Summary
Background
The increasing prevalence of type 2 diabetes poses a major public health challenge. Population-based screening and early treatment for type 2 diabetes could reduce this growing burden. However, uncertainty persists around the benefits of screening for type 2 diabetes. We assessed the effect of a population-based stepwise screening programme on mortality.
Methods
In a pragmatic parallel group, cluster-randomised trial, 33 general practices in eastern England were randomly assigned by the method of minimisation in an unbalanced design to: screening followed by intensive multifactorial treatment for people diagnosed with diabetes (n=15); screening plus routine care of diabetes according to national guidelines (n=13); and a no-screening control group (n=5). The study population consisted of 20 184 individuals aged 40–69 years (mean 58 years), at high risk of prevalent undiagnosed diabetes, on the basis of a previously validated risk score. In screening practices, individuals were invited to a stepwise programme including random capillary blood glucose and glycated haemoglobin (HbA1c) tests, a fasting capillary blood glucose test, and a confirmatory oral glucose tolerance test. The primary outcome was all-cause mortality. All participants were flagged for mortality surveillance by the England and Wales Office of National Statistics. Analysis was by intention-to-screen and compared all-cause mortality rates between screening and control groups. This study is registered, number ISRCTN86769081.
Findings
Of 16 047 high-risk individuals in screening practices, 15 089 (94%) were invited for screening during 2001–06, 11 737 (73%) attended, and 466 (3%) were diagnosed with diabetes. 4137 control individuals were followed up. During 184 057 person-years of follow up (median duration 9·6 years [IQR 8·9–9·9]), there were 1532 deaths in the screening practices and 377 in control practices (mortality hazard ratio [HR] 1·06, 95% CI 0·90–1·25). We noted no significant reduction in cardiovascular (HR 1·02, 95% CI 0·75–1·38), cancer (1·08, 0·90–1·30), or diabetes-related mortality (1·26, 0·75–2·10) associated with invitation to screening.
Interpretation
In this large UK sample, screening for type 2 diabetes in patients at increased risk was not associated with a reduction in all-cause, cardiovascular, or diabetes-related mortality within 10 years. The benefits of screening might be smaller than expected and restricted to individuals with detectable disease.
Funding
Wellcome Trust; UK Medical Research Council; National Health Service research and development support; UK National Institute for Health Research; University of Aarhus, Denmark; Bio-Rad.
Introduction
Type 2 diabetes poses a major public health challenge. The high proportion of undiagnosed cases of diabetes, the substantial number of patients with complications at clinical diagnosis, and the long latent phase of the disease are strong arguments for screening.1 Assessment of diabetes risk is currently included in the UK National Health Service (NHS) Health Checks programme for all individuals aged 40–74 years.2 Findings from studies nested in the ADDITION-Cambridge trial3 suggest that screening does not seem to be associated with psychological harm,4 nor does it falsely reassure individuals with negative results.5 However, uncertainty persists concerning the benefits of population-based screening for type 2 diabetes, and more established screening tests such as mammography, with some suggesting that apparent benefits are explained in part by overdiagnosis and improvements in treatment.6
Mortality reduction is a robust measure of the effectiveness of a screening programme as shown for screening for prostate7 and cervical cancer.8 Mortality provides an overall assessment of the potential benefits associated with population risk assessment, provision of risk information to patients and practitioners, invitation to testing, and early detection and treatment, as well as potential harms such as false reassurance. Identification of those at high risk of diabetes also provides opportunities for primary prevention of both diabetes and cardiovascular disease.9
Modelling studies suggest that a programme of screening for diabetes would reduce both diabetes-related and overall mortality,10–12 but these estimates depend on several key assumptions that need confirmation in a randomised trial. We report mortality over a median 9·6 years in a population-based cluster-randomised trial of screening in patients aged 40–69 years at high risk of having undiagnosed diabetes in general practices in eastern England.
Methods
Study design
ADDITION-Cambridge is a primary care-based screening and intervention study for type 2 diabetes. The study has been described in detail elsewhere;3,13 further details and the protocol are available. ADDITION-Cambridge consists of two phases: a pragmatic parallel group, unbalanced, cluster-randomised trial of screening; and a cluster-randomised trial comparing the effects of intensive multifactorial therapy with routine care in individuals with screen-detected type 2 diabetes. We report the results from the trial of screening from the first phase of the study.
Ethics approval was granted by the Eastern Multi-Regional Ethics Committee (02/5/54). Flagging of the records of individuals at high risk of having prevalent undiagnosed diabetes for mortality was approved under section 60 of the UK Health and Social Care Act 2001 (Reference MR798).
