This study aimed to identify independent pretreatment factors that predict for control of local brain metastases in a large single-institution series of patients with brain metastases who received stereotactic radiosurgery. Lesion aspect/phenotype and radiotherapy schedule were independent factors associated with progression outcomes.
Keywords: Brain metastases, Stereotactic radiosurgery, Recursive partitioning analysis, Predictive modeling, Local control
Learning Objectives
Describe the results of a new recursive partitioning analysis (RPA) predicting for SRS lesion control.
Discuss the SRS lesion in the light of other literature assessing predictors of lesion control in SRS for brain metastases.
Abstract
Purpose.
The objective of this investigation was to identify independent pretreatment factors that predict for control of local brain metastases (BM) in a large single-institution series of patients receiving stereotactic radiosurgery (SRS). Recursive partitioning analysis was used to potentially identify a class of patients with durable lesion control characteristics.
Methods.
A retrospective SRS database containing baseline characteristics, treatment details, and follow-up data of newly diagnosed patients with 1–3 BM (on magnetic resonance imaging) treated with linear accelerator-based SRS was created. Three study endpoints were used: time to progression (primary endpoint, individual lesion progression; n = 536), time to first progression (secondary endpoint, first lesion progression on an individual patient basis; n = 380), and overall survival (secondary endpoint; n = 380). Recursive partitioning analysis (RPA) was performed to identify predictors of time to progression.
Results.
Multivariable analysis demonstrated that lesion aspect/phenotype and radiotherapy schedule were independent factors associated with both progression outcomes. Presence of tumor necrosis was found to be associated with a significant hazard of progression (hazard ratio >3), whereas use of the most intense radiotherapy fractionation schedule (21 Gy in one fraction) was associated with significant reductions in progression (hazard ratio <0.3). RPA using SRS dose and lesion aspect/phenotype was created and described three distinct prognostic groups.
Conclusions.
RPA of a large retrospective database of patients receiving SRS confirmed previous observations regarding the importance of SRS dose and lesion aspect/phenotype in lesion control and overall survival. The SRS lesion analysis may help to stratify future clinical trials and better define patient care options and prognosis.
Implications for Practice:
The importance of this work is primarily in the confirmation of previously reported associations between lesion dose and MRI phenotype with local lesion control after stereotactic radiosurgery. This manuscript extends these associations into a clinical useful risk stratification system to relate how lesion dose and MRI phenotype can relate to lesion control and overall survival. This new risk stratification system may assist in clinical care and clinical trial design by better defining expected treatment outcomes after stereotactic radiosurgery.
Introduction
The diagnosis of brain metastases (BM) is frequently related to the natural history of the spread of many primary tumors, including those arising in the lung, breast, colorectal, renal, and skin (i.e., melanoma) [1]. Development of metastatic disease in the brain can lead to clinically significant reductions in health-related quality of life, neurological/neurocognitive compromise, and life expectancy [2]. Treatment selection is highly dependent on pretreatment clinical factors, prognosis (as estimated by various published risk stratification prognostic indices), and patient treatment preferences [3, 4].
A published randomized controlled trial (RCT) comparing whole brain radiotherapy (WBRT) plus stereotactic radiotherapy (SRS) boost versus WBRT alone supported the use of the SRS technique in oligometastatic (1–3 brain metastases ≤3.0 cm) patients [5]. This clinical trial demonstrated clinically important improvements in lesion control, performance status, and survival (in the solitary metastasis subgroup). Subsequent published RCTs have addressed the issue of whether or not the WBRT component is advisable for the initial treatment of de novo brain metastases [6–9]. In general, inclusion of WBRT has been shown to improve regional intracranial control but at the expense of additional neurocognitive effects.
Durable lesion control, ideally lasting during the expected patient lifespan, is an important goal of high-quality SRS to prevent symptomatic recurrence that would mandate consideration of one or more salvage procedures including WBRT, neurosurgical resection, and various forms of drug therapy [10]. Various investigations have been published on the topic of predictive factors associated with lesion control in the context of SRS treatment [11–18]. Factors that have been shown in at least one publication to be a significant predictor of lesion control include lesion dose, lesion radiological characteristics (i.e., lesion aspect), lesion target volume, patient performance status, presence of extracranial disease, cancer histology, and inclusion of WBRT.
