Fat | 15.9 | 14.3 | |||
| 2 | Mass (g) | 120.4 | 105.1 | 111.4 | 113.6 |
| Lean mass (g) | 87.7 | 91.4 | |||
| Fat mass (g) | 13.9 | 12.8 | |||
| % Fat | 12.4 | 11.0 | |||
| 3 | Mass (g) | 167.7 | 177.1 | ||
| 4 | Mass (g) | 202.8 | 175.9 | ||
| 6 | Mass (g) | 285.1 | 246.8 | 273.6 | 226.6 |
| Lean mass (g) | 198.4 | 174.7 | |||
| Fat mass (g) | 45.5 | 24.9 | |||
| % Fat | 15.9 | 10.6 | |||
| 12 | Mass (g) | 402.9 | 257.5 | ||
| Lean mass (g) | 276.9 | 201.8 | |||
| Fat mass (g) | 82.6 | 21.4 | |||
| % Fat | 20.5 | 8.3 | |||
| 24 | Mass (g) | 514.6 | 383.3 | ||
In order to address this question, we designed a study in which growth in overall, lean, and fat mass was tracked over the first year of life in animals with either obese or non-obese mothers and who were provided or not provided access to higher fat diets during the post-weaning period (Power et al., 2012). Table 2 summarizes some of the findings from this study. In this case, because we had body composition information, obese juveniles were defined as those whose relative body fat exceeded 14% - i.e., the degree of body fat associated with metabolic dysfunction in our previous studies of adult marmosets.
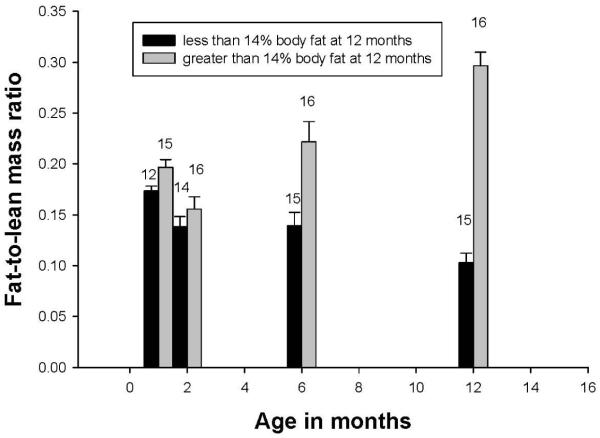
Juveniles classified as obese by relative body composition at 12 months already had greater lean mass and fat mass by 6 months. Body mass did not differ between groups prior to 6 months; however, by 1 month subjects destined to become obese had greater percent body fat. In addition, the pattern of fat growth relative to lean growth was different in the two groups – percent body fat decreased between 1 and 12 months in normal subjects while in obese subjects it increased (Fig. 3).
Figure 3.
Relation of average fat/lean mass ratios to age in normal (less than 14% body fat) and obese (>14% body fat) year-old marmosets. From Power, et al., 2012 Am J Primatol
The differences in the findings in this study compared to the previous one may stem from the more stringent definition used for obesity in the weight-based study – with only animals that exceeded the 90th percentile of weight being defined as obese. Those subjects weighed 514 grams on average as young adults, in contrast to an average weight of 461 grams for those subjects for which relative fat mass, rather than weight, was used to define obesity (Tardif, et al., 2009). These contrasting data suggest that there may be a subset of the obese population that is “super-obese” and for which associations with early life body mass may be even stronger.
Diet and maternal obesity appear to have potentially independent effects that may also vary with developmental age. Diet had a significant effect on juvenile adiposity at 6 months of age – 58% of juveniles offered a higher-fat diet mix were classified as obese at 6 months versus 25% offered the normal diet mix. Juveniles offered the higher-fat mix had greater gains in lean (0.99±0.05 g/day vs. 0.86±0.03 g/day, F(1,27)=5.685, p=.024) and fat mass (0.25±0.04 g/day vs. 0.12±0.02 g/day, F(1,27)=9.067, p=.006) from 1 to 6 months, and a greater ratio of fat-to-lean gain (0.236±.035 vs. .139±.014, F(1,27)=7.695, p=.010). At 12 months of age, however, there was no longer an effect on diet. This was due to the fact that 4 animals showed gains in fat mass between 6 and 12 months that moved them from the normal to the obese category – all four of these subjects were offspring of relatively fat mothers – with maternal body fat ranging from 12.9% to 17.0%. Combined, these results suggest that exposure to higher fat diets increases the relative rate of fat accumulation in early life in marmosets, but that even animals with no higher-fat diet exposure can become obese with time and that risk is associated with being the offspring of an obese mother. The sample size of this study, at present, is insufficient for additional generalized linear modeling approaches to further elucidate this point.
