Abstract
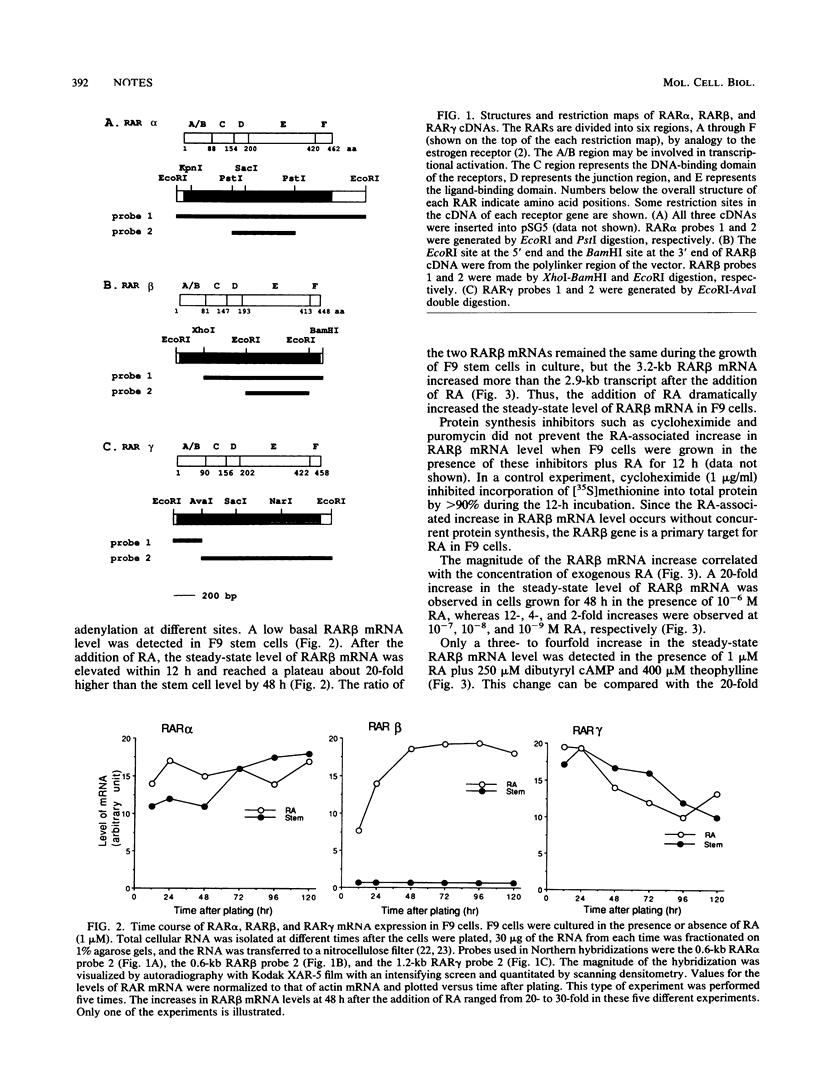
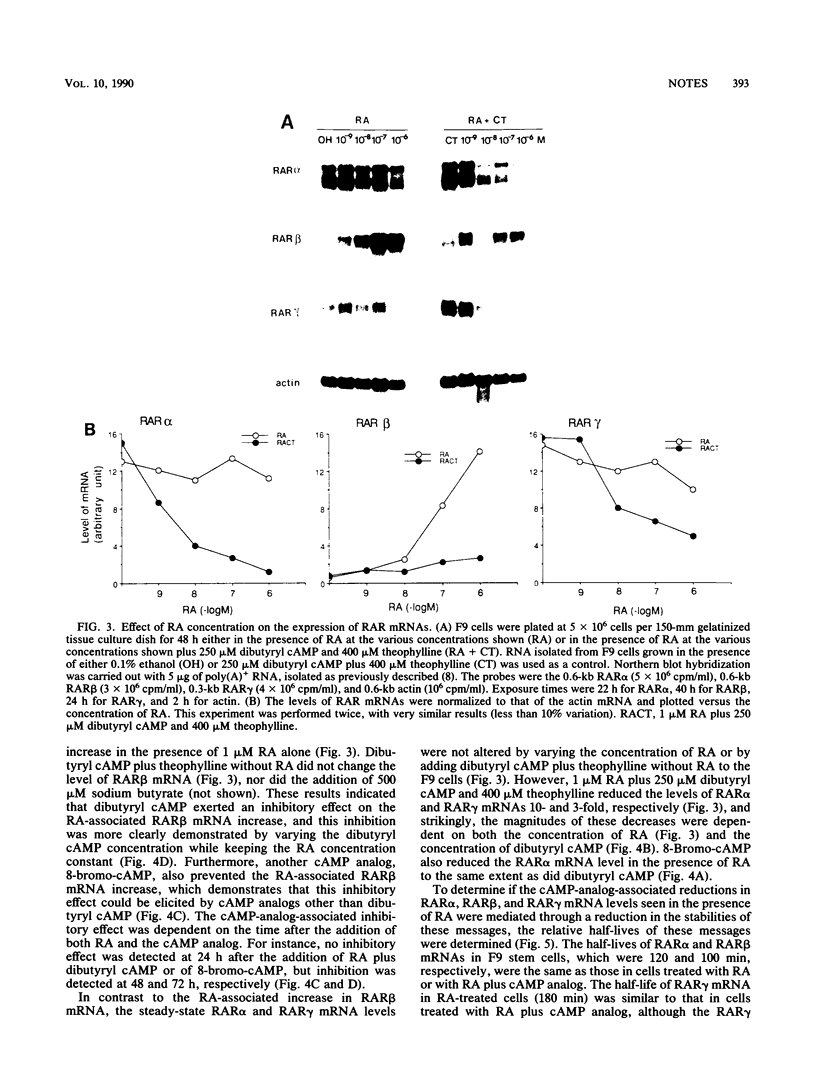
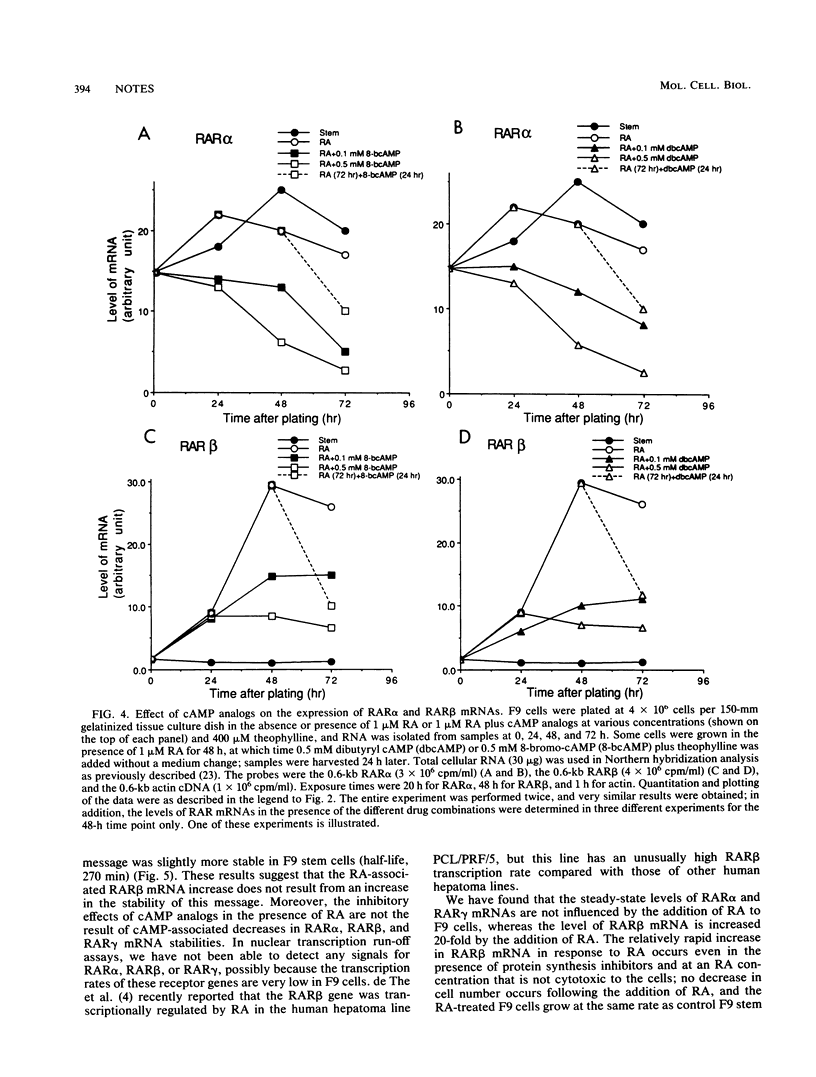
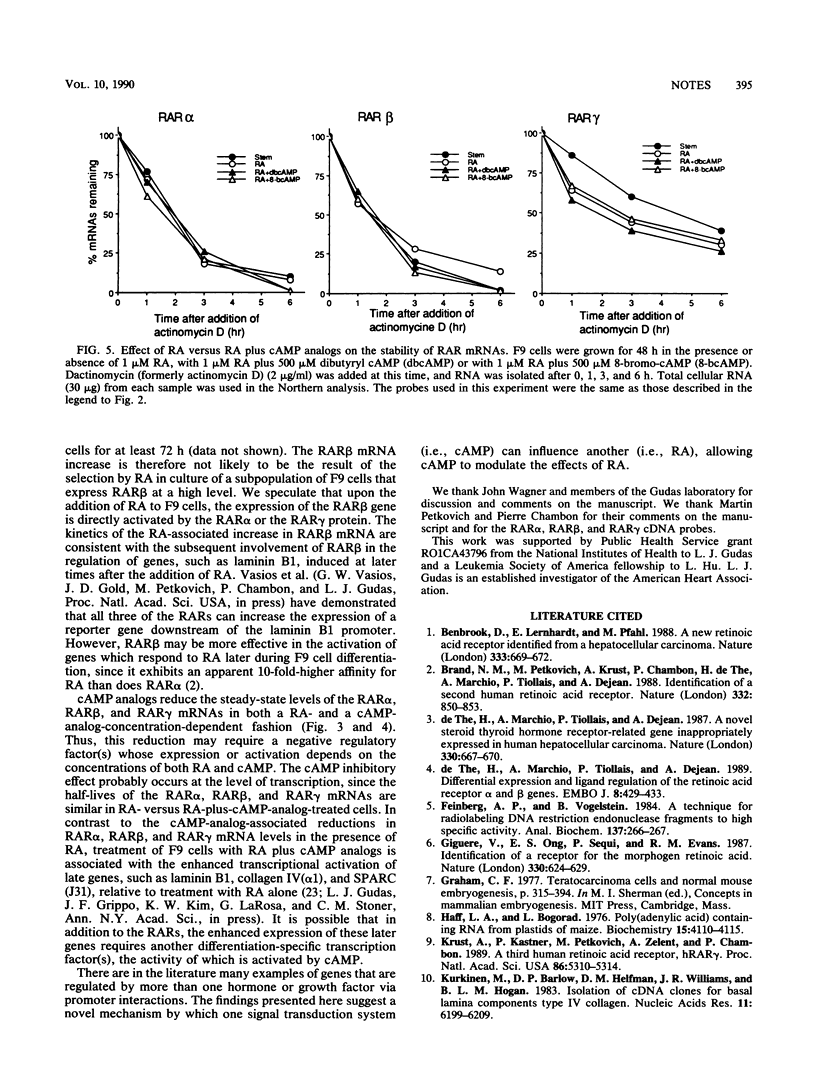
Retinoic acid (RA) receptor alpha (RAR alpha) and RAR gamma steady-state mRNA levels remained relatively constant over time after the addition of RA to F9 teratocarcinoma stem cells. In contrast, the steady-state RAR beta mRNA level started to increase within 12 h after the addition of RA and reached a 20-fold-higher level by 48 h. This RA-associated RAR beta mRNA increase was not prevented by protein synthesis inhibitors but was prevented by the addition of cyclic AMP analogs. In the presence of RA, cyclic AMP analogs also greatly reduced the RAR alpha and RAR gamma mRNA levels, even though cyclic AMP analogs alone did not alter these mRNA levels. The addition of either RA or RA plus cyclic AMP analogs did not result in changes in the three RAR mRNA half-lives. These results suggest that agents which elevate the internal cyclic AMP concentration may also affect the cellular response to RA by altering the expression of the RARs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Haff L. A., Bogorad L. Poly(adenylic acid)-containing RNA from plastids of maize. Biochemistry. 