The objective of this investigation is to identify independent pretreatment factors that predict for lesion control in the context of a large single-institution series of SRS for brain metastases. Recursive partitioning analysis was used to potentially identify a class of patients with durable lesion control characteristics. Our findings are discussed in the context of previously published investigations.
Methods
The SRS database contains baseline characteristics, treatment details, and follow-up data of patients who were newly diagnosed with BM and treated with linear accelerator (LINAC)-based SRS. Patients with 1–3 newly diagnosed BM (including those near the brainstem and posterior fossa) that were confirmed on high-resolution contrast-enhanced magnetic resonance imaging (MRI) were eligible for single-modality SRS. Patients with recurrent disease after previous radiotherapy were not included in this series.
SRS was delivered using five dynamic conformal arcs either on a Novalis linear accelerator (2002–2008) or a Novalis TX linear accelerator (2008 onwards; BrainLAB, Feldkirchen, Germany). Patient fixation was performed using the relocatable Gill-Thomas-Cosman frame (2002–2008) or with BrainLAB's frameless mask system (2008 onwards; BrainLAB, Feldkirchen, Germany).
The SRS target volumes consisted of the outer contrast-enhancing border of the lesions contoured on the planning MRI with a 1-mm margin to correct for residual setup error. All lesion target volumes were prescribed to the 80% isodose line. SRS was generally prescribed using a prospectively defined risk-adapted fractionation scheme, with the smallest lesions (≤7.5 cm3) receiving 21 Gy, lesions measuring 7.5–25 cm3 or those BM adjacent to the brainstem receiving 18 Gy, and the largest lesions (>25 cm3) receiving either a single fraction of 15 Gy or 24 Gy in three fractions of 8 Gy. The prescription dose was always determined after contouring the target volume in the planning system. Because brain metastases are often spherical, the 21-Gy cutoff value generally corresponds to lesions with a maximum diameter of approximately 2.5 cm; the 18-Gy cutoff value corresponds to lesions of approximately 3.6 cm.
The recommended follow-up for patients receiving SRS consisted of clinic visits including neurological examination with contrast-enhanced MRI every 3 months during the first 2 years, followed by clinic visits and MRI scans every 6 months thereafter. The median follow-up duration of the database calculated using the reverse Kaplan-Meier method was 32 months [19].
Lesional aspect was classified based on the pattern of contrast enhancement on gadolinium-enhanced T1 sequences of the MRI, as was previously described by Goodman et al. [18]. Lesions were classified as follows: (a) lesions with homogeneous (i.e., uniform) contrast enhancement; (b) lesions with heterogeneous contrast enhancement, if there were areas of nonhomogeneous contrast enhancement; (c) thin-walled cystic lesions (either simple cystic or multicystic); and (d) lesions with a necrotic center. Radiological evidence of lesional progressive disease was defined according to the criteria described by Shiau et al. [17] and specifically was defined as “at least 25% increase in the product of three perpendicular diameters (craniocaudal, anterior-posterior, and mediolateral)”. Date of death and intracranial and extracranial disease status at time of death was also captured in the retrospective database.
Endpoints
Three separate endpoints were used in conjunction with this predictive analysis. Time to progression (n = 536 lesions) was the primary endpoint, defined as time from initiation of stereotactic radiosurgery to development of progressive disease on a per-lesion level. Each lesion was evaluated according to the previously described follow-up guidelines to determine whether or not radiological and/or clinical evidence of progressive disease was indeed present (yes/no) as well as the date of evaluation.
Time to first progression (n = 380 patients) was one of the secondary endpoints. This endpoint was derived from the time-to-progression endpoint at a per-patient level. Patients were first evaluated as having either progressive intracranial disease at any lesion/site (yes/no), then further evaluated to determine in which lesion(s) progression first occurred. If two or three lesions showed progression at same time, the largest lesion was used in the final analysis [20]. This analysis was performed to further assess the stability of findings from the primary analysis of time to progression (per lesion) in terms of possible nonindependence issues resulting from the inclusion of more than one lesion per patient.
