Abstract
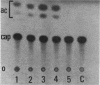
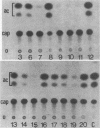
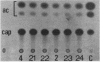
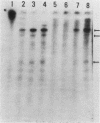
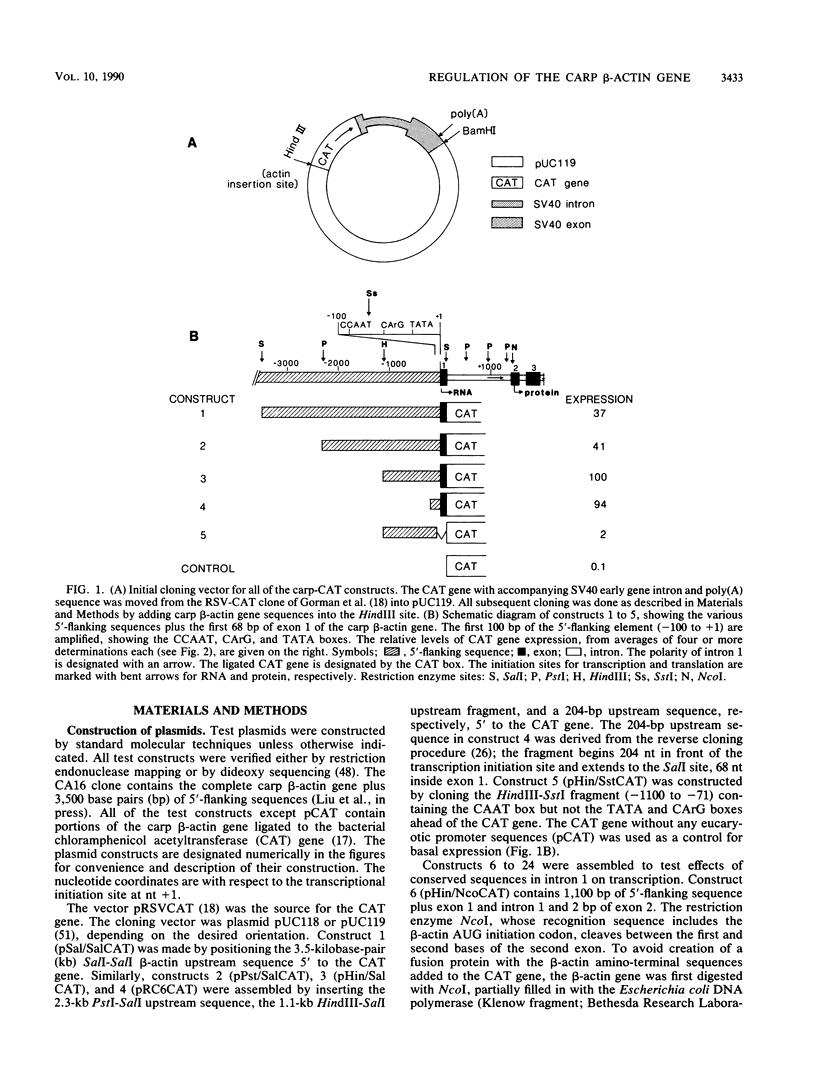
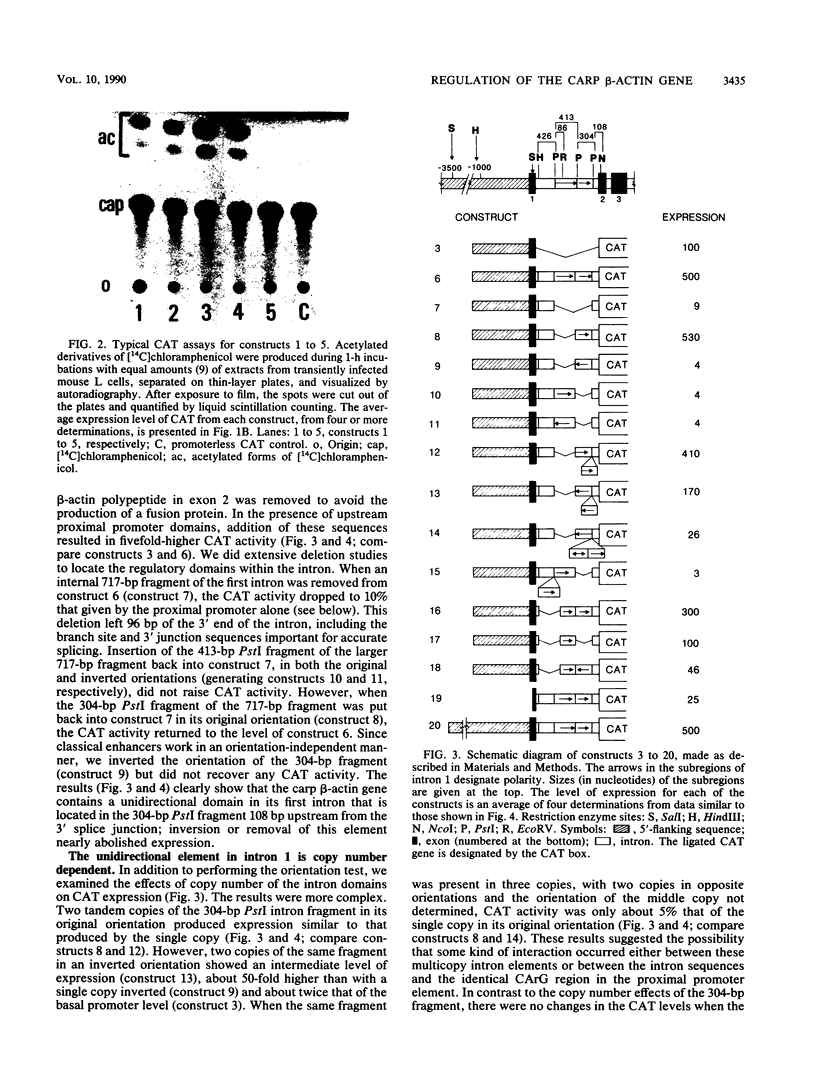
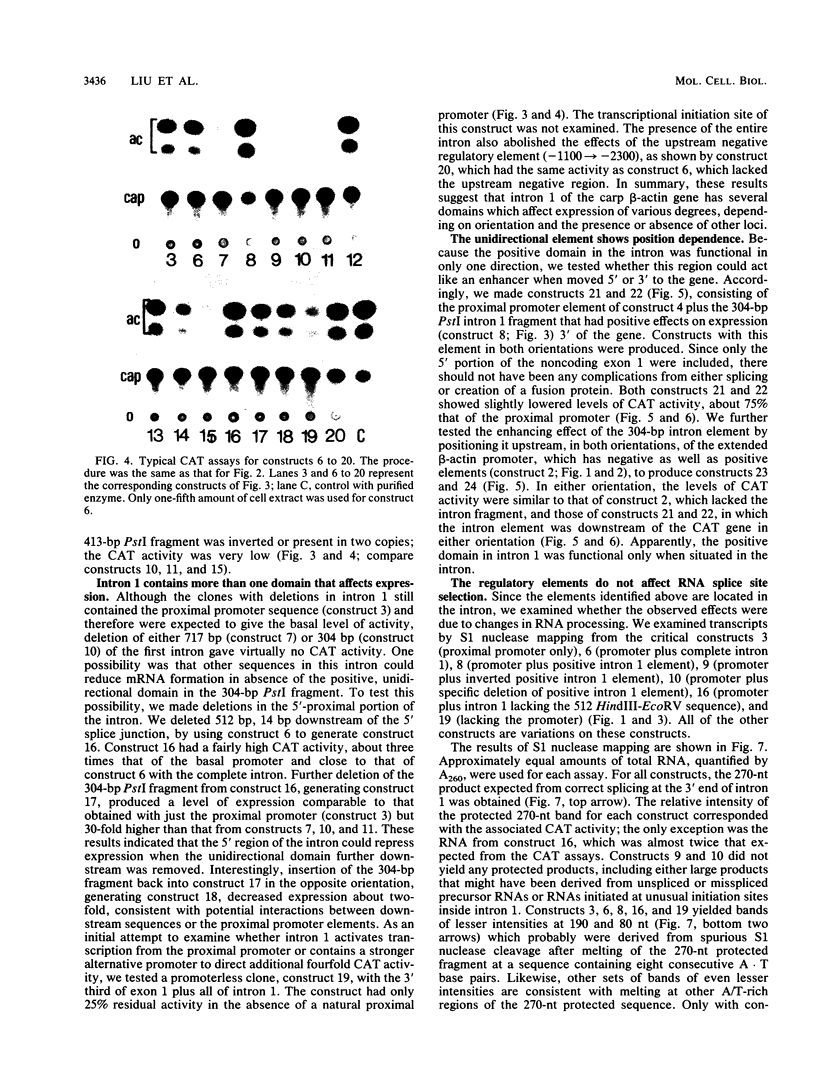
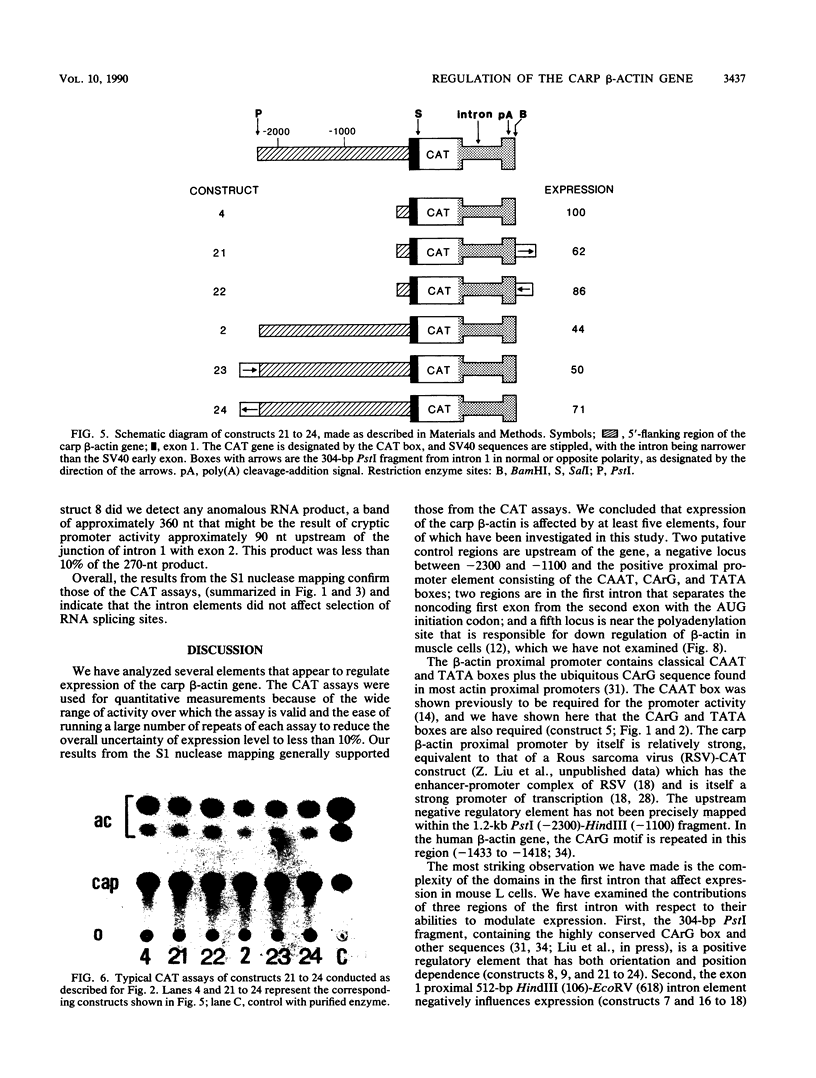
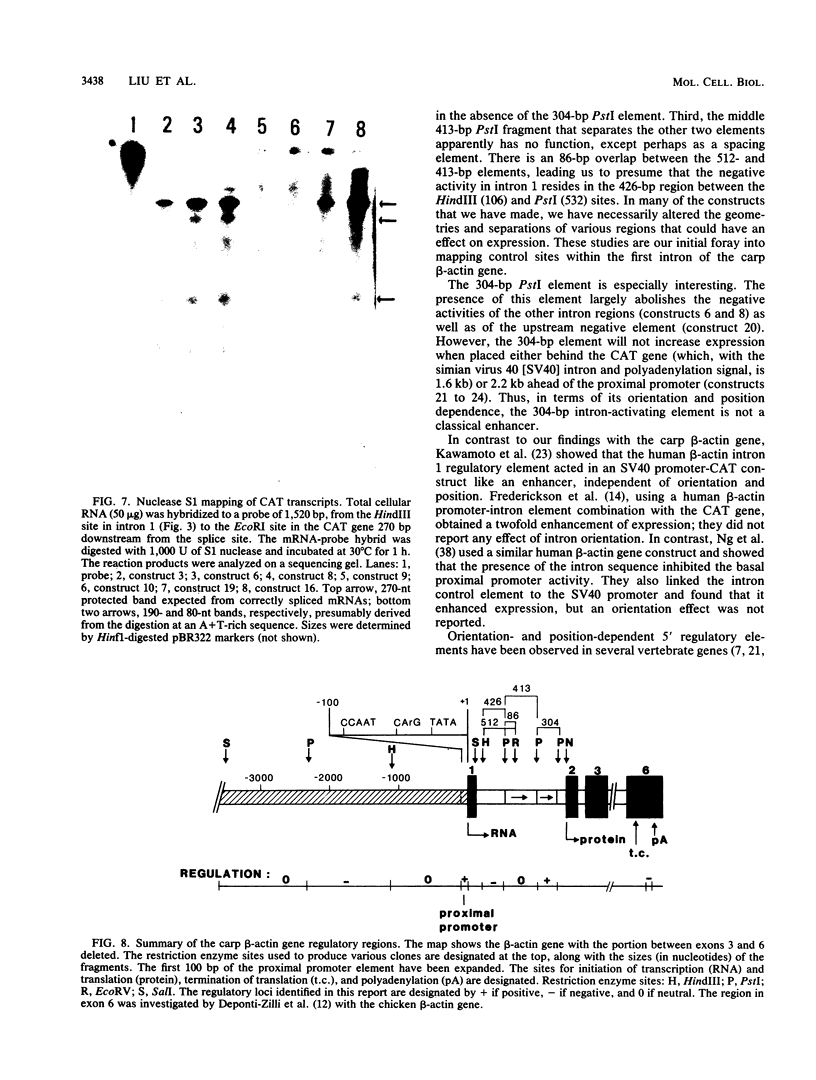
Regulatory regions of the beta-actin gene of the common carp (Cyprinus carpio) have been examined by linking upstream, 5'-flanking sequences and regions of the first intron to a bacterial chloramphenicol acetyltransferase (CAT) reporter gene. By analysis of the mRNA products and encoded CAT activity, we have identified four putative regions that influence expression: (i) a negative regulatory region 2,300 to 1,100 base pairs (bp) ahead of the gene; (ii) a proximal promoter element, containing the highly conserved CCAAT, CC(A/T)6GG, and TATA boxes, that is within the first 204 bp upstream of the initiation site; (iii) a negative element of 426 bp in the 5' region of the first intron; and (iv) a positive 304-bp element near the end of the first intron that contains highly conserved sequences found in all characterized beta-actin genes. The positive intron element is not a classical enhancer; it is position and orientation dependent, as has been observed in other housekeeping genes in vertebrates. Depending on the elements joined together, CAT gene expression can be modulated more than 500-fold in transfected mouse cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Sakonju S., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3' border of the region. Cell. 1980 Jan;19(1):27–35. doi: 10.1016/0092-8674(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Liska D. J., Apone S., Devarayalu S. Interactions between the promoter and first intron are involved in transcriptional control of alpha 1(I) collagen gene expression. Mol Cell Biol. 1988 Nov;8(11):4851–4857. doi: 10.1128/mcb.8.11.