This suggestion is further reinforced by comparisons of food intake at 6 months and 12 months of age. There was an association between higher fat food intake and adiposity at 6 months, with young that were classified as obese by 6 months consuming more, higher fat food. At 12 months, there was no longer an association of adiposity with higher fat food intake, but there was an association with overall food intake, with 12-month obese subjects consuming more food, overall (Ross et al., in press).
Another factor of interest in obesity development is sex. Adult females are generally reported to have higher relative adiposity in most nonhuman primates, similar to humans. Rutenberg and Coelho (1988) reported that when baboons were fed high caloric density formula during infancy, they displayed an increase in body mass gain that was retained as a higher body mass into adulthood in females, but not in males. This finding suggests the possibility of significant pre-pubescent sex differences in the accumulation and retention of fat mass in nonhuman primates, as has been reported in some human studies (Whelton et al., 2002; Fukuyama et al., 2005; Sanigorski et al., 2007). As opposed to baboons, marmosets are generally described as sexually monomorphic, with minor or no differences in body size historically being reported. However, with the development of obesity in captive populations, we observed that adult female marmosets had significantly higher relative body fat than did males (Tardif, et al., 2009). In addition, we reported a significantly higher percentage of early adulthood obesity in females than in males. However, in our subsequent study of lean versus fat mass growth (Power et al., 2012), there were roughly equal numbers of fat males and females at 6 and 12 months of age. Continued surveillance of these subjects may yet show a sex difference, post puberty. We reported in 2009 (Tardif, et al., 2009) that birth weight was associated with weight in early adulthood for females but not for males. However, in our subsequent study (Power, et al., 2012), we found that there was no difference between males and females in the relation of birth weight to subsequent weight. There was, however, a positive relationship of birth weight to degree of fat gain by 6 month in obese infants, regardless of sex. It is possible that our previous result did not represent a true sex difference in the association between birth condition and later adiposity; but rather there is such an association among individuals that become obese, male or female, that was not evident in males due to the few obese males in the 2009 study.
Conclusions and Future Directions
Common marmosets display variance in early life growth patterns that is related to both pre- and post-natal factors. In terms of prenatal factors, the interaction of litter size and maternal condition defines birth body mass. Birth body mass is under stabilizing selection, such that very small or very large birth masses will be selected against. However, within that constraint, changes in the average birth weight of triplet infants in association with increases in pre-pregnancy maternal mass, suggest that the marmoset uterine environment is exquisitely designed to reflect resources available for the gestation of multiple offspring. This “natural experiment” in which both resources (e.g., maternal energy reserves in terms of fat stores, maternal food type and availability) and competitive demand (litter size) may be varied offers interesting opportunities to explore the role of the prenatal environment in determining growth and ultimate adult size and adiposity. Future studies will concentrate on characterization of adult life outcomes in animals with differing resource and competitive prenatal environments.
As with other nonhuman primates, a subset of the captive marmoset population will become obese and that obesity is associated with metabolic and cardiovascular dysfunction. We now know that this obesity can be generated in very early life in captive marmosets, making this species a potentially valuable model in which to study pediatric obesity and its sequelae. Our studies to date, on a relatively small population of young, growing marmosets, have identified early life exposure to higher fat diets as a factor that will enhance the chances of post-weaning obesity development. However, overall higher food consumption, regardless of food type, is also associated with obesity development, but at a later age. Future studies are necessary to more thoroughly define the relative roles of energy intake and energy output on development of early life adiposity in this species, but findings, to date, suggest that intake is at least one factor that is driving obesity development.
One unexpected finding in our studies has been the relatively high body fat percentage of neonatal (12–18%) marmosets. These are significantly higher values that those reported for nonhuman primates by Kuzawa (1998). Human neonates are reported to have approximately 15% body fat, increasing to 25% at weaning initiation and then declining post weaning to about 16% prepuberty and this pattern of fat deposition is proposed to be unusual to humans (Kuzawa, 1998). Our study suggests that neonatal fat mass in marmosets may be not as dissimilar to humans as previously thought, though the pattern of rapidly increasing fat deposition seen in normal human infants is not seen in normal marmoset infants. A cautionary caveat is that the QMR methodology has not been validated against chemical extraction for marmosets. Tinsley and colleagues (2004) reported that QMR and DEXA estimated body fat values in rodents that exceeded those of chemical analysis by around 20%. Therefore, differences in methods of assessing body fat mass may account for some of the differences between studies. However, even with a potential overestimate of 20% by QMR compared to chemical analysis, the percent body fat of the neonatal marmosets would far exceed the value reported in the literature for baboons (Lewis et al., 1983). Future studies should better characterize the nature and distribution of adipose tissue in neonatal and peri-weaning marmosets to determine the extent to which it is similar or different from humans or from other non-human primates.