Randomisation and masking
138 general practices in eastern England were invited by letter to participate, and 63 agreed, of which three served as pilots. Practices were stratified by the number of known diabetic patients (<160 and ≥160 patients) and their local referring district hospital. Randomisation was undertaken by a statistician using the method of minimisation. In the first stage of randomisation, 33 recruited practices were allocated (1:3:3) to one of three groups: no screening (control; five practices), screening followed by intensive treatment of patients with screen-detected diabetes (IT; 15 practices), and screening plus routine care of patients with screen-detected diabetes (RC; 13 practices). These practices are included in the main trial analysis of screening versus control presented here. One of the 28 screening practices dropped out before screening commenced because of logistical difficulties with identification of people at high risk. The need to achieve the required sample size of patients with screen-detected diabetes for the treatment trial warranted the uneven randomisation ratio with a disproportionate number of screening practices and a second stage of randomisation. 27 practices were subsequently randomly assigned (1:1) to IT (n=14) and RC (n=13). The final group allocation after the two stages of randomisation included 28 practices to IT, 27 to RC, and five to control (no screening). A further six randomised practices (two IT and four RC) dropped out after recruitment, but before screening commenced because of other commitments or unforeseen difficulties in setting up the practice-based screening programme. Results from all practices included in the final group allocation are also presented in a parallel cohort analysis. This design has the advantage of increasing the sample size for the comparison of screened versus control practices, but increases the possibility of confounding and selection bias. The investigators assessing outcomes and analysing data were masked to group assignment.
Eligibility criteria
Practices were recruited from September, 2001 to October, 2002. From November, 2001 to April, 2003, we identified eligible individuals by searching the electronic medical records of 151 464 patients aged 40–69 years in 54 general practices for information about age, sex, body-mass index, and prescribed steroid and antihypertensive medication. We used this information to calculate a previously validated score14 to predict the risk of undiagnosed diabetes. Practices were eligible to take part if they could provide data for calculation of the risk score for at least 70% of their patients. Eligible participants had a diabetes risk score of 0·17 or higher but were not known to have diabetes. This cutoff corresponds to the top 25% of the risk distribution in the participating practices. Information about the diabetes risk score was withheld from practitioners and patients in the control practices. In screening practices, patients deemed unsuitable for screening by their general practitioner were not invited for biochemical testing. Exclusion criteria were pregnancy, lactation, an illness with a likely prognosis of less than a year, or a psychiatric illness likely to restrict study involvement or invalidate informed consent.
Procedures
The invitation list for screening was defined at the outset of the study; practices were asked to invite only the patients on the list that we provided. In the screening group (IT and RC), eligible individuals were sent a personal invitation from their general practitioner to attend their practice for stepwise screening including random capillary blood glucose and glycated haemoglobin (HbA1c) tests and a fasting capillary blood glucose test, followed by a confirmatory oral glucose tolerance test undertaken in a local outpatient facility (figure 1). Individuals attending the final glucose tolerance test stage also underwent assessment of cardiovascular risk factors including measurement of body-mass index, waist circumference, blood pressure, and blood lipids. Diagnosis of diabetes was based on the 1999 WHO criteria.15 Screening took place between January, 2002 and March, 2006. Practice teams were notified of the results of all clinical and biochemical measures with a clear statement of whether or not the individual met diagnostic criteria for type 2 diabetes, and they then informed patients of the test results. Non-attendance was defined as failure to attend the initial screening step after one reminder letter.
Figure 1.
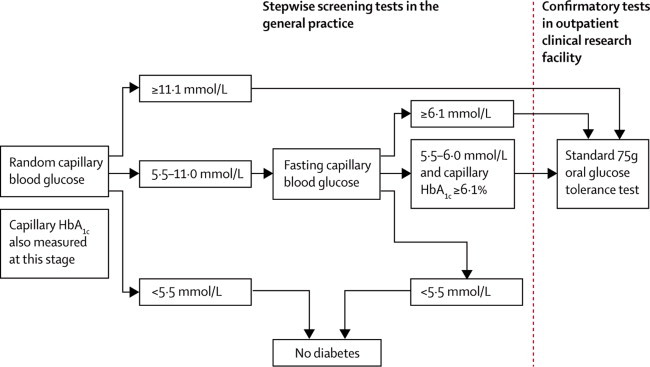
ADDITION-Cambridge screening and diagnostic procedure
HbA1c=glycated haemoglobin.
Participants diagnosed with type 2 diabetes were subsequently managed according to the treatment regimen to which their practice was allocated: RC or IT. The characteristics of the interventions to promote intensive treatment have been described previously3 (appendix). We aimed to educate and support general practitioners, practice nurses, and participants in target-driven management (with medication and promotion of healthy lifestyles) of hyperglycaemia, blood pressure, and cholesterol, similar to the stepwise regimen used in the Steno-2 study.16 Treatment targets and algorithms were based on trial data showing the benefits of intensive treatment of cardiovascular risk factors in people with type 2 diabetes.16–20 In the RC group, participants with screen-detected diabetes received usual diabetes care through the UK NHS based on current recommendations.21
The primary outcome was all-cause mortality; secondary outcomes were death from cardiovascular disease, cancers, and other causes, and diabetes-related death. All eligible participants were flagged for mortality surveillance by the England and Wales Office of National Statistics, with unique NHS patient numbers. The Office of National Statistics provided a copy of the death certificate for deceased participants. The General Register Office of Scotland and the Central Statistics Office of Ireland were contacted to obtain the vital status of participants who moved to those areas. A trace of NHS records for current address in April, 2012 suggested that 1% of ADDITION-Cambridge participants might have been lost to follow-up. Those lost to follow-up were assumed to be alive.