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Morishima J. K., Devarayalu S., Gelinas R. E. Regulatory elements in the first intron contribute to transcriptional control of the human alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8869–8873. doi: 10.1073/pnas.84.24.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J. The first intron of the alpha 1(I) collagen gene contains several transcriptional regulatory elements. J Biol Chem. 1988 Feb 5;263(4):1603–1606. [PubMed] [Google Scholar]
- Bouvagnet P. F., Strehler E. E., White G. E., Strehler-Page M. A., Nadal-Ginard B., Mahdavi V. Multiple positive and negative 5' regulatory elements control the cell-type-specific expression of the embryonic skeletal myosin heavy-chain gene. Mol Cell Biol. 1987 Dec;7(12):4377–4389. doi: 10.1128/mcb.7.12.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung S., Perry R. P. Importance of introns for expression of mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2075–2082. doi: 10.1128/mcb.9.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePonti-Zilli L., Seiler-Tuyns A., Paterson B. M. A 40-base-pair sequence in the 3' end of the beta-actin gene regulates beta-actin mRNA transcription during myogenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1389–1393. doi: 10.1073/pnas.85.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Jackson P. D., Felsenfeld G. Analysis of the tissue-specific enhancer at the 3' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4786–4790. doi: 10.1073/pnas.84.14.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson R. M., Micheau M. R., Iwamoto A., Miyamoto N. G. 5' flanking and first intron sequences of the human beta-actin gene required for efficient promoter activity. Nucleic Acids Res. 1989 Jan 11;17(1):253–270. doi: 10.1093/nar/17.