Abstract
Enzyme dynamics are being incorporated into soil carbon cycling models and accurate representation of enzyme kinetics is an important step in predicting belowground nutrient dynamics. A scarce number of studies have measured activation energy (Ea) in soils and fewer studies have measured Ea in arctic and tropical soils, or in subsurface soils. We determined the Ea for four typical lignocellulose degrading enzymes in the A and B horizons of seven soils covering six different soil orders. We also elucidated which soil properties predicted any measurable differences in Ea. β-glucosidase, cellobiohydrolase, phenol oxidase and peroxidase activities were measured at five temperatures, 4, 21, 30, 40, and 60°C. Ea was calculated using the Arrhenius equation. β-glucosidase and cellobiohydrolase Ea values for both A and B horizons in this study were similar to previously reported values, however we could not make a direct comparison for B horizon soils because of the lack of data. There was no consistent relationship between hydrolase enzyme Ea and the environmental variables we measured. Phenol oxidase was the only enzyme that had a consistent positive relationship between Ea and pH in both horizons. The Ea in the arctic and subarctic zones for peroxidase was lower than the hydrolases and phenol oxidase values, indicating peroxidase may be a rate limited enzyme in environments under warming conditions. By including these six soil types we have increased the number of soil oxidative enzyme Ea values reported in the literature by 50%. This study is a step towards better quantifying enzyme kinetics in different climate zones.
Introduction
In recent years, increasingly complex and realistic soil carbon models explicitly include microbial processes [1]–[5]. However, most soil carbon models remain largely simplified constructs because of the difficulty in ascertaining microbial responses to different variables [6]. One difficulty in modeling the soil carbon system is understanding the microbial mechanisms that are influenced by temperature, such as enzymatic reactions (extra- and intracellular), diffusion of substrates, and microbial substrate utilization efficiency [7]–[9]. The temperature response of reactions at all scales of life can be determined by calculating the activation energy (Ea) [10]–[15]. Activation energy is the difference in energy between reactants and the transitional species that decay into products, and this determines the change in a reaction's rate with temperature. However, estimating this parameter for enzymatic reactions is challenging because activity and Ea are specific to the type of enzyme [16], in situ temperature range, substrate, and other edaphic characteristics under consideration [9].
At the ecosystem scale, extracellular enzyme activity is influenced by organic matter abundance and composition [17]. Lignocellulose, a main component of plant litter is comprised primarily of cellulose, hemicellulose, and lignin [14] and is broken down extracellularly by a suite of enzymes produced by many organisms [9]. In order to convert cellulose into glucose, three categories of hydrolases are produced by microbes: cellobiohydrolase, endoglucanase and β-glucosidase [18]–[19]. Lignin is broken down by a class of oxidoreductases called ligninases [20]. Typical ligninases include peroxidases and phenol oxidases [17], [21]. Enzymes that depolymerize high molecular weight compounds, such as lignin require more enzymatic steps and have been shown to have higher Ea than enzymes that break down simpler compounds [16], [22]. A higher Ea for complex compounds indicates that there may be a disproportionate effect of increasing temperatures on the depolymerization of high molecular weight components of organic matter [6].
In situ temperature also influences the temperature optima of enzymes. Several studies have demonstrated that enzymes produced by microorganisms in colder climates have lower temperature optima [23]–[24]. The stability of an enzyme's structure is dependent on the in situ temperature range, with enzymes in colder climates having more flexible structures than enzymes in warmer climates [25]–[26]. The ability of enzymes to change structural conformation with temperature [27], thus altering the active site, could impact the Ea of enzymatic reactions in soils from different climates because isoenzymes for the same reaction do have different substrate affinities [28] and may have different Ea. Extracellular enzymes can be sorbed to clays [29]–[30] which can affect their activity. Studies have shown both increases and decreases in activity once enzymes become immobilized on clays [31]–[32]. If sorption alters the structure of the enzymes, particularly the active site, it could change the kinetic properties of the enzyme [33].
Surprisingly, a scarce amount of studies have been carried out to determine the Ea of soil enzymes [16], [34]–[35], especially for oxidative enzymes. More often, it is easier to find studies carried out on pure enzymes, however these studies do not indicate how edaphic conditions might alter Ea [36]–[43]. Because of the paucity of data we chose to determine the Ea for four typical lignocellulose degrading enzymes in surface and subsurface horizons of seven soils covering six different soil orders. We also elucidated which soil properties predicted any measurable differences in Ea. We hypothesized that Ea for all enzymes would be greatest in cold regions (arctic and subarctic), followed by temperate and tropical regions. Tropical regions have very stable temperatures so that enzymes may be adapted to the constant warm temperatures whereas temperatures fluctuate daily and seasonally in the temperate and cold regions. In addition, we hypothesized that (2) the ambient temperature 30 days prior to sampling would influence the enzyme pool, so that potential enzyme activity would be lower in soils collected during warmer periods compared to colder periods due to enzymatic efficiency, and (3) Ea for oxidative enzymes would be greater than hydrolytic enzymes, regardless of climate regions, due to the difference in substrate complexity.