1976 Sep 7;15(18):4110–4115. doi: 10.1021/bi00663a030. [DOI] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Helfman D. M., Williams J. G., Hogan B. L. Isolation of cDNA clones for basal lamina components: type IV procollagen. Nucleic Acids Res. 1983 Sep 24;11(18):6199–6209. doi: 10.1093/nar/11.18.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982 Feb 25;295(5851):672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Murphy S. P., Garbern J., Odenwald W. F., Lazzarini R. A., Linney E. Differential expression of the homeobox gene Hox-1.3 in F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5587–5591. doi: 10.1073/pnas.85.15.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Soprano D. R., Soprano K. J., Goodman D. S. Retinol-binding protein and transthyretin mRNA levels in visceral yolk sac and liver during fetal development in the rat. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7330–7334. doi: 10.1073/pnas.83.19.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Takahashi Y. I., Smith J. E., Winick M., Goodman D. S. Vitam A deficiency and fetal growth and development in the rat. J Nutr. 1975 Oct;105(10):1299–1310. doi: 10.1093/jn/105.10.1299. [DOI] [PubMed] [Google Scholar]
- Tickle C., Lee J., Eichele G. A quantitative analysis of the effect of all-trans-retinoic acid on the pattern of chick wing development. Dev Biol. 1985 May;109(1):82–95. doi: 10.1016/0012-1606(85)90348-3. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Isolation of cDNA clones specific for collagen IV and laminin from mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5880–5884. doi: 10.1073/pnas.80.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- de The H., Marchio A., Tiollais P., Dejean A. Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J. 1989 Feb;8(2):429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Marchio A., Tiollais P., Dejean A. A novel steroid thyroid hormone receptor-related gene inappropriately expressed in human hepatocellular carcinoma. Nature. 1987 Dec 17;330(6149):667–670. doi: 10.1038/330667a0. [DOI] [PubMed] [Google Scholar]