Survival (n=380 patients) was used as the other secondary endpoint. Survival was defined as time from initiation of stereotactic radiosurgery to date of last follow-up and/or death, whichever came first. This endpoint was reported for descriptive purposes only and was not used for any predictive modeling.
Statistical Methodology
Univariable Cox regressions were constructed for time to progression (model 1) and time to first progression (model 2) to identify significant predictors of progressive disease; they were performed at the lesion level (n = 536) and patient level (n = 380), respectively. Multivariable Cox regression analyses were performed, incorporating all factors found to be somewhat significant from univariable Cox regression (i.e., p < .30), followed by automated backward elimination technique to sequentially remove factors until all remaining covariates had p values less than .15. Adjustment for clustering was performed for univariable and multivariable analyses related to time to progression due to the fact that each patient contributed different numbers of lesions to the analysis.
Recursive partitioning analysis (RPA) was performed at the lesion level (n = 536), incorporating significant predictors identified from multivariable Cox regression (model 1 factors: lesion radiological phenotype [aspect] and radiation dose) [21]. The SRS lesion RPA was performed in two ways: (a) primarily as a time-to-event outcome (taking into account time to progressive disease) and (b) modeling progressive disease as a binary outcome (yes/no) to assess the robustness of the SRS lesion RPA model created. Kaplan-Meier estimates of time to progression (n = 536) and time to first progression (n = 380) were performed. Kaplan-Meier estimates stratified by baseline characteristics and proposed SRS lesion RPA stratifications were also calculated (for all three endpoints including overall survival) and different classes were compared using log-rank test statistic. Statistical analysis was performed using SAS (version 9.2; SAS Institute, Cary, NC) and the open-source R software platform (www.r-project.org).
Results
Between December 2002 and July 2011, a total of 380 patients with 536 newly diagnosed BM were treated with LINAC-based SRS as a single modality. Patient, tumor, and treatment-related descriptive statistics organized per patient (n = 380) and per lesion (n = 536) are summarized in Table 1. A variety of dose fractionation schedules were used for the 536 lesions treated: 21 Gy in one fraction (292 lesions, 54.5%), 18 Gy in one fraction (170 lesions, 31.7%), 24 Gy in three fractions (47 lesions, 8.8%), and 15 Gy in one fraction (27 lesions, 5.0%). Progressive disease was identified in 71 of 536 lesions (13%) in 65 of 380 patients (17%). Fifty-five patients (15%) were alive at last follow-up.
Table 1.
Baseline characteristics of patients and lesions
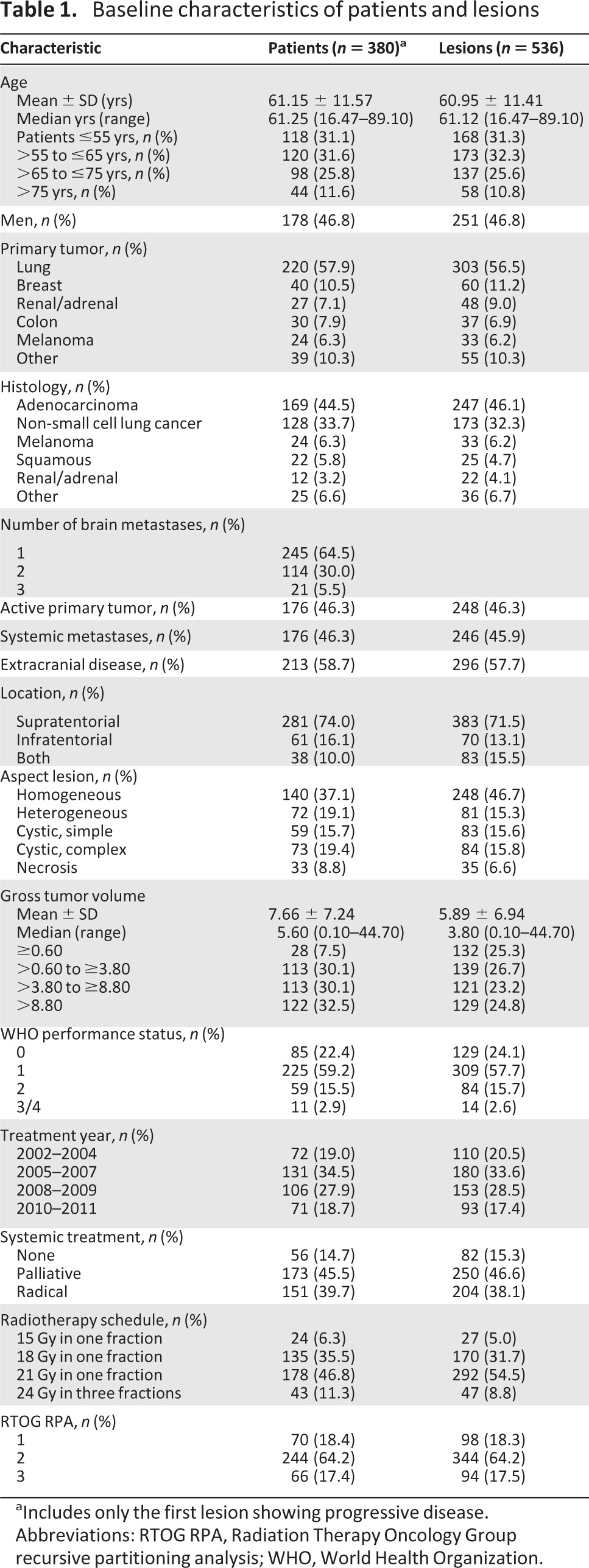
aIncludes only the first lesion showing progressive disease.