Materials and Methods
Site Descriptions
Soil samples were collected from a broad range of climatic zones across the western hemisphere (Table 1). From each location, two or three soil samples were taken from both A and B horizons, composited and 2 mm sieved. From the seven soils tested, six soil orders (Alfisol, Andisol, Gelisol, Mollisol, Oxisol, and Utlisol) and four major climate zones (arctic, subarctic, temperate, and tropical) were represented. No specific permits were required to collect soil samples from the field locations, which were on public, non protected land. In the case of the Icelandic site The Environment Agency of Iceland was informed of the soil sampling and according to regulation B, no. 520/1975 a permit is not required for soil sampling for scientific purposes. All necessary shipping permits were obtained for the described field samples, a USDA APHIS quarantine permit for shipment of soils from outside the United States and a compliance agreement for the shipment of domestic soils. The temperate Ultisol and temperate Mollisol are both US-DOE sites, and the arctic Gellisol is a US-ACE site. The sample collection did not involve or harm any endangered or protected species.
Table 1. Soil characteristics and environmental variables.
| Soils | Location | Order | Sample Year | Horizon | depth (cm) | Clay (%) | pH | Total C (%) | avg T (°C) | GWC | MAT (°C) | MAP (mm) |
| Arctic 1 | Fairbanks, AK | Gelisol | Dec-10 | Active | 0–30 | 13 | 7.03 | 2.54 | −12.0 | 0.362 | −2.9 | 572 |
| Permafrost | 50–75 | 13 | 8.05 | 1.82 | 0.453 | −2.9 | 572 | |||||
| Subarctic 1 | Krýsuvík, Iceland | Andisol | July-11 | A | 0–15 | 12 | 5.84 | 8.49 | 8.6 | 0.775 | 5.0 | 1600 |
| B | 35–55 | 10 | 6.13 | 8.14 | 1.053 | 5.0 | 1600 | |||||
| Temperate 1 | Kane, IL | Mollisol | July-11 | A | 0–15 | 35 | 6.70 | 3.72 | 19.9 | 0.354 | 11.3 | 996 |
| B | 55–70 | 26 | 6.80 | 1.66 | 0.291 | 11.3 | 996 | |||||
| Temperate 2 | Gibson, TN | Alfisol | April-11 | A | 0–15 | 29 | 5.50 | 1.06 | 12.2 | 0.217 | 16.9 | 1381 |
| B | 50–75 | 32 | 5.80 | 0.52 | 0.12 | 16.9 | 1381 | |||||
| Temperate 3 | Blount, TN | Ultisol | May-11 | A | 0–10 | 25 | 6.17 | 1.97 | 16.6 | 0.239 | 14.7 | 1225 |
| B | 40–60 | 45 | 5.11 | 0.26 | 0.203 | 14.7 | 1225 | |||||
| Tropical 1 | Lavras, Brazil | Oxisol | March-11 | A | 0–12 | 67 | 4.42 | 5.85 | 24.0 | 0.299 | 19.3 | 1343.3 |
| B | 42–65 | 79 | 4.68 | 2.33 | 0.272 | 19.3 | 1343.3 | |||||
| Tropical 2 | Lavras, Brazil | Ultisol | March-11 | A | 0–10 | 45 | 5.42 | 3.17 | 24.0 | 0.24 | 19.3 | 1343.3 |
| B | 37–50 | 42 | 5.17 | 1.07 | 0.227 | 19.3 | 1343.3 |
Total C = total carbon, avg T = average air temperature (°C) for the month preceding sampling, GWC = gravimetric water content, MAT = mean annual temperature, MAP = mean annual precipitation.
Soil Processing
Field moist soil samples were composited, 2 mm sieved, and then stored at −10°C for a maximum of 1 week until enzyme analyses could be performed. Gravimetric water content (GWC) was determined in triplicate by oven drying the pre-weighed subsamples for 24 hr at 105°C, and then reweighing each subsample. Total carbon and nitrogen analyses were performed on air dried, ground soil using a LECO TruSpec CN analyzer (LECO Corp., St. Joseph, MI). Particle size analysis was performed using the Buoycous hydrometer method [44]. Soil pH was measured on the supernatant of a 5 mM CaCl2 solution in a 2∶1 solution to solid ratio.
Enzyme Assays
Enzymatic assays were performed, in 96-well microplates on two hydrolases, β-glucosidase (BG, EC 3.2.1.21) and cellobiohydrolase (CB, EC 3.2.1.91), and two oxidases, peroxidase (PER, EC 1.11.1.7) and phenol oxidase (POX, EC 1.10.3.2) [45]. Each assay was performed at 4, 21, 30, 40, and 60°C in 50 mM pH 5 sodium acetate buffer. Typically assays are performed at the in situ soil pH, however Wang et al. (2012b) have demonstrated that most soil enzymes have pH optima around 5. Also, oxidative enzyme activity is very difficult to measure at a higher pH due to abiotic oxidation of substrates, which can lead to incorrect calculation of Ea. Through laboratory trials, it was determined that a pH 5 buffer would be an adequate pH for these soils despite the wide range of in situ soil pH (Table 1). For every soil, three 1 g subsamples were taken to represent the heterogeneity at the site. Each subsample was mixed with 125 mL of 50 mM pH 5 acetate buffer were mixed with a hand blender for two minutes. The suspension was then added to a 150 mm petri dish and maintained using a magnetic stir rod.
