Abstract