Deaths were coded into three categories (cardiovascular, cancer, and other) on the basis of the underlying cause of death. Cardiovascular death was defined by an International Classification of Diseases, tenth edition (ICD-10) code in the range I00–I99, and cancer death by a code in the range C00–D48. We also noted whether diabetes was included anywhere on each death certificate. This classification was independently done by an assessor masked to randomisation group status. 50% of the deaths were classified by a second assessor with 98% agreement. Consensus was reached by discussion.
Statistical analysis
All analyses were pre-specified.3 Analyses were done on an intention-to-screen basis at the population level. All eligible high-risk individuals were considered in analyses irrespective of their participation in the screening programme (this population included non-attenders and high-risk patients deemed unfit for screening by their general practitioner). Comparison of baseline practice and patient characteristics between randomised groups was done with t tests for continuous data and χ2 tests for categorical tests. To generate mortality rates, the number of person-years in the screening and control groups was calculated from the date of randomisation of each practice to the date of death or last information about the vital status of the patient. The earliest date of entry was Nov 21, 2001; deaths were confirmed up to Nov 28, 2011.
Hazard ratios (HR) comparing the screening (IT and RC) and control groups were estimated with a Cox proportional hazards model. Since randomisation was at the practice level, robust standard errors were calculated that take into account the two-level structure of the data (individuals clustered within practices)22 and any potential correlation between individuals within practices. We calculated the intraclass correlation coefficients (ICCs) for each mortality endpoint. We also did a parallel group cohort analysis that compared screening practices from both the first and second rounds of randomisation (n=49) with control practices (n=5). This analysis enabled us to include an additional 19 246 individuals in the comparison of screening and control practices. We did a sensitivity analysis to assess potential effects of antecedent disease through exclusion of participants who died within 3 months of randomisation, and to examine the effect of reclassifying individuals lost to tracking by the Office of National Statistics as deceased. We also undertook an analysis that compared mortality between attenders and non-attenders for screening. All analyses were done with Stata (version 12.1).
The study sample size was originally estimated to quantify the effectiveness of intensive treatment in screen-detected patients via detection of a 20% relative difference in the UK Prospective Diabetes Study (UKPDS) modelled 10-year risk of cardiovascular disease between patients with screen-detected diabetes in the IT and RC groups.3
This trial is registered, ISRCTN86769081.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the study data and final responsibility for submission for publication.
Results
Figure 2 shows the trial profile. Practices that declined to take part were similar to those who agreed to take part in terms of list size, prevalence of undiagnosed diabetes, number of general practitioner or nurse whole time equivalents, and the Index of Multiple Deprivation score23 (data not shown). However, participating practices had a smaller list size, lower crude diabetes prevalence, and fewer general practitioner or nurse whole time equivalents than did the average English practice (data taken from the UK National Primary Care Database). Furthermore, the Index of Multiple Deprivation scores for ADDITION-Cambridge practices (median [IQR] 11·7 [7·1–17·7]) suggested that they served less deprived communities than the average English practice (21·3 [12·3–36·1]).
Figure 2.
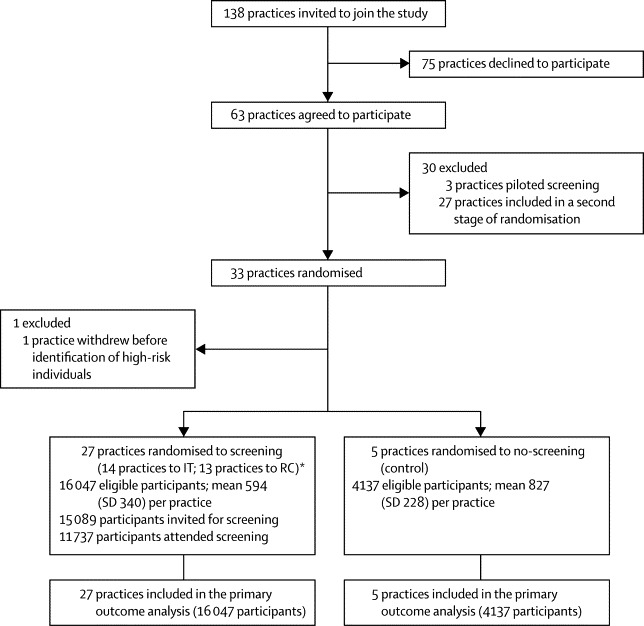
ADDITION-Cambridge trial profile
RC=screening followed by routine care of patients with screen-detected diabetes according to national guidelines. *IT=screening followed by intensive treatment of patients with screen-detected diabetes.
Table 1 shows the baseline characteristics of practices and participants. The screening and control groups were well balanced except for participant age and the proportion of participants prescribed steroids, which were slightly higher in the screening group.
Table 1.