The hydrolase assays were performed in replicates of eight in black 96-well microplates. The blank wells on each plate received 250 µL of acetate buffer, reference-standard wells received 200 µL of acetate buffer and 50 µL of 10 µM 4-methylumbelliferone (MUB) standard, and negative-control wells received 200 µL acetate buffer and 50 µL 200 µM 4-MUB-linked substrates (4-MUB-β-D-glucoside and 4-MUB-β-D-cellobioside). For each soil, quench-control wells received 200 µL of soil suspension and 50 µL 10 µM 4-MUB standard, sample control wells received 200 µL soil suspension and 50 µL of 50 mM acetate buffer. Activity assay wells received 200 µL of soil suspension and 50 µL of 200 µM 4-MUB-linked substrate. After incubating for 2 h, each black microplate received 10 µL 0.5N NaOH in every well in order to raise the pH and enhance the fluorescence to a detectable level. Fluorescence was then detected at an excitation wavelength 365 nm, emission wavelength 450 nm, and sensitivity of 50 with a BioTec Synergy™MX Multi-Mode Microplate Reader.
The oxidase assays were performed in clear 96-well UV microplates. For each plate, 8 blank wells received 250 µL of acetate buffer and 16 reference-standard wells received 200 µL acetate buffer and 50 µL of L-3,4-dihydroxyphenylalanine (L-DOPA). For each soil, 8 homogenate control wells received 200 µL of soil suspension and 50 µL of acetate buffer and 16 assay wells received 200 µL soil suspension and 50 µL L-DOPA. Additionally, each well of the peroxidase assay plates received 10 µL of 0.3% H2O2. After incubation for 24 h, clear microplates were read spectrophotometrically at 460 nm with a BioTec Synergy™MX Multi-Mode Microplate Reader.
Data Analysis
For enzymatic assays, the activities for both hydrolases and oxidases were expressed as nmol of substrate converted per g dry soil per h (nmol g dry soil−1 h−1). An Arrhenius plot was created to estimate activation energy according to the Arrhenius equation:
 |
(1) |
where A, a constant, is the frequency factor, Ea is the activation energy, R is the gas constant and T is the absolute temperature (°K)
Relationships between Ea and environmental parameters in Table 1 were determined using both linear and polynomial regressions. In addition, PROC GLM (SAS Inc., Cary NC) was used to determine if biome type had a significant effect on Ea(P<0.05), using the three enzyme subsamples from each location.
Results
In most cases, β-glucosidase Ea tended to be higher in the B horizon compared to the A horizon horizon, except in the arctic system where they were similar (Table 2; P<0.05). Phenol oxidase Ea, was consistently greater in the A horizon than B horizon soil in the tropics (P<0.05). Cellobiohydrolase and peroxidase Ea showed no discernible trend with soil depth.
Table 2. Activation Energies (kJ mol−1) with standard error in parentheses (analytical replicates n = 8 for BG, CB; n = 16 for PER, POX) for extracellular enzymes. “*” indicated n = 1.
| Soil | Ea (kJ/mol) | ||||||||
| Horizon | BG | CB | PER | POX | |||||
| Arctic | A | 35.4 | (1.34) | 39.4 | (3.93) | 12.7 | (0.52) | 81.8 | (7.38) |
| P | 34.8 | (1.05) | 18.7 | (4.22) | 21.3 | (0.92) | 74.2 | (3.16) | |
| Subarctic | A | 36.5 | (0.73) | 38.6 | (0.59) | 21.2 | (2.16) | 45.7 | (5.56) |
| B | 52.2 | (2.08) | 41.5 | (0.62) | 22.4 | (2.20) | 39.4 | (10.10) | |
| Temperate 1 | A | 40.9 | (1.46) | 38.0 | (1.05) | 64.9 | (2.24) | 102.0 | (9.22) |
| B | 49.4 | (2.48) | 21.2 | (9.97) | 28.0 | (6.50) | 94.8 | (6.81) | |
| Temperate 2 | A | 31.0 | (0.69) | 43.4 | (0.81) | 25.4 | (1.80) | 49.5 | (5.88) |
| B | 40.9 | (2.48) | 39.9 | (7.48) | 19.8 | (1.82) | 47.5 | (3.75) | |
| Temperate 3 | A | 51.5 | (2.17) | 53.6 | (2.51) | 28.8 | (1.69) | 73.2 | (6.09) |
| B | 58.8 | (4.98) | 46.7 | (2.36) | 54.2 | (8.84) | 29.0 | (4.58) | |
| Tropical 1 | A | 47.8 | (0.93) | 50.5 | (7.58) | 26.5 | (4.25) | 47.7 | (10.00) |
| B | 56.6 | (2.66) | 47.0 | * | 47.1 | (4.58) | 27.1 | (11.90) | |
| Tropical 2 | A | 39.3 | (1.51) | 42.5 | (2.27) | 58.3 | (5.12) | 82.5 | (9.96) |
| B | 42.8 | (1.91) | 43.3 | (2.46) | 22.8 | (3.09) | 45.5 | (3.55) | |
Ea for all enzymes was affected by biome type (Figure 1; P<0.1 and 0.05). In the A horizon, Ea for the hydrolases was similar for arctic and subarctic biomes, while in the B horizon the hydrolase's Ea for the subarctic was more similar to temperate and tropical biomes. In the A horizon, within a biome the Ea of the hydrolytic enzymes was similar, whereas the Ea of the two oxidative enzymes were very different from each other. In the B horizon, the Ea of both hydrolases (β-glucosidase and cellobiohydrolase) were always lower in the arctic biome (Alaska) than subarctic, temperate and tropical biomes (P<0.1 and 0.05). Phenol oxidase Ea was similar in arctic and temperate biomes in both horizons, and lowest in the subarctic biome (P<0.1).