Human pediatric obesity is increasing and will exact growing costs, both personal and financial, related to early onset of diseases that have been associated historically with middle- and late-age in humans. A recent meta-analysis of medical and lifestyle intervention effects on pediatric obesity suggests very limited short term effects of any interventions tested to date (McGovern, et al., 2008). Therefore, continued research into the etiology of pediatric obesity is warranted. Humans, themselves, are the species that offers the most insights into this phenomenon. However, while epidemiological studies and intervention trials are key pieces of deciphering the pediatric obesity puzzle, there are significant limitations on what human studies provide due to limits upon control and assessment of both energy intake and energy output in human studies (Reilly, et al., 2007; Collins, 2010). There remains a role for animal models in which manipulation and control of genetic, epigenetic and environmental factors can be more readily accomplished. With the demonstration that obesity can be generated in infant and juvenile marmoset monkeys and that the occurrence of early life obesity can be altered by maternal condition and early life high fat diet exposure, the marmoset is poised for development as a pediatric obesity model. In particular, the natural occurrence of a variable litter size in this primate offers some unusual opportunities for exploring both pre- and post-natal effects on obesity development.
Acknowledgements
The authors would like to thank D. Layne-Colon and J. Artavia for their efforts in the studies described herein. The studies reported upon in this review were supported by NIH grants R01-RR02022 (Tardif), R01-DK077639 (Power, Tardif). This project was also supported by the National Center for Research Resources P51-RR013986 and is currently supported by the Office of Research Infrastructure Programs/OD P51-OD011133 (Southwest National Primate Research Center). The studies described here comply with all regulations, institutional or governmental, regarding the ethical treatment of nonhuman primate research subjects and comply with the American Association of Physical Anthropologists Code of Ethics as it pertains to living human and nonhuman subjects.
Grant sponsorship: NIH grant R01-RR002022, R01-DK077639, and P51-RR013986 (now P51- OD011133)
Literature Cited
- Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free-living baboons reflect food availability and activity levels. Am J Primatol. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Whitaker RC. Prevalence of obesity among US preschool children in different racial and ethnic groups. Arch Pediatr Adolesc Med. 2009;163:344–348. doi: 10.1001/archpediatrics.2009.18. [DOI] [PubMed] [Google Scholar]
- Araujo A, Arruda M, Alencar A, et al. Body weight of wild and captive common marmosets. Int J Primatol. 2000;21:317–324. [Google Scholar]
- Arner P. Resistin: yet another adipokine tells us that men are not mice. Diabetologia. 2005;48:2203–2205. doi: 10.1007/s00125-005-1956-3. [DOI] [PubMed] [Google Scholar]
- Ausman LM, Rasmussen KM, Gallina DL. Spontaneous obesity in maturing squirrel monkeys fed semipurified diets. Am J Physiol. 1981;241:R316–R321. doi: 10.1152/ajpregu.1981.241.5.R316. [DOI] [PubMed] [Google Scholar]
- Baird J, Fisher D, Lucas P, et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929–936. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin N, Hannah J, Ortmeyer H, Hansen B. Central obesity in rhesus monkeys: association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? Int J Obes Rel Metab Disord. 1993;17:53–61. [PubMed] [Google Scholar]
- Coelho AM, Glassman DM. Subcutaneous fat in well-nourished olive baboons: an assessment of age/sex differences and the correlation to body weight and crown-rump length. Am J Phys Anthropol. 1984;63:146–147. [Google Scholar]
- Coelho AM. Baboon dimorphism: growth in weight, length and adiposity from birth to 8 years of age. In: Watts ES, editor. Nonhuman primate models for human growth and development. Alan R. Liss; New York: 1985. pp. 125–159. [Google Scholar]
- Collins CE, Watson J, Burrows T. Measuring dietary intake in children and adolescents in the context of overweight and obesity. Intl J Obes. 2010;34:1103–1115. doi: 10.1038/ijo.2009.241. [DOI] [PubMed] [Google Scholar]