Baseline characteristics of practices and eligible individuals at high risk of undiagnosed diabetes in the ADDITION-Cambridge trial
| No screening (control) | Screening (intervention) | |
|---|---|---|
| Practices | ||
| Number | 5 | 27 |
| Practice list size | 9351 (3038) | 7271 (582) |
| Unadjusted prevalence of diabetes (%) | 3·3% (0·8) | 3·0% (1·0) |
| General practitioner whole time equivalents | 4·8 (2·0) | 3·8 (1·6) |
| Nurse whole time equivalents | 2·1 (0·8) | 2·0 (0·8) |
| Index of multiple deprivation score* | 16·1 (9·0) | 12·9 (7·7) |
| Participants | ||
| Number | 4137 | 16 047 |
| Age (years) | 57·9 (7·8) | 58·2 (7·7) |
| Men, n (%) | 2641 (63·9%) | 10 260 (63·9%) |
| BMI (kg/m2) | 30·6 (4·6) | 30·5 (4·6) |
| Diabetes risk score median (IQR) | 0·34 (0·24–0·51) | 0·35 (0·24–0·52) |
| Prescribed antihypertensive medication, n (%) | 1853 (44·8%) | 7372 (45·9%) |
| Prescribed steroids, n (%) | 154 (3·7%) | 866 (5·4%) |
Data are mean (SD) unless otherwise indicated. BMI=body-mass index.
The Index of Multiple Deprivation combines a number of indicators, chosen to cover a range of economic, social, and housing issues, into one deprivation score for each small area in England. This score allows each area to be ranked relative to one another according to their level of deprivation. A high Index of Multiple Deprivation score indicates a high level of deprivation.
After the first stage of randomisation, 20 184 individuals (median age 59 years; IQR 53–65) were eligible for screening on the basis of their diabetes risk score in the 32 participating practices (screening and IT 14 practices; screening and RC 13 practices; no-screening control five practices). Of 16 047 eligible people in the screening group, 15 089 (94%) were invited and 11 737 (73%) attended the first stage of screening. 466 participants (3% of those eligible for screening) were diagnosed with diabetes. 4137 individuals were in the no-screening control group.
Median duration of follow-up was 9·6 years (IQR 8·9–9·9; 184 057 person-years). From November, 2001 to November, 2011, there were 1532 deaths in the screening group and 377 in the control group (table 2). The ICC values were very small (all-cause mortality 0·007; cardiovascular disease mortality 0·024; cancer mortality 0·0000011; diabetes-related mortality 0·000015). The most common cause of death was cancer (table 2). All-cause mortality did not differ significantly between the screening and the control groups (HR 1·06, 95% CI 0·90–1·25; p=0·46; figure 3). Sensitivity analyses showed that the results were not affected by the exclusion of people (n=24) who died within 3 months of randomisation (1·07, 0·90–1·26; p=0·44). The proportion of individuals lost to Office of National Statistics tracking was equally distributed between trial groups; results were not affected if these individuals were assumed to have died (data not shown). We noted no significant difference between groups in cardiovascular mortality, cancer mortality, or other causes of death (table 2). Diabetes was listed among other causes of death in 91 individuals (75 in the screening group and 16 in the control group). There was no significant difference between groups in diabetes-related mortality (1·26, 0·75–2·10). None of these estimates were affected by adjustment for IT or RC trial group (data not shown). Compared with attenders, non-attenders for screening were younger, more obese, more likely to be men, less likely to be taking antihypertensive drugs, and had a higher all-cause mortality (adjusted HR 2·01, 95% CI 1·74–2·32).
Table 2.
Incidence of death by study group and hazard ratios for mortality in the ADDITION-Cambridge trial
|
No-screening control group |
Screening group |
Hazard ratio (95%CI)* | |||||
|---|---|---|---|---|---|---|---|
| Number of deaths | Person-years of follow-up | Rate per 1000 person-years (95% CI) | Number of deaths | Person-years of follow up | Rate per 1000 person-years (95% CI) | ||
| All-cause mortality | 377 | 38 126 | 9·89 (8·94–10·94) | 1532 | 145 930 | 10·50 (9·99–11·04) | 1·06 (0·90–1·25) |
| Cardiovascular mortality | 124 | 38 126 | 3·25 (2·73–3·88) | 482 | 145 930 | 3·30 (3·02–3·61) | 1·02 (0·75–1·38) |
| Cancer mortality | 169 | 38 126 | 4·43 (3·81–5·15) | 697 | 145 930 | 4·78 (4·43–5·14) | 1·08 (0·90–1·30) |
| Other causes of death | 84 | 38 126 | 2·20 (1·78–2·73) | 353 | 145 930 | 2·42 (2·18–2·68) | 1·10 (0·87–1·39) |
Accounting for clustering.
Figure 3.
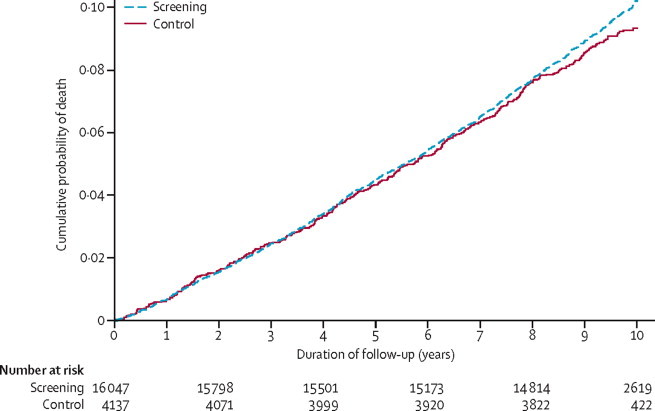
Cumulative incidence of death in the screening and no screening control groups in the ADDITION-Cambridge trial
In the parallel group cohort analysis, there were 2769 deaths in the 49 screening practices from November, 2001 to November, 2011. Mortality rates compared with the no-screening control group were similar to the trial analysis results presented above (data not shown). The most common cause of death was cancer. We noted no significant differences between the screening and the control groups for all-cause mortality (HR 0·98, 95% CI 0·84–1·14), cardiovascular mortality (0·92, 0·70–1·22), cancer mortality (1·00, 0·84–1·20), other causes of death (1·00, 0·80–1·25), or diabetes related mortality (0·97, 0·58–1·61).