Figure 1. Activation energy for hydrolytic and oxidative enzymes.
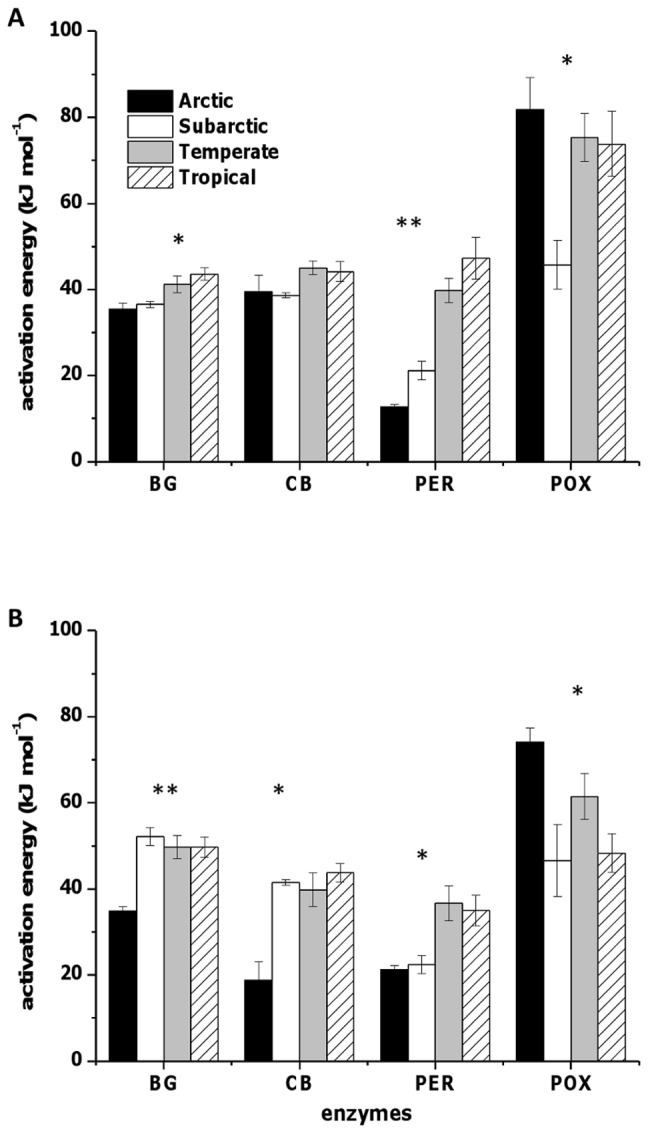
Ea in (a) A horizon and (b) B horizon for β-glucosidase (BG), cellobiohydrolase (CB), peroxidase (PER) and phenol oxidase (POX). Activation energy calculated from three subsamples taken from each soil and depth combination. The number of study locations per biome were tropical n = 2; temperature n = 3; subarctic n = 1; arctic n = 1. The “*” indicates a significant effect of biome with a 0.05≤P≤0.1 and “**” indicates a significant effect of biome with a P≤0.01.
The Ea of β-glucosidase and cellobiohydrolase have positive relationships with average air temperature in the month preceding sampling and mean annual temperature (Table 3). Soil pH had a strong negative relationship on the Ea of hydrolases in the B horizon, and a strong positive relationship with phenol oxidase Ea in both horizons. The relationship between clay and Ea was positive for β-glucosidase and peroxidase in both horizons, but negative for phenol oxidase Ea in the B horizon. Significant regressions are shown in Supplemental Figure 1.
Table 3. Linear and polynomial regression statistics relating Ea for four enzymes to four different environmental variables.
| A HORIZON | B HORIZON | ||||||||
| enzyme | variable | regression | r2 | F | P | regression | r2 | F | P |
| β-glucosidase | clay | y = 0.21x+31.21 | 0.28 | 3.35 | 0.13 | y = 0.25x+35.76 | 0.22 | 2.67 | 0.16 |
| pH | y = −4.87x+75.01 | 0.54 | 8.09 | 0.04 | |||||
| avg T | y = 31.8−0.04x+0.02x2 | 0.35 | 2.62 | 0.19 | y = 0.40x+40.44 | 0.54 | 8.23 | 0.04 | |
| MAT | y = 41.54+1.96x−0.09x2 | 0.56 | 4.87 | 0.08 | |||||
| cellobiohydrolase | clay | ||||||||
| pH | y = −4.95x+71.1 | 0.72 | 16.43 | 0.01 | |||||
| avg T | y = 34.74+1.03x−0.03x2 | 0.71 | 7.17 | 0.07 | |||||
| MAT | y = 0.39x+36.50 | 0.37 | 4.49 | 0.09 | y = 30.57+2.58x−0.09x2 | 0.71 | 8.30 | 0.04 | |
| peroxidase | clay | y = 1.01x+0.99 | 0.46 | 6.15 | 0.06 | y = 24.44−0.34x+0.008x2 | 0.48 | 3.82 | 0.12 |
| pH | |||||||||
| avg T | y = 0.83x+22.28 | 0.62 | 11.13 | 0.02 | |||||
| MAT | y = 1.2x+16.56 | 0.44 | 5.73 | 0.06 | |||||
| phenol oxidase | clay | y = −0.79x+79.75 | 0.31 | 3.73 | 0.11 | ||||
| pH | y = 14.71x−21.78 | 0.23 | 2.77 | 0.16 | y = 12.45x−2.41 | 0.61 | 10.40 | 0.02 | |
| avg T | |||||||||
| MAT | y = −1.37x+69.98 | 0.34 | 4.15 | 0.10 | |||||
Data are shown for A and B horizon regressions in Supplemental Figure 1 , avg T = average air temperature (°C) for the month preceding sampling; MAT = mean annual temperature (°C).