Abbreviations: RTOG RPA, Radiation Therapy Oncology Group recursive partitioning analysis; WHO, World Health Organization.
Results from the univariable Cox regression analysis for both the lesional (time to progression) and patient (time to first progression) analyses are depicted in Table 2. Results of multivariable Cox regression analysis for both time to progression and time to first progression are summarized in supplemental online Tables 1 and 2, respectively. Both models demonstrate that lesion aspect/phenotype and radiotherapy schedule are independent factors associated with both progression outcomes. Presence of tumor necrosis was found to be associated with a significant hazard of progression (hazard ratio >3), whereas use of the most intense radiotherapy fractionation schedule (21 Gy in one fraction) was associated with significant reductions in progression (hazard ratio <0.3).
Table 2.
Univariable Cox regression models examining relationship between individual predictors of time to progression (n = 536) and time to first progression (n = 380)
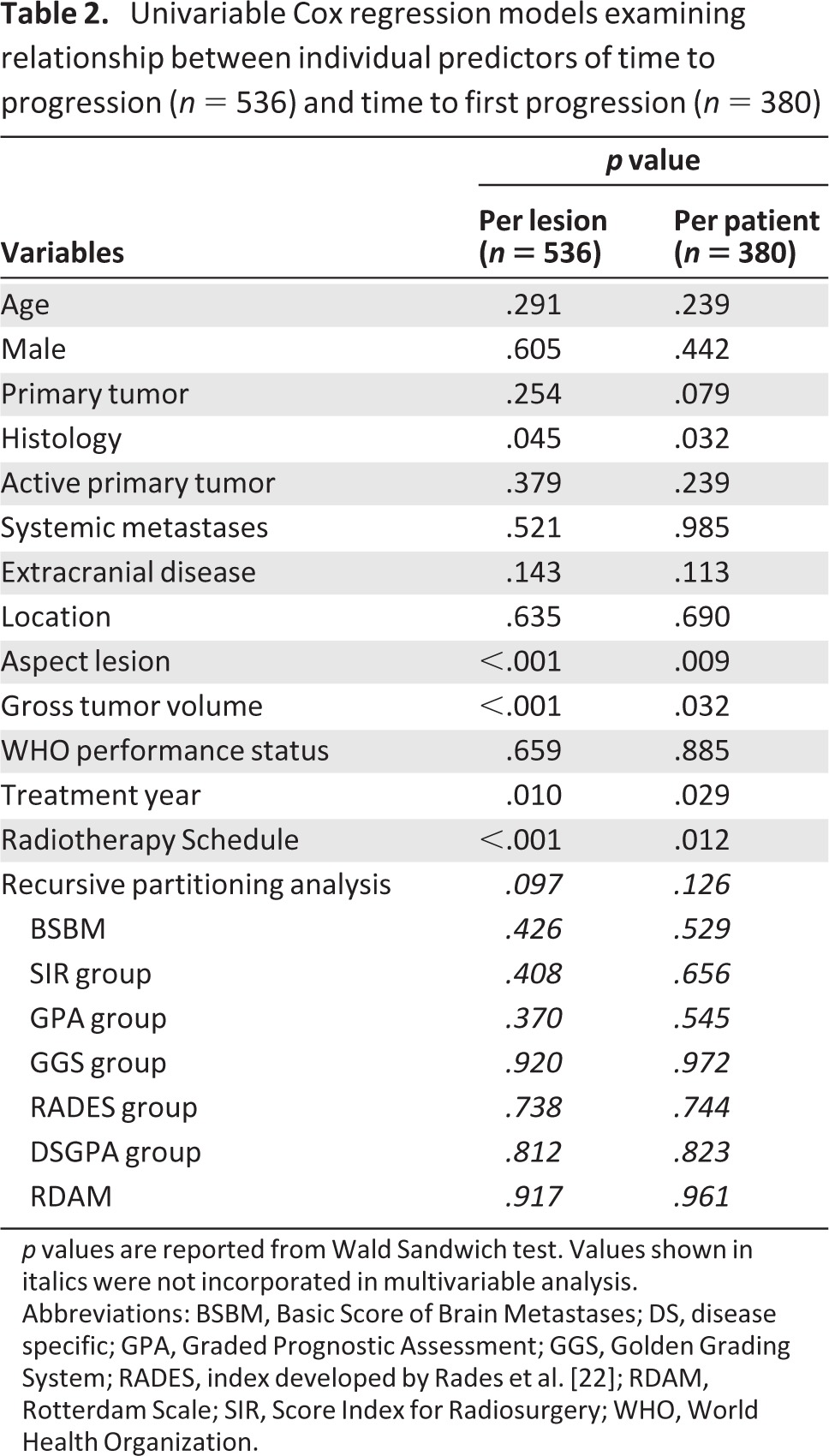
p values are reported from Wald Sandwich test. Values shown in italics were not incorporated in multivariable analysis.
Abbreviations: BSBM, Basic Score of Brain Metastases; DS, disease specific; GPA, Graded Prognostic Assessment; GGS, Golden Grading System; RADES, index developed by Rades et al. [22]; RDAM, Rotterdam Scale; SIR, Score Index for Radiosurgery; WHO, World Health Organization.