Discussion
In this large, population-based UK sample, all-cause mortality over a median 9·6 years was not reduced by one round of screening for type 2 diabetes in people at high risk of prevalent undiagnosed diabetes. Similarly, invitation to screening was not associated with a reduction in cardiovascular, cancer, diabetes-related, or other causes of death. Results from the parallel cohort analysis were similar to those from the main trial analysis, suggesting that population-based screening for diabetes is not associated with a reduction in all-cause, cardiovascular, or diabetes-related mortality within 10 years in this age group.
Modelling studies indicate that a programme of screening for diabetes every 3–5 years would reduce diabetes-related mortality in Taiwan by 26–40%,10,11 and would prevent up to five deaths per 1000 people in the USA.12 An examination of the mortality experience of the Ely cohort24 suggested that individuals who were invited to diabetes screening every 5 years between 1990 and 1999 had a non-significant 21% lower all-cause mortality than did individuals who were not invited to screening. However, the parameters used in these non-randomised studies differed from the characteristics of the screening programme in our study—eg, the frequency and nature of the screening tests, length of follow-up, and population subgroups in which screening was undertaken. The finding of a higher risk of mortality in those not attending an appointment for screening compared with those who attended accords with findings from other population-based screening programmes,25 and is likely to indicate healthy volunteer bias and exclusion of unhealthy individuals by general practitioners.
There are several possible explanations for the lack of difference in mortality recorded in this trial. There might have been a dilution of the effect of screening. There is ongoing ad-hoc opportunistic screening for type 2 diabetes in primary care in the UK, following recommendations from organisations such as Diabetes UK.26 Opportunistic screening, combined with 27% non-attendance for screening and less than 100% sensitivity at each stage of the screening programme, could have contributed to the fairly low yield of screen-detected patients. However, evidence suggests that the extent of opportunistic screening is limited in the UK.27 There has been a continuing improvement in the detection and management of cardiovascular disease risk factors including diabetes in UK primary care,28 an occurrence enhanced by the Quality and Outcomes Framework system of remuneration for general practitioners.29 This improvement might have contributed to the mortality in high-risk individuals in the control group being 50% lower than expected on the basis of observational data from the Netherlands.30 Furthermore, the prevalence of undiagnosed diabetes might have been previously overestimated.
Evidence is growing for the benefit of intensive treatment of risk factors early in the course of the disease.31 Results from ADDITION-Europe, a cluster-randomised trial of intensive, target-driven management of screen-detected patients, showed that individuals diagnosed and treated earlier had a mortality experience that was similar to that reported for people of the same age without diabetes in the general population in Denmark.32 However, although earlier detection could benefit the few people diagnosed with diabetes, the proportion might have been too small to affect population mortality in ADDITION-Cambridge. In the event of a lower than expected prevalence of undiagnosed diabetes, effects of screening on mortality might therefore be limited. In populations with higher rates of undiagnosed prevalent diabetes, the effects of screening on mortality could be larger. However, recent estimates of a fairly short lead time (around 3 years) between detection by screening and clinical diagnosis suggest that the benefits of screening might have been overestimated.33
For prostate cancer, the effect of screening on population mortality is established largely by the effects of earlier diagnosis and treatment. Identification of individuals at high risk of developing diabetes offers other opportunities for health promotion. Effects on mortality might have been greater if cardiovascular disease risk factors had been assessed alongside glucose at the initial screening appointment, as proposed in the UK Health Checks programme,2 if lifestyle advice had been provided with risk information to participants who screened negative for diabetes at any stage, and if uptake of screening had been higher. Only one screening round was undertaken. Repeated screening might be associated with greater benefits, although response rates could fall over time and costs would be greater. Finally, population-based screening for type 2 diabetes might be associated with little benefit at the population level (in all those invited to screening). An individual high-risk approach to reducing the burden of type 2 diabetes may therefore need to be complemented by a population-based strategy targeting the underlying determinants of disease risk.
Diabetes was stated as cause of death on only 90 death certificates. The underreporting of diabetes-related mortality could have led to an underestimate of the effect of screening.34 However, up to 60% of deaths in type 2 diabetes are explained by cardiovascular disease,35 and we did not find a difference in cardiovascular disease mortality between study groups. The low absolute number of deaths due to cardiovascular disease compared with deaths attributable to cancer probably indicates the secular decline in cardiovascular mortality in England.36 This decline is believed to be due to both improved preventive treatment (primary and secondary prevention) and changes in lifestyle and risk factors such as the reduction in the prevalence of smoking. This decline may be steeper in the east of England, because of the affluence of its population.23 As the result of this decline, cancers are now the most common cause of death in this region of England.37 Furthermore, the risk factors that formed part of the diabetes risk score that we used to define the study population (eg, age and obesity) also predispose to cancer.