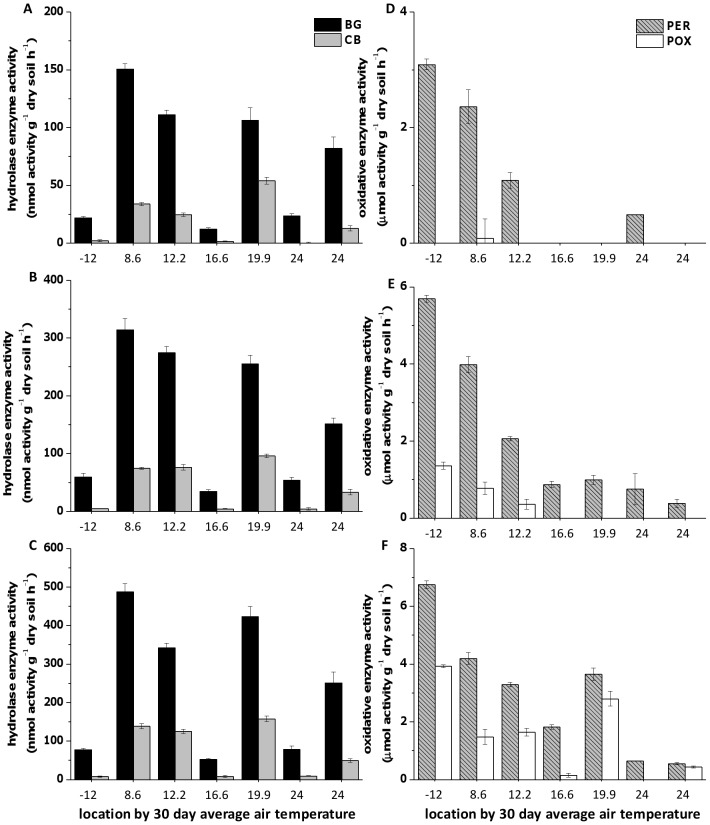
There was no clear relationship between potential hydrolase activities and average air temperature in the month preceding sampling (Figure 2, A horizon only). The arctic location, one temperate, and one tropical location all showed low potential β-glucosidase and cellobiohydolase. Potential activity at 4, 21, 30, 40 and 60°C were used to calculate Ea but activity for 40 and 60°C are not shown in Figure 2 because those temperatures are well outside the range of native soil temperatures in all locations and in some locations no activity was detected despite being present at lower temperature assays, likely due to enzyme inactivation at high temperatures. Activity increased for β-glucosidase (42–68%), cellobiohydrolase (37–57%), peroxidase (0–75%), and phenol oxidase (0–55%) in the A horizon for assays performed between 4°C and 21°C. β-glucosidase activity was always the highest followed by cellobiohydrolase activity, peroxidase activity and phenol oxidase activity. Phenol oxidase activity was not detected at 4° and 21°C in the temperate and tropical locations but was detected in the arctic and subarctic locations. Enzyme activity for both oxidative enzymes showed a distinct trend with decreased activity as average air temperature in the month preceding sampling increased (Figure 2; P<0.2). B horizon enzyme activity increased with assay temperature, however there was no clear trend for activity with average air temperature (data not shown).
Figure 2. Hydrolytic and oxidative enzyme activities in the A horizon.
Enzyme activities at (a) 4°C, (b) 21°C, and (c) 30°C for hydrolytic enzyme activities in order by average air temperature 30 days prior to sampling. Oxidative enzyme activity at (d) 4°C, (e) 21°C, and (f) 30°C in order by average air temperature 30 days prior to sampling.
Discussion
This study is one of the few that has looked at the Ea for hydrolytic and oxidative enzymes across soil orders and soil depth. In particular, by including arctic and tropical soils we have increased the breadth of Ea values beyond the temperate zone. Despite the importance of arctic and tropical ecosystem to the carbon cycle, there is little information regarding Ea of hydrolase and oxidative enzymes [46]–[47]. Overall, the Ea values for the hydrolases in all climatic zones were similar or slightly higher than other reported values [16], [46], [48], with average Ea values of 44 and 40.3 for β-glucosidase and cellobiohydrolase across all biomes, respectively. The cellobiohydrolase reaction occurs before the β-glucosidase reaction, but they had similar Ea indicating no differential response to temperature or a rate limiting step.
In several cases the Ea in the B horizon was higher than other reported values from A horizon soils, but B horizon soils are often not considered in enzyme studies, so we have little data for direct comparison. Most enzyme studies consider only the portion of soil near the surface (top 15 cm, usually A horizon), but there is a large portion of the terrestrial carbon stored below. It is unclear why enzyme studies tend to ignore enzymes dynamics below the first 15–20 cm, perhaps it is assumed that enzymes in the B horizon would respond similarly to those in the A horizon or that they are less affected by environmental disturbance due to their depth.