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Birnstiel M. L. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature. 1981 Dec 17;294(5842):626–631. doi: 10.1038/294626a0. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Miwa T., Boxer L. M., Kedes L. Interaction of nuclear proteins with muscle-specific regulatory sequences of the human cardiac alpha-actin promoter. Mol Cell Biol. 1988 Oct;8(10):4110–4119. doi: 10.1128/mcb.8.10.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton W., Miyashita T., Kohno K., Hassell J. R., Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., Johnson J. E., Buskin J. N., Gartside C. L., Hauschka S. D. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988 Jan;8(1):62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski B. A., Yang L. H., Cacheris P., Morle G. D., Leiden J. M. The first intron of the 4F2 heavy-chain gene contains a transcriptional enhancer element that binds multiple nuclear proteins. Mol Cell Biol. 1989 Jun;9(6):2588–2597. doi: 10.1128/mcb.9.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Makino K., Niwa H., Sugiyama H., Kimura S., Amemura M., Nakata A., Kakunaga T. Identification of the human beta-actin enhancer and its binding factor. Mol Cell Biol. 1988 Jan;8(1):267–272. doi: 10.1128/mcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Lee K. F., Atiee S. H., Rosen J. M. Differential regulation of rat beta-casein-chloramphenicol acetyltransferase fusion gene expression in transgenic mice. Mol Cell Biol. 1989 Feb;9(2):560–565. doi: 10.1128/mcb.9.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. J., Hackett P. B. Rapid generation of subclones for DNA sequencing using the reverse cloning procedure. Biotechniques. 1989 Jul-Aug;7(7):722–728. [PubMed] [Google Scholar]