To the best of our knowledge, this is the first trial to evaluate the effect of a screening programme for type 2 diabetes on population mortality (panel). The strengths of our study include the randomised design reducing the possibility of selection, lead, and length time bias and confounding seen in observational studies of screening.38 Trial groups were well balanced at baseline; the small difference in participants' age and the proportion of prescribed steroids is unlikely to have affected the findings. The invitation list for screening was defined at the outset, thereby minimising contamination and selection bias due to participant recruitment varying by trial group. The observed ICC values were much smaller than anticipated, contributing to tighter 95% CIs and a more robust negative finding for the primary outcome. Furthermore, we incorporated valid outcome measurement with minimal loss to tracking and a high level of agreement for classification of cause of death. We also included a sufficiently large number of high-risk individuals to detect clinically important effects on mortality as suggested by previous modelling studies. Follow-up was 10 years from practice randomisation, albeit slightly less from attendance for screening.
Panel. Research in context.
Systematic review
A modelling study12 showed that, compared with no diabetes screening, screening individuals every 3–5 years between the ages of 30 and 45 years would reduce the incidence of myocardial infarction, prevent a substantial number of deaths, and be cost effective. These estimates are dependent on several key assumptions that require confirmation in a randomised trial. We searched PubMed for relevant articles with “diabetes mellitus, type 2” and “mortality” as Medical Subject Headings (MeSH) and the term “randomised controlled trial” in any heading, in combination with the term “screen*”. We placed no restriction on language, year of publication, or study quality. We found no published trial evidence of the effects of a screening programme for diabetes on mortality.
Interpretation
Invitation to one round of screening for type 2 diabetes in high-risk individuals was not associated with a reduction in all-cause or diabetes-related mortality over 10 years. The benefits of screening might be smaller than expected and restricted to individuals with detectable disease. Benefits to the population could be increased by: detection and management of related cardiovascular risk factors alongside assessment of diabetes risk; repeated rounds of screening; and identification of non-attenders and strategies to maximise uptake of screening.
Our sample was representative of the eastern England population, since up to 99% of people in the UK are registered with a general practice. However, participating practices served less deprived areas than the average English practice. Caution should therefore be exercised in extrapolation of our results to more socioeconomically disadvantaged communities in which the disease risk might be higher although attendance for screening is likely to be lower.39 Most participants were white (the main ethnic group in the region), which also limits generalisability. In view of the higher absolute risk of diabetes and its consequences among other ethnic groups, the benefits of screening might be greater in these populations, assuming equivalent uptake. We did not have ethical or research governance permission to extract information from NHS records of individuals diagnosed with diabetes in the no-screening control group or from individuals who were clinically diagnosed with diabetes in the screening group following a negative screening test. Consequently we were unable to quantify lead time or compare outcomes between screen-detected and clinically diagnosed patients.
In conclusion, invitation to a single round of screening for type 2 diabetes in high-risk individuals in UK general practice might benefit the minority with detectable disease but was not associated with a reduction in all-cause or diabetes-related mortality over 10 years. If population-based screening for diabetes is to be implemented, it should be undertaken alongside assessment and management of risk factors for diabetes and cardiovascular disease and population level preventive strategies targeting underlying determinants of these diseases.
For the protocol see http://www.mrc-epid.cam.ac.uk/Research/Studies/ADDITION/index.html
Acknowledgments
Acknowledgments
ADDITION-Cambridge was supported by the Wellcome Trust (grant reference No G061895) the Medical Research Council (grant reference no: G0001164), National Health Service R&D support funding (including the Primary Care Research and Diabetes Research Networks), and the National Institute for Health Research. JBE-T was funded by a scholarship from the Gates Cambridge Trust. We received an unrestricted grant from University of Aarhus, Denmark, to support the ADDITION-Cambridge trial. Bio-Rad provided equipment to undertake capillary glucose screening by HbA1c in general practice. ALK is a National Institute for Health Research (NIHR) Senior Investigator. The Primary Care Research Unit is supported by NIHR Research funds. SJG receives support from the Department of Health NIHR Programme Grant funding scheme (RP-PG-0606-1259). ATP was supported by the NIHR Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. This article presents independent research funded by the NIHR under the Programme Grants for Applied Research programme (RP-PG-0606-1259]. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. We thank the independent trial steering committee (Nigel Stott [Chair], John Weinman, Richard Himsworth, and Paul Little) and all participants, practice nurses, and general practitioners in the ADDITION-Cambridge study. The Primary Care Research Unit at the University of Cambridge and the Medical Research Council Epidemiology Unit in Cambridge jointly coordinated the study. Aside from the authors, the ADDITION-Cambridge study team has included Rebecca Abbott, Amanda Adler, Judith Argles, Gisela Baker, Rebecca Bale, Ros Barling, Daniel Barnes, Mark Betts, Sue Boase, Clare Boothby, Sandra Bovan, Ryan Butler, James Brimbicombe, Parinya Chamnan, Sean Dinneen, Pesheya Doubleday, Sue Emms, Mark Evans, Tom Fanshawe, Francis Finucane, Philippa Gash, Julie Grant, Wendy Hardeman, Robert Henderson, Susie Hennings, Garry King, Georgina Lewis, Christine May Hall, Joanna Mitchell, Richard Parker, Nicola Popplewell, Emanuella De Lucia Rolfe, Megan Smith, Stephen Sutton, Liz White and Fiona Whittle. We also wish to thank the Cambridge University Hospitals NHS Foundation Trust Department of Clinical Biochemistry and the NIHR Cambridge Biomedical Research Centre Core Biochemistry Assay Laboratory for carrying out the biochemical assays and the following groups within the MRC Epidemiology Unit: data management (Adam Dickinson), information technology (Iain Morrison), technical (Matt Sims), and field epidemiology (Paul Roberts, Kim Mwanza, and James Sylvester). The National Primary Care Database is a product of the National Primary Care Research and Development Centre (NPCRDC) at the University of Manchester. It was devised by Deborah Baker. The database was constructed by Justin Hayes at the Regional Research Laboratory, School of Geography, University of Manchester; SEE IT consultancy designed and built the map interface. We thank Andrew Wagner, Mark Hann, and David Reeves (NPCRDC) for cleaning and validating the data sets. Andrew Wagner is the database manager.