Across enzymes there was no consistent relationship between Ea and the environmental variables we measured. We found that in the A horizon there were fewer relationships between Ea and the environmental variables we used (MAT, pH, clay and average 30 day temperature) compared to the B horizon. Only the positive linear relationship between phenol oxidase Ea and pH was maintained in both A and B horizons. There are other environmental variables (mineral content, clay type) not measured in this study that could be measured in the future to determine their influence if any over Ea.
The information to date on Ea for oxidative enzymes is scant from soil environments [14], [47]–[48] with most of Ea estimates coming from pure cultures of isolated enzymes [41]. A total of 11 values for phenol oxidase Ea and 14 values for peroxidase Ea were identified in [46], with most of those values coming from one study [48]. Previously estimated values for peroxidase Ea in soil samples ranged from 30.5 kJ mol−1 [48] up to 60 kJ mol−1 [14] with an average of 54 kJ mol−1 [47]. The value for peroxidase Ea in this study averaged across all climate regions was lower, 32 kJ mol−1, than previously estimated, but particularly in the arctic and subarctic regions, which had an average Ea of 19 kJ mol−1. Phenol oxidase activity ranged from 29–102 kJ mol−1 at our study sites and averaged 59.5 KJ mol−1 which was similar to previously recorded phenol oxidase Ea values 37–57 kJ mol−1 [48], with an average of 54 kJ mol−1 [47]. The low peroxidase Ea in colder regions was counter to our hypothesis that enzymes in colder regions would be more temperature sensitive than those in warmer region, however this does not hold true for phenol oxidase. The large difference in Ea between peroxidase and phenol oxidase indicate that not all oxidative enzymes respond similarly to temperature, whereas the two hydrolytic enzymes had similar temperature responses. Grouping oxidative enzymes together, as we did in our hypothesis, was incorrect because peroxidases appear to be much less temperature sensitive than hydrolytic enzymes, whereas phenol oxidases are much more temperature sensitive.
In the soils we used, phenol oxidase had a greater Ea than peroxidase indicating that the phenol oxidase reactions are more sensitive to temperature increases than peroxidase reactions, thus phenol oxidase reaction rates may increase more than peroxidase reaction rates with warming. Peroxidase also had very low Ea compared to the hydrolytic enzymes, indicating that the reaction rate is less sensitive to changes in temperature. In this study, peroxidase was the dominant oxidative enzyme in most environments, thus if temperatures rise, it may be the rate limiting step in decomposition because of its reduced sensitivity to temperature.
Enzyme activity for both oxidative enzymes showed a distinct trend with decreasing activity as the 30 day average temperature increased in accordance with our hypothesis that there would be lower potential enzyme activity in soils collected during warmer time periods because of increased enzymatic efficiency (Figure 2d–f; P<0.2). There was very little measurable peroxidase and no phenol oxidase activity in the soils from the warmer locations during the 4°C incubations and no measurable phenol oxidase activity in soils warmer locations during the 21°C incubations. This may be due to the structural conformation of the peroxidase and phenol oxidase enzymes. Enzymes in warmer locations tend to have more rigid or stable conformations whereas enzymes in colder environments tend to have more fluid conformations [25], [26]. The initially rigid conformation of the oxidative enzymes from warmer climates may have made it more difficult for enzymes to interact with substrates in the colder incubation temperature because the low temperature increased the rigidity of the enzyme. However, we do not see this clear trend for hydrolytic enzyme activity.
Enzyme activity and thus Ea calculations are made by adding simple substrates to soil slurries. The substrates are similar in structure to the substrates depolymerized in nature by enzymes but different in complexity. As mentioned earlier, plant material is made up of many different substrates but primarily lignin and cellulose which form a lignocelluloses complex. The lignocellulose complex has the lignin and cellulose intertwined so that enzymes need to work in conjunction to break down the material. The substrates we add in assays are single substrates, not in a complex, so the enzymes likely break down the substrates at a faster rate than in nature. Since Ea is calculated using activity rates the use of single substrates may decrease the Ea over what occurs in nature when a consortium of enzymes are required to complete the depolymerization of lignocelluloses complexes.
This study is by no means a comprehensive list of enzyme Ea across the globe, out of the thirty possible enzymes to be assayed we selected only four, but we did select enzymes representative of two major groups, hydrolases and oxidases. Enzyme activity has been measured in arctic and tropical biomes, however the Ea for enzymes in these systems has rarely been measured before [46], [49]. By including these six soil types we have increased the number of soil oxidative enzyme Ea values reported in the literature by fifty percent. This study is a step towards better understanding and comparing enzyme kinetics in different climate zones. Also, it points out that the classification of enzymes by reaction types may not be indicative of their responses to temperature. Enzyme dynamics are being incorporated into models [5] and having accurate representation of enzyme kinetics from different regions is an important step in predicting nutrient dynamics.
Supporting Information
Activation energy for all enzymes in both soil horizons. Ea in the A horizon (a–d), B horizon (e–h), and combined A and B horizons (i–l) across four soil characteristics: clay, pH, average air temperature (°C) for the month preceding sampling, and mean annual temperature (MAT). Significant and marginally significant linear and polynomial regressions are shown for each enzyme and each soil characteristic, P<0.2.
(TIF)
Acknowledgments
We thank Stan Wullschleger, Anna Wagner, Julie Jastrow, Yuri Zinn, and Guðrún Gísladóttir for providing soil samples. We would like to thank Dr. Gangsheng Wang and two anonymous reviewers for their helpful suggestions on the writing of this manuscript.