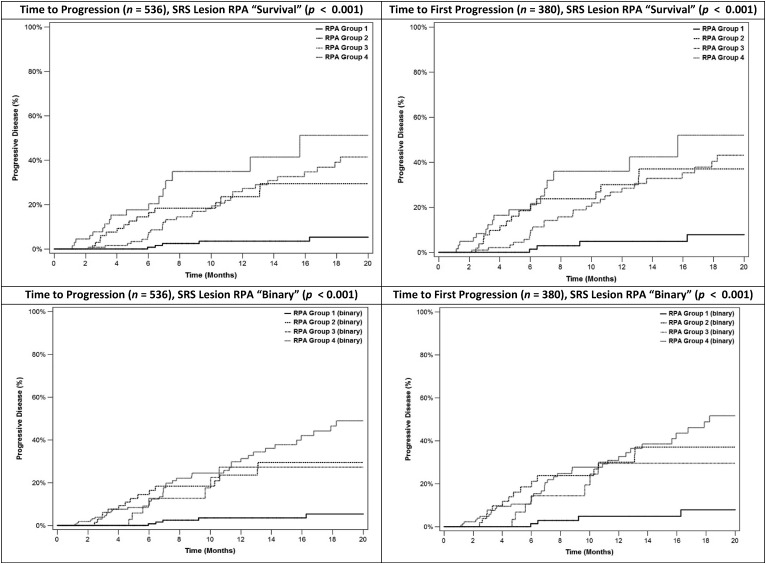
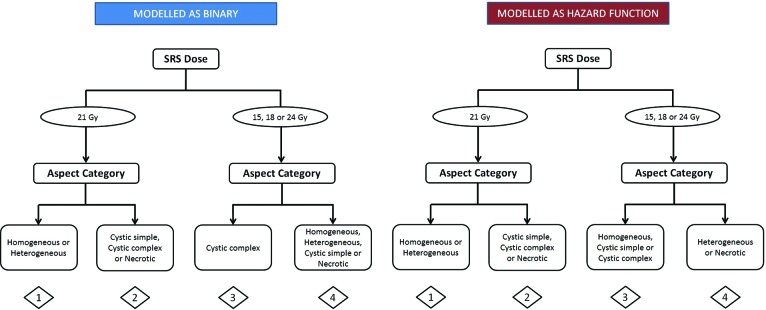
Kaplan-Meier curves for all eligible patients and subdivided into relevant groups for both progression outcomes are summarized in supplemental online Fig. 1. As depicted in Fig. 1, SRS lesion RPA time-to-event and binary analyses partitioned the patient population into four possible groupings based on radiotherapy schedule (21 Gy in one fraction vs. the other less intense regimens) and lesion aspect/phenotype (different combinations observed; however, the 21 Gy/1 fraction arm was split identically between homogeneous/heterogeneous vs. cystic/necrotic). Kaplan-Meier curves for each of the SRS lesion RPA groups were created for both progression outcomes using both RPA approaches (time to event and binary; Fig. 2).
Figure 1.
Survival-based (hazard function) and binary recursive partitioning analysis of the time-to-progression endpoint (n = 536).
Abbreviation: SRS, stereotactic radiosurgery.
Figure 2.
Time to progression (n = 536) and time to first progression (n = 380) Kaplan-Meier curves for derived recursive partitioning classes.
Abbreviations: RPA, recursive partitioning analysis; SRS, stereotactic radiosurgery.
Inspection of these Kaplan-Meier depictions demonstrated that three distinct groups of patients were present: a good prognosis group (group 1), intermediate groups consisting of the two middle SRS lesion RPA groups (groups 2 and 3), and a poor prognosis group (group 4). Individual Kaplan-Meier progression curves for each aspect/phenotype combination with the 21 Gy/1 fraction schedule versus other fractionation schedules are shown in supplemental online Fig. 2. Supplemental online Fig. 3 summarizes the Kaplan-Meier overall survival curves for all patients and curves divided by lesion/aspect and highest radiation biological equivalent dose fractionation. Kaplan-Meier overall survival estimates were affected by the SRS lesion RPA group, with a statistically significant log-rank test (p = .05) in terms of overall survival.
Discussion
This study summarized the progression and survival outcomes of a large SRS database, with a particular focus on the determination of factors that predict for brain metastasis local lesion control. Lesion aspect/phenotype and radiation dose schedule were both found to be critical independent factors both in the prediction of time to lesion progression and also time to first lesion progression at the patient level. The presence of extracranial disease was found to have a borderline significant effect on radiological confirmation of lesional progression. This may be due to a confounding effect in which patients with extracranial disease may suffer from extracranial progression and death prior to intracranial lesion progression. Regine et al. previously observed this relationship in a cohort of 36 patients in which presence of extracranial disease reduced the rate of observed symptomatic brain recurrence by more than half [13]. Tumor histology was not found to inform the SRS lesion RPA despite statistical significance on univariable analysis. It is hypothesized that, in the context of SRS dosing, histology may not be important given the ablative doses being used and/or that the prognostic impact of histology may be exerted indirectly through lesion phenotype (i.e., necrotic lesions) having inferior local control.