Contributors
SJS had full access to all of the data in the study and takes responsibility for the accuracy of the data analysis. SJG and NJW take responsibility for the integrity of the data. SJG acts as guarantor for this paper. SJG, NJW, and ALK designed the study, and are principal investigators for the trial. SJG, KMW, and LAS participated in the acquisition of the data. JBE-T, SJG, RKS, LAS, ATP, ALK, and SJS participated in the analysis and interpretation of data. JBE-T, RKS, LAS, and SJG drafted the report. ATP, SJS, NJW, ALK, and KMW participated in the critical revision of the report for important intellectual content. JBE-T, RKS, SJG, LAS, ATP, SJS, and KMW provided administrative, technical, and material support for the study. ADDITION-Cambridge practices: Acorn Community Health Centre, Arbury Road Surgery, Ashwell Surgery, Birchwood Surgery, Bridge Street Medical Centre, Brookfields & Cherry Hinton, Broomfields, Buckden Surgery, Burwell Surgery, Cambridge Surgery, Cedar House Surgery, Charles Hicks Centre, Chequers Lane Surgery, Clarkson Surgery, Cornerstone Practice, Cornford House Surgery, Cottenham Surgery, Cromwell Place Surgery, Dr Smith and Partner (Cambridge), East Field Surgery, Ely Surgery, Freshwell Health Centre, George Clare Surgery, Great Staughton Surgery, Harston Surgery, Health Centre (Eaton Socon), Hilton House, John Tasker House, Lensfield Medical Practice, Manea Surgery, Mercheford House, Milton Surgery, Nene Valley Medical Practice, Nevells Road Surgery, New Roysia Surgery, Northcote House Surgery, Nuffield Road Medical Centre, Orchard Surgery, Orchard House Surgery, Orton Medical Practice, Park Medical Centre, Paston Health Centre, Peterborough Surgery, Petersfield Medical Practice, Prior's Field Surgery, Queen Edith's Medical Practice, Queen Street Surgery, Rainbow Surgery, Ramsey Health Centre, Riverside Practice, Roman Gate Surgery, Rosalind Franklin House, South Street Surgery, Thaxted Surgery, The Health Centre (Bury St Edmunds), The Old Exchange, The Surgery Stanground, Townley Close Health Centre, Trumpington Street Medical Practice, Werrington Health Centre, York Street Medical Practice.
Conflicts of interest
SJG received an honorarium and reimbursement of travel expenses from Ely Lilly associated with membership of an independent data monitoring committee for a randomised trial of a medication to lower glucose. He has received payment from Novo Nordisk for a lecture given at a postgraduate educational meeting in 2010. ALK has received payment from Novo Nordisk for giving a lecture. The remaining authors declare that they have no conflicts of interest.