- Comuzzie A, Cole C, Martin L, et al. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- de Onis M, Blössner, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, et al. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002;132:3155–3160. doi: 10.1093/jn/131.10.3155. [DOI] [PubMed] [Google Scholar]
- Eckel R, Grundy S, Zimmer P. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Ford S, Davis L. Systematics and body size: implications for feeding adaptations in New World monkeys. Am J Phys Anthropol. 1992;88:415–468. doi: 10.1002/ajpa.1330880403. [DOI] [PubMed] [Google Scholar]
- Fukuyama S, Inaoka T, Matsumura Y, et al. Anthropometry of 5-19-year-old Tongan children with species interest in the high prevalence of obesity among adolescent girls. Ann Hum Biol. 2005;32:714–723. doi: 10.1080/03014460500273275. [DOI] [PubMed] [Google Scholar]
- Gardner DSL, Hosking J, Metcalf BS, Jefrey AN, Voss LD, Wilkins TJ. Contribution of early weight gain to childhood overweight and metabolic health: A longitudinal study (Early Bird 36) Pediatr. 2009;123:e67–e73. doi: 10.1542/peds.2008-1292. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Kirtland KA, Cadwell BL, Rios Burrows N, Barker LE, Thompson TJ, Geiss L. Estimated county-level prevalence of diabetes and obesity – United States, 2007. Morbid Mortal Weekly Rep. 2009;58:1259–1263. [PubMed] [Google Scholar]
- Hansen B, Bodkin N. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes. 1993;42:1809–1814. doi: 10.2337/diab.42.12.1809. [DOI] [PubMed] [Google Scholar]
- Henrichs S. Mouse feeding behavior: ethology, regulatory mechanisms and utility for mutant phenotyping. Behav Brain Res. 2001;125:81–88. doi: 10.1016/s0166-4328(01)00287-x. [DOI] [PubMed] [Google Scholar]
- Jaquish CE, Tardif SD, Cheverud JM. Interactions between infant growth and survival: evidence for selection on age-specific body weight in captive common marmosets (Callithrix jacchus) Am J Primatol. 1997;42:269–280. doi: 10.1002/(SICI)1098-2345(1997)42:4<269::AID-AJP2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Fairbanks L, Bailey J, et al. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obes. 2007;15:1666–1674. doi: 10.1038/oby.2007.199. [DOI] [PubMed] [Google Scholar]
- Kemnitz J. Obesity in macaques: spontaneous and induced. Adv Vet Sci Comp Med. 1984;28:81–114. doi: 10.1016/b978-0-12-039228-5.50009-7. [DOI] [PubMed] [Google Scholar]
- Kirkwood JK. Patterns of growth in primates. J Zool Lond. 1985;205:123–136. [Google Scholar]
- Knight B, Shields BM, Hill A, Powell RJ, et al. The impact of maternal glycemia and obesity on early postnatal growth in a nondiabetic Caucasian population. Diabet Care. 2007;30:777–783. doi: 10.2337/dc06-1849. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Yearb Phys Anthropol. 1998;27:177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Levin B, Dunn-Meynell D, Balkan B, Keesey R. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–30. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Levin B, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275:R1374–79. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- Lewis DS, Bertrand HA, Masoro EJ, et al. Preweaning nutrition and fat development in baboons. J Nutr. 1983;113:2253–2259. doi: 10.1093/jn/113.11.2253. [DOI] [PubMed] [Google Scholar]
- May R, Kim D, Mote-Watson D. Weight gain and weight status during the first 3 months: relationships to birth size and feeding. Am J Phys Anthropol – this symposium volume. 2012 doi: 10.1002/ajpa.22190. [DOI] [PubMed] [Google Scholar]
- McGovern L, Johnson JN, Remberto P, Hettinger A, et al. Treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2008;93:4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- Merker H-J, Bremer D, Csato W, Heger W, Gossrau R. Development of the marmoset placenta. In: Neubert D, Merker H-J, Hendrickx AG, editors. Non-human primates: developmental biology and toxicology. Ueberreuter Wissenschaft; Berlin: 1988. pp. 245–272. [Google Scholar]
- Oiken E, Gillman M. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- Power ML, Oftedal OT, Tardif SD. Does the milk of Callitrichid monkeys differ from that of larger anthropoids? Am J Primatol. 2002;56:117–127. doi: 10.1002/ajp.1068. [DOI] [PubMed] [Google Scholar]