- Liu Z., Zhu Z., Roberg K., Faras A. J., Guise K. S., Kapuscinski A. R., Hackett P. B. The beta-actin gene of carp (Ctenopharyngodon idella). Nucleic Acids Res. 1989 Jul 25;17(14):5850–5850. doi: 10.1093/nar/17.14.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire P., Wuarin J., Schibler U. The role of cis-acting promoter elements in tissue-specific albumin gene expression. Science. 1989 Apr 21;244(4902):343–346. doi: 10.1126/science.2711183. [DOI] [PubMed] [Google Scholar]
- Mason J. O., Williams G. T., Neuberger M. S. Transcription cell type specificity is conferred by an immunoglobulin VH gene promoter that includes a functional consensus sequence. Cell. 1985 Jun;41(2):479–487. doi: 10.1016/s0092-8674(85)80021-0. [DOI] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T., Boxer L. M., Kedes L. CArG boxes in the human cardiac alpha-actin gene are core binding sites for positive trans-acting regulatory factors. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6702–6706. doi: 10.1073/pnas.84.19.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T., Kedes L. Duplicated CArG box domains have positive and mutually dependent regulatory roles in expression of the human alpha-cardiac actin gene. Mol Cell Biol. 1987 Aug;7(8):2803–2813. doi: 10.1128/mcb.7.8.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N. G. Nucleotide sequence of the human beta-actin promoter 5' flanking region. Nucleic Acids Res. 1987 Nov 11;15(21):9095–9095. doi: 10.1093/nar/15.21.9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Gustafson T. A., Kedes L. A common factor regulates skeletal and cardiac alpha-actin gene transcription in muscle. Mol Cell Biol. 1988 Oct;8(10):4120–4133. doi: 10.1128/mcb.8.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Kedes L. Multiple 5'-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol. 1987 Nov;7(11):4089–4099. doi: 10.1128/mcb.7.