Supplementary Material
References
- 1.Simmons RK, Echouffo-Tcheugui JB, Griffin SJ. Screening for type 2 diabetes: an update of the evidence. Diabetes Obes Metab. 2010;12:838–844. doi: 10.1111/j.1463-1326.2010.01244.x. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health . Putting prevention first. Vascular checks: risk assessment and management. Department of Health; London: 2008. [Google Scholar]
- 3.Echouffo-Tcheugui JB, Simmons RK, Williams KM. The ADDITION-Cambridge trial protocol: a cluster-randomised controlled trial of screening for type 2 diabetes and intensive treatment for screen-detected patients. BMC Public Health. 2009;9:136. doi: 10.1186/1471-2458-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eborall HC, Griffin SJ, Prevost AT, Kinmonth AL, French DP, Sutton S. Psychological impact of screening for type 2 diabetes: controlled trial and comparative study embedded in the ADDITION (Cambridge) randomised controlled trial. BMJ. 2007;335:486. doi: 10.1136/bmj.39303.723449.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paddison CA, Eborall HC, Sutton S. Are people with negative diabetes screening tests falsely reassured? Parallel group cohort study embedded in the ADDITION (Cambridge) randomised controlled trial. BMJ. 2009;339:b4535. doi: 10.1136/bmj.b4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autier P, Boniol M, Gavin A, Vatten LJ. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ. 2011;343:d4411. doi: 10.1136/bmj.d4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroder FH, Hugosson J, Roobol MJ. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 8.Sankaranarayanan R, Nene BM, Shastri SS. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 9.Uusitupa M, Peltonen M, Lindstrom J. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study—secondary analysis of the randomized trial. PLoS One. 2009;4:e5656. doi: 10.1371/journal.pone.0005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo HS, Chang HJ, Chou P, Teng L, Chen TH. A Markov chain model to assess the efficacy of screening for non-insulin dependent diabetes mellitus (NIDDM) Int J Epidemiol. 1999;28:233–240. doi: 10.1093/ije/28.2.233. [DOI] [PubMed] [Google Scholar]
- 11.Chang HJ, Kuo HS, Tung TH, Chou P, Chen TH. Evaluation of a population-based screening for type 2 diabetes: a community-based screening project in Puli, Taiwan. Prev Med. 2000;31:396–402. doi: 10.1006/pmed.2000.0728. [DOI] [PubMed] [Google Scholar]
- 12.Kahn R, Alperin P, Eddy D. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet. 2010;375:1365–1374. doi: 10.1016/S0140-6736(09)62162-0. [DOI] [PubMed] [Google Scholar]
- 13.Sargeant LA, Simmons RK, Barling RS. Who attends a UK diabetes screening programme? Findings from the ADDITION-Cambridge study. Diabet Med. 2010;27:995–1003. doi: 10.1111/j.1464-5491.2010.03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin SJ, Little PS, Hales CN, Kinmonth AL, Wareham NJ. Diabetes risk score: towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev. 2000;16:164–171. doi: 10.1002/1520-7560(200005/06)16:3<164::aid-dmrr103>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications; Part 1: diagnosis and classification of diabetes mellitus. World Health Organisation; Switzerland: 1999. [DOI] [PubMed] [Google Scholar]
- 16.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 17.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 19.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 20.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S) Diab Care. 1997;20:614–620. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 21.McIntosh A, Hutchinson A, Home PD. Clinical guidelines and evidence review for type 2 diabetes: management of blood glucose. University of Sheffield; Sheffield: 2001. [Google Scholar]
- 22.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability; 1967; Berkeley, CA: University of California Press; 1967: 221–33.
- 23.Hain D. Briefing 18. The index of multiple deprivation. Eastern Region Public Health Observatory; Cambridge: 2007. [Google Scholar]
- 24.Simmons RK, Rahman M, Jakes RW. Effect of population screening for type 2 diabetes on mortality: long-term follow-up of the Ely cohort. Diabetologia. 2011;54:312–319. doi: 10.1007/s00125-010-1949-8. [DOI] [PubMed] [Google Scholar]
- 25.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 26.Diabetes United Kingdom Early identification of people with type 2 diabetes. Position statement. September 2008. http://www.diabetes.org.uk (accessed Sept 16, 2012).
- 27.Waugh N, Scotland G, McNamee P. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess. 2007;11:iii–iiv. doi: 10.3310/hta11170. ix-xi, 1–125. [DOI] [PubMed] [Google Scholar]
- 28.Campbell SM, Roland MO, Middleton E, Reeves D. Improvements in quality of clinical care in English general practice 1998–2003: longitudinal observational study. BMJ. 2005;331:1121. doi: 10.1136/bmj.38632.611123.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doran T, Fullwood C, Gravelle H. Pay-for-performance programs in family practices in the United Kingdom. N Engl J Med. 2006;355:375–384. doi: 10.1056/NEJMsa055505. [DOI] [PubMed] [Google Scholar]
- 30.Spijkerman A, Griffin S, Dekker J, Nijpels G, Wareham NJ. What is the risk of mortality for people who are screen positive in a diabetes screening programme but who do not have diabetes on biochemical testing? Diabetes screening programmes from a public health perspective. J Med Screen. 2002;9:187–190. doi: 10.1136/jms.9.4.187. [DOI] [PubMed] [Google Scholar]
- 31.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 32.Griffin SJ, Borch-Johnsen K, Davies MJ. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378:156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman M, Simmons RK, Hennings SH, Wareham NJ, Griffin SJ. How much does screening bring forward the diagnosis of type 2 diabetes and reduce complications? Twelve year follow-up of the Ely cohort. Diabetologia. 2012;55:1651–1659. doi: 10.1007/s00125-011-2441-9. [DOI] [PubMed] [Google Scholar]
- 34.Thomason MJ, Biddulph JP, Cull CA, Holman RR. Reporting of diabetes on death certificates using data from the UK Prospective Diabetes Study. Diabet Med. 2005;22:1031–1036. doi: 10.1111/j.1464-5491.2005.01584.x. [DOI] [PubMed] [Google Scholar]
- 35.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(suppl 2):S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 36.Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–1107. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- 37.ERPHO http://www.erpho.org.uk/ (accessed Sept 16, 2012).
- 38.Hennekens CH, Buring JE. Epidemiology in medicine. Lippincott, Williams & Wilkins; Philadelphia: 1987. [Google Scholar]
- 39.Marteau TM, Mann E, Prevost AT. Impact of an informed choice invitation on uptake of screening for diabetes in primary care (DICISION): randomised trial. BMJ. 2010;340:c2138. doi: 10.1136/bmj.c2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