Funding Statement
This research was funded by the Laboratory Directed Research and Development (LDRD) Program of the Oak Ridge National Laboratory (ORNL), managed by UT-Battelle, LLC, for the U.S. Department of Energy under contract DE-AC05-00OR22725. Collection and processing of soil samples from Brazil was supported by CNPq. The submitted manuscript has been authored by a contractor of the U.S. Government under contract DE-AC05-00OR22725. Accordingly, the U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce the published form of this contribution, or allow others to do so, for U.S. Government purposes. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lawrence CR, Neff JC, Schimel JP (2009) Does adding microbial mechanisms of decomposition improve soil organic matter models? A comparison of four models using data from a pulsed rewetting experiment. Soil Biology & Biochemistry 41: 1923–1934. [Google Scholar]
- 2. Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecological Monographs 76: 151–174. [Google Scholar]
- 3. Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biology & Biochemistry 35: 549–563. [Google Scholar]
- 4. Wang G, Post WM (2012) A theoretical reassessment of microbial maintenance and implications for microbial ecology modeling. FEMS Microbiology Ecology 81: 610–617. [DOI] [PubMed] [Google Scholar]
- 5. Wang G, Post WM, Mayes MA (2012) Development of microbial-enzyme-mediated decomposition model parameters through steady-state and dynamic analyses. Ecological Applications In press. [DOI] [PubMed] [Google Scholar]
- 6. Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440: 165–173. [DOI] [PubMed] [Google Scholar]
- 7. Skopp J, Jawson MD, Doran JW (1990) Steady-State Aerobic Microbial Activity as a Function of Soil-Water Content. Soil Science Society of America Journal 54: 1619–1625. [Google Scholar]
- 8. Steinweg JM, Plante AF, Conant RT, Paul EA, Tanaka DL (2008) Patterns of substrate utilization during long-term incubations at different temperatures. Soil Biology & Biochemistry 40: 2722–2728. [Google Scholar]
- 9.Wallenstein MD, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh RL (2011) Controls on the Temperature Sensitivity of Soil Enzymes: A Key Driver of In Situ Enzyme Activity Rates. In: Shukla G, Varma A, editors. Soil Enzymology. Berlin: Springer-Verlag. pp. 245–258.
- 10. Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293: 2248–2251. [DOI] [PubMed] [Google Scholar]
- 11. Enquist BJ, Economo EP, Huxman TE, Allen AP, Ignace DD, et al. (2003) Scaling metabolism from organisms to ecosystems. Nature 423: 639–642. [DOI] [PubMed] [Google Scholar]
- 12. Wallenstein MD, Weintraub MN (2008) Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biology & Biochemistry 40: 2098–2106. [Google Scholar]
- 13. Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Functional Ecology 8: 315–323. [Google Scholar]
- 14. Di Nardo C, Cinquegrana A, Papa S, Fuggi A, Fioretto A (2004) Laccase and peroxidase isoenzymes during leaf litter decomposition of Quercus ilex in a Mediterranean ecosystem. Soil Biology & Biochemistry 36: 1539–1544. [Google Scholar]
- 15. Craine JM, Fierer N, McLauchlan KK (2010) Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nature Geosciences 3: 854–857. [Google Scholar]
- 16. Trasar-Cepeda C, Gil-Sotres F, Leiros MC (2007) Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biology & Biochemistry 39: 311–319. [Google Scholar]
- 17. Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, et al. (2008) Stoichiometry of soil enzyme activity at global scale. Ecology Letters 11: 1252–1264. [DOI] [PubMed] [Google Scholar]
- 18. Baldrian P, Valaskova V (2008) Degradation of cellulose by basidiomycetous fungi. FEMS Microbiology Reviews 32: 501–521. [DOI] [PubMed] [Google Scholar]
- 19. Stricker A, Mach R, de Graaff L (2008) Regulation of transcription of cellulases- and hemicellulases-encoding genes inAspergillus niger and Hypocrea jecorina (Trichoderma reesei). Applied Microbiology and Biotechnology 78: 211–220. [DOI] [PubMed] [Google Scholar]
- 20.Shi W (2011) Agricultural and ecological significance of soil enzymes: soil carbon sequenstration and nutrient cycling. In: Shukla G, Varma A, editors. Soil Enzymology. Berlin: Springer-Verlag. pp. 43–60.
- 21. Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 49: 637–644. [Google Scholar]
- 22. Koch O, Tscherko D, Kandeler E (2007) Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Global Biogeochemical Cycles 21: GB4017. [Google Scholar]
- 23. Feller G (2003) Molecular adaptations to cold in psychrophilic enzymes. Cellular and Molecular Life Sciences 60: 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huston AL, Krieger-Brockett BB, Deming JW (2000) Remarkably low temperature optima for extracellular enzyme activity from Arctic bacteria and sea ice. Environmental Microbiology 2: 383–388. [DOI] [PubMed] [Google Scholar]
- 25. Závodszky P, Kardos J, Svingor Á, Petsko GA (1998) Adjustment of conformational flexibility is a key event in the thermal adaptation of proteins. Proceedings of the National Academy of Sciences 95: 7406–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochachka P, Somero G (1984) Biochemical adaptation. Princeton, NJ: Princeton University Press.