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. Y., Gunning P., Eddy R., Ponte P., Leavitt J., Shows T., Kedes L. Evolution of the functional human beta-actin gene and its multi-pseudogene family: conservation of noncoding regions and chromosomal dispersion of pseudogenes. Mol Cell Biol. 1985 Oct;5(10):2720–2732. doi: 10.1128/mcb.5.10.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. Y., Gunning P., Liu S. H., Leavitt J., Kedes L. Regulation of the human beta-actin promoter by upstream and intron domains. Nucleic Acids Res. 1989 Jan 25;17(2):601–615. doi: 10.1093/nar/17.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe R. A., Lorenzen S. I., Brenner D. A., Breindl M. Regulatory elements in the 5'-flanking region and the first intron contribute to transcriptional control of the mouse alpha 1 type I collagen gene. Mol Cell Biol. 1989 May;9(5):2224–2227. doi: 10.1128/mcb.9.5.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers B. L., Sobnosky M. G., Saunders G. F. Transcriptional enhancer within the human placental lactogen and growth hormone multigene cluster. Nucleic Acids Res. 1986 Oct 10;14(19):7647–7659. doi: 10.1093/nar/14.19.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P., de Crombrugghe B. Identification of a cell-specific transcriptional enhancer in the first intron of the mouse alpha 2 (type I) collagen gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5590–5594. doi: 10.1073/pnas.84.16.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw C. M., Vergeer W. P., du Plooy S. J., Bernard M. P., Ramirez F., de Wet W. J. DNA sequences in the first intron of the human pro-alpha 1(I) collagen gene enhance transcription. J Biol Chem. 1987 Nov 5;262(31):15151–15157. [PubMed] [Google Scholar]