- Ong KK, Diderholm B, Salzano G, et al. Pregnancy insulin, glucose and BMI contribute to birth outcomes in nondiabetic mothers. Diabet Care. 2008;31:2193–2197. doi: 10.2337/dc08-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power ML, Ross CN, Schulkin J, Tardif SD. The development of obesity begins at an early age in captive common marmosets (Callithrix jacchus) Am J Primatol. 2012;74:261–269. doi: 10.1002/ajp.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault N, Botton J, Forhan A, Hankard R, et al. Determinants of early ponderal and statural growth in full-term infants in the EDEN mother-child cohort study. Am J Clin Nutr. 2010;92:594–602. doi: 10.3945/ajcn.2010.29292. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Ness AR, Sherriff A. Epidemiological and physiological approaches to understanding the etiology of pediatric obesity: finding the needle in the haystack. Pediatr Res. 2007;61:646–652. doi: 10.1203/pdr.0b013e3180536667. [DOI] [PubMed] [Google Scholar]
- Ross CN, Power ML, Tardif SD. Relation of food intake behaviors and obesity development in young common marmoset monkeys. Am J Primatol (abstract) doi: 10.1002/oby.20432. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenberg GW, Coelho AM. Neonatal nutrition and longitudinal growth from birth to adolescence in baboons. Am J Phys Anthropol. 1988;75:529–539. doi: 10.1002/ajpa.1330750410. [DOI] [PubMed] [Google Scholar]
- Rutherford JN, Tardif SD. Placental efficiency and intrauterine resource allocation strategies in the common marmoset pregnancy. Am J Phys Anthropol. 2008;137:60–68. doi: 10.1002/ajpa.20846. [DOI] [PubMed] [Google Scholar]
- Rutherford JN, Tardif SD. Developmental plasticity of the microscopic placental architecture in relation to litter size variation in the common marmoset monkey (Callithrix jacchus) Placenta. 2009;30:105–110. doi: 10.1016/j.placenta.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanigorski A, Bell A, Dreme P, Swinburn B. High childhood obesity in an Australian population. Obes. 2007;15:1908–1912. doi: 10.1038/oby.2007.226. [DOI] [PubMed] [Google Scholar]
- Smith CA, Moore HDM. The morphology of early development and implantation in vivo and in vitro in the marmoset monkey. In: Neubert D, Merker H-J, Hendrickx AG, editors. Non-human primates: developmental biology and toxicology. Ueberreuter Wissenschaft; Berlin: 1988. pp. 171–190. [Google Scholar]
- Speakman J, Hambly C, Mitchell S, Krol E. Animal models of obesity. Obes Rev. 2007;8(S1):55–61. doi: 10.1111/j.1467-789X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Spurlock M, Gabler N. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford University Press; Oxford: 1992. pp. 72–90. [Google Scholar]
- Tardif SD, Layne DG, Cancino L, Smucny DA. Neonatal behavioral scoring of common marmosets (Callithrix jacchus): relation to physical condition and survival. J Med Primatol. 2002;31:147–151. doi: 10.1034/j.1600-0684.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus) Comp Med. 2003;53:364–368. [PubMed] [Google Scholar]
- Tardif SD, Bales KL. Relations among birth condition, maternal condition, and postnatal growth in captive common marmoset monkeys (Callithrix jacchus) Am J Primatol. 2004;62:83–94. doi: 10.1002/ajp.20009. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Power ML, Ross CN, et al. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus) Obes. 2009;17:1499–1505. doi: 10.1038/oby.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- Wachtman LM, Kramer JA, Miller AD, Hachey AM, Curran EH, Mansfield KG. Differential contribution of dietary fat and monosaccharide to metabolic syndrome in the common marmoset (Callithrix jacchus) Obes. 2011;19:1145–1156. doi: 10.1038/oby.2010.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Kavanagh K, Ward G, et al. Old World primate models of type 2 diabetes mellitus. ILAR J. 2006;47:259–271. doi: 10.1093/ilar.47.3.259. [DOI] [PubMed] [Google Scholar]
- Whelton H, Harrington J, Crowley E, et al. Prevalence of overweight and obesity on the island of Ireland: results from the North South survey of children's height, weight and body mass index. BMC Public Health. 2002;7:187. doi: 10.1186/1471-2458-7-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijlaars LPMM, Johnson L, van Jaarsveld CHM, Wardle J. Socioeconomic status and weight gain in early infancy. Int J Obes. 2011;35:963–970. doi: 10.1038/ijo.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]