An RPA approach was used to further investigate the relationship between lesion aspect/phenotype and RT schedule with progression outcomes. This analysis demonstrated that three distinct groups exist that predict for time to progression, time to first progression, and overall survival. Specifically, this SRS lesion RPA has identified a high-risk subgroup of patients that potentially do not fully benefit from SRS therapy. This high-risk patient population may have been alternatively treated with whole brain radiotherapy alone, with the potential advantages of treatment simplicity and lower cost and/or resource expenditure (dependent on practice setting). However, it is important to note that identifying this high-risk patient population may lead to future treatment innovation research. The therapeutic ratio could be improved by optimization of dose escalation, dose targeting, treatment delivery, and novel drug therapy.
The three SRS lesion groups are summarized as follows:
Low progression risk: Homogeneous or heterogeneous lesion treated with 21 Gy in one fraction
Intermediate progression risk: Any cystic lesion treated between 15–21 Gy in one or 24 Gy in three fractions or necrotic lesions treated with 21 Gy in 1 fraction or homogeneous lesions treated with 15–18 Gy in one fraction or 24 Gy in three fractions
High progression risk: Heterogeneous or necrotic lesions treated with 15–18 Gy in one fraction or 24 Gy in three fractions
Shiau et al. previously reported the interaction of radiotherapy schedule and lesion aspect on lesion control [17]. In this retrospective series of 119 patients with 219 lesions treated with SRS from 1991–1994, patients were treated with a median SRS dose of 18.5 Gy (range: 10–22 Gy) with a median lesion size of 1.3 cc (range: 0.02–30.9 cc). Multivariable Cox proportional hazards analysis demonstrated that SRS dose ≥18 Gy, lesion aspect/phenotype, and interval between diagnosis and SRS therapy were independent predictors of freedom from progression. The authors suggest that SRS dose could be a surrogate for lesion dose; however, tumor volume was not found to be independently significant on multivariable analysis. Our analysis further demonstrated a correlation between SRS dose and lesion size and identified SRS dose as the independent predictor of progression over lesion size.
Other investigators have studied the interaction of SRS dose, lesion size, and various patient outcomes [11, 12, 14]. Breneman et al. published a predictive analysis of 84 patients and 145 lesions, which observed that patients receiving SRS dose ≥18 Gy and/or melanoma histology had improved local control. Similarily, Schomas et al. [14] observed that dose prescription, minimum tumor dose, histology, and tumor volume were predictive of local control on univariable analysis. Minimum tumor dose was the only factor predictive of local control on multivariable analysis (p = .03).
Molenaar et al. also observed that SRS dose, planning target volume, and patient performance status were predictive of time to local failure [12]. Shetata et al. suggested that the use of whole-brain radiation therapy in conjunction with SRS improved lesion control [15]; however, this claim has not been reported elsewhere in literature. A systematic review formally exploring the relationship between dose and local control was conducted by Wiggenraad et al. [16]. This review assessed 11 papers and demonstrated that 6-month local control was greater than 80% irrespective of SRS dose fractionation schedule. One-year local control rates with single-dose SRS treatment were observed to be more variable and depended on dose: >80% for ≥ 21 Gy), >60% for ≥18 Gy), and <50% for <15 Gy.
The major limitation of this work is that the database is derived from a retrospective analysis of patients who received SRS. Mitigating the issue of the retrospective nature of the database was the prospective approach regarding patient selection, treatment simulation/delivery, dose-fractionation selection, and follow-up procedures relating to this patient population. Future work in this area will include validation of our findings in other existing SRS databases. Additionally, modeling of regional (out-of-field) failure risk may provide insight into patients better served with the integration of whole-brain radiation therapy in conjunction with SRS therapy.
Conclusions
A recursive partitioning analysis of a large retrospective SRS database has confirmed previous observations regarding the importance of SRS dose and lesion aspect/phenotype in lesion control. The SRS lesion RPA describes three distinct prognostic groups of patients in terms of time to lesion progression. Use of the SRS lesion RPA groups also predicted for overall survival using an actuarial log-rank test analysis. The SRS lesion RPA analysis may help to stratify future SRS clinical trials and better define patient care options and prognosis in conjunction with SRS therapy.