- Rudnicki M. A., Ruben M., McBurney M. W. Regulated expression of a transfected human cardiac actin gene during differentiation of multipotential murine embryonal carcinoma cells. Mol Cell Biol. 1988 Jan;8(1):406–417. doi: 10.1128/mcb.8.1.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford T., Nienhuis A. W. Human globin gene promoter sequences are sufficient for specific expression of a hybrid gene transfected into tissue culture cells. Mol Cell Biol. 1987 Jan;7(1):398–402. doi: 10.1128/mcb.7.1.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer J. D., Thurston S. J. A negative regulatory element with properties similar to those of enhancers is contained within an Alu sequence. Mol Cell Biol. 1989 Feb;9(2):355–364. doi: 10.1128/mcb.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Rossi P., de Crombrugghe B. Transcriptional control of the mouse alpha 2(I) collagen gene: functional deletion analysis of the promoter and evidence for cell-specific expression. Mol Cell Biol. 1986 Feb;6(2):347–354. doi: 10.1128/mcb.6.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Eldridge J. D., Paterson B. M. Expression and regulation of chicken actin genes introduced into mouse myogenic and nonmyogenic cells. Proc Natl Acad Sci U S A. 1984 May;81(10):2980–2984. doi: 10.1073/pnas.81.10.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Walsh K. Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol. 1989 May;9(5):2191–2201. doi: 10.1128/mcb.9.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B. Transcription elements and factors of RNA polymerase B promoters of higher eukaryotes. CRC Crit Rev Biochem. 1988;23(2):77–120. doi: 10.3109/10409238809088317. [DOI] [PubMed] [Google Scholar]
- Yutzey K. E., Kline R. L., Konieczny S. F. An internal regulatory element controls troponin I gene expression. Mol Cell Biol. 1989 Apr;9(4):1397–1405. doi: 10.1128/mcb.9.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]