- 27. Lonhienne T, Gerday C, Feller G (2000) Psychrophilic enzymes: revisiting the thermodynamic parameters of activation may explain local flexibility. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 1543: 1–10. [DOI] [PubMed] [Google Scholar]
- 28. Marx MC, Kandeler E, Wood M, Wermbter N, Jarvis SC (2005) Exploring the enzymatic landscape: distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biology & Biochemistry 37: 35–48. [Google Scholar]
- 29.Burns RG (1986) Interactions of enzymes with soil mineral and organic colloids. In: Guang PM, Schnitzer M, editors. Interactions of soil minerals with natural organics and microbes. Madison, WI: Soil Science Society of America. pp. 429–451.
- 30.Nannipieri P, Sequi P, Fusi P (1996) Humus and enzyme activity. In: Piccolo A, editor. Humic substances in terrestrial ecosystems. New York: Marcel Dekker. pp. 293–328.
- 31. Sarkar JM, Leonowicz A, Bollag J-M (1989) Immobilization of enzymes on clays and soils. Soil Biology & Biochemistry 21: 223–230. [Google Scholar]
- 32. Gianfreda L, Rao MA, Violante A (1992) Adsorption, activity and kinetic-properties of urease on montmorillonite, aluminum hydroxide and Al(OH)X-montmorillonite. Soil Biology & Biochemistry 24: 51–58. [Google Scholar]
- 33.Quiquampoix H (2000) Mechanisms of protein adsorption on surfaces and consequences for extracellular enzyme activity in soil. In: Bollag J, Stotzky G, editors. Soil Biochemistry. New York: Marcel Dekker. pp. 171–206.
- 34. Deng SP, Tabatabai MA (1994) Cellulase Activity of Soils. Soil Biology & Biochemistry 26: 1347–1354. [Google Scholar]
- 35. Parham JA, Deng SP (2000) Detection, quantification and characterization of beta-glucosaminidase activity in soil. Soil Biology & Biochemistry 32: 1183–1190. [Google Scholar]
- 36. Aktaş N, Çiçek H, Taşpınar Ünal A, Kibarer G, Kolankaya N, et al. (2001) Reaction kinetics for laccase-catalyzed polymerization of 1-naphthol. Bioresource Technology 80: 29–36. [DOI] [PubMed] [Google Scholar]
- 37. Brown-Peterson NJ, Salin ML (1993) Purification of a catalase-peroxidase from Halobacterium halobium: characterization of some unique properties of the halophilic enzyme. Journal of Bacteriology 175: 4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghori MI, Iqbal MJ, Hameed A (2011) Characterization of a novel lipase from Bacillus sp. isolated from tannery wastes. Brazilian Journal of Microbiology 42: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hewson WD, Dunford HB (1975) Horseradish Peroxidase. XVIII. The Arrhenius Activation Energy for the Formation of Compound I. Canadian Journal of Chemistry 53: 1928–1932. [Google Scholar]
- 40. Kuznetsov AM, Bogdanovskaya VA, Tarasevich MR, Gavrilova EF (1987) The mechanism of cathode reduction of oxygen in a carbon carrier-laccase system. FEBS Letters 215: 219–222. [DOI] [PubMed] [Google Scholar]
- 41. Niemetz R, Gross GG (2003) Oxidation of pentagalloylglucose to the ellagitannin, tellimagrandin II, by a phenol oxidase from Tellima grandiflora leaves. Phytochemistry 62: 301–306. [DOI] [PubMed] [Google Scholar]
- 42. Toscano G, Colarieti ML, Greco G Jr (2003) Oxidative polymerisation of phenols by a phenol oxidase from green olives. Enzyme and Microbial Technology 33: 47–54. [Google Scholar]
- 43. Gonçalves EM, Pinheiro J, Abreu M, Brandão TRS, Silva CLM (2007) Modelling the kinetics of peroxidase inactivation, colour and texture changes of pumpkin (Cucurbita maxima L.) during blanching. Journal of Food Engineering 81: 693–701. [Google Scholar]
- 44.Dane JD, Topp GC (2004) Hydrometer Method. Methods of Soil Analysis, Part 4, Physical Methods. Madison, Wisconsin USA: Soil Science Society of America, Inc. pp. 278–283.
- 45. Štursová M, Baldrian P (2011) Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant and Soil 338: 99–110. [Google Scholar]
- 46. German DP, Marcelo KRB, Stone MM, Allison SD (2012) The Michaelis–Menten kinetics of soil extracellular enzymes in response to temperature: a cross-latitudinal study. Global Change Biology 18: 1468–1479. [Google Scholar]
- 47. Wang G, Post WM, Mayes MA, Frerichs JT, Sindhu J (2012) Parameter estimation for models of ligninolytic and cellulolytic enzyme kinetics. Soil Biology and Biochemistry 48: 28–38. [Google Scholar]
- 48. McClaugherty CA, Linkins AE (1990) Temperature Responses of Enzymes in 2 Forest Soils. Soil Biology & Biochemistry 22: 29–33. [Google Scholar]
- 49. Stone MM, Weiss MS, Goodale CL, Adams MB, Fernandez IJ, et al. (2012) Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Global Change Biology 18: 1173–1184. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activation energy for all enzymes in both soil horizons. Ea in the A horizon (a–d), B horizon (e–h), and combined A and B horizons (i–l) across four soil characteristics: clay, pH, average air temperature (°C) for the month preceding sampling, and mean annual temperature (MAT). Significant and marginally significant linear and polynomial regressions are shown for each enzyme and each soil characteristic, P<0.2.
(TIF)