See www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Author Contributions
Conception/Design: George Rodrigues, Frank Lagerwaard
Provision of study material or patients: Jaap Zindler, Frank Lagerwaard
Collection and/or assembly of data: George Rodrigues, Jaap Zindler, Andrew Warner, Frank Lagerwaard
Data analysis and interpretation: George Rodrigues, Jaap Zindler, Andrew Warner, Frank Lagerwaard
Manuscript writing: George Rodrigues, Jaap Zindler, Andrew Warner, Frank Lagerwaard
Final approval of manuscript: George Rodrigues, Jaap Zindler, Andrew Warner, Frank Lagerwaard
Disclosures
Jaap Zindler: Varian Medical Systems, BrainLAB (RF); Frank Lagerwaard: BrainLAB (H, RF); Varian (RF). The other authors indicated no financial relationships.
Section editors: Suresh Senan: Lilly and Varian Medical Systems (C/A); Varian Medical Systems (H, RF); Andrew Turrisi: None
Reviewer “A”: None
Reviewer “B”: None
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;4:CD003869. doi: 10.1002/14651858.CD003869.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao MN, Lloyd NS, Wong RK, et al. Radiotherapeutic management of brain metastases: A systematic review and meta-analysis. Cancer Treat Rev. 2005;31:256–273. doi: 10.1016/j.ctrv.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Nieder C, Mehta MP. Prognostic indices for brain metastases: Usefulness and challenges. Radiat Oncol. 2009;4:10. doi: 10.1186/1748-717X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 6.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 8.Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 10.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breneman JC, Warnick RE, Albright RE, Jr., et al. Stereotactic radiosurgery for the treatment of brain metastases. Results of a single institution series. Cancer. 1997;79:551–557. doi: 10.1002/(sici)1097-0142(19970201)79:3<551::aid-cncr18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Molenaar R, Wiggenraad R, Verbeek-de Kanter A, et al. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg. 2009;23:170–178. doi: 10.1080/02688690902755613. [DOI] [PubMed] [Google Scholar]
- 13.Regine WF, Huhn JL, Patchell RA, et al. Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: Results and implications. Int J Radiat Oncol Biol Phys. 2002;52:333–338. doi: 10.1016/s0360-3016(01)02645-1. [DOI] [PubMed] [Google Scholar]
- 14.Schomas DA, Roeske JC, MacDonald RL, et al. Predictors of tumor control in patients treated with LINAC-based stereotactic radiosurgery for metastatic disease to the brain. Am J Clin Oncol. 2005;28:180–187. doi: 10.1097/01.coc.0000143017.69880.04. [DOI] [PubMed] [Google Scholar]
- 15.Shehata MK, Young B, Reid B, et al. Stereotatic radiosurgery of 468 brain metastases ≤2 cm: Implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:87–93. doi: 10.1016/j.ijrobp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Wiggenraad R, Verbeek-de Kanter A, Kal HB, et al. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol. 2011;98:292–297. doi: 10.1016/j.radonc.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Shiau CY, Sneed PK, Shu HK, et al. Radiosurgery for brain metastases: Relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys. 1997;37:375–383. doi: 10.1016/s0360-3016(96)00497-x. [DOI] [PubMed] [Google Scholar]
- 18.Goodman KA, Sneed PK, McDermott MW, et al. Relationship between pattern of enhancement and local control of brain metastases after radiosurgery. Int J Radiat Oncol Biol Phys. 2001;50:139–146. doi: 10.1016/s0360-3016(00)01584-4. [DOI] [PubMed] [Google Scholar]
- 19.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 21.Cook EF, Goldman L. Empiric comparison of multivariate analytic techniques: Advantages and disadvantages of recursive partitioning analysis. J Chronic Dis. 1984;37:721–731. doi: 10.1016/0021-9681(84)90041-9. [DOI] [PubMed] [Google Scholar]
- 22.Rades D, Dunst J, Schild SE, et al. A new scoring system to predict the survival of patients treated with whole-brain radiotherapy for brain metastases. Strahlenther Onkol. 2008;184:251–255. doi: 10.1007/s00066-008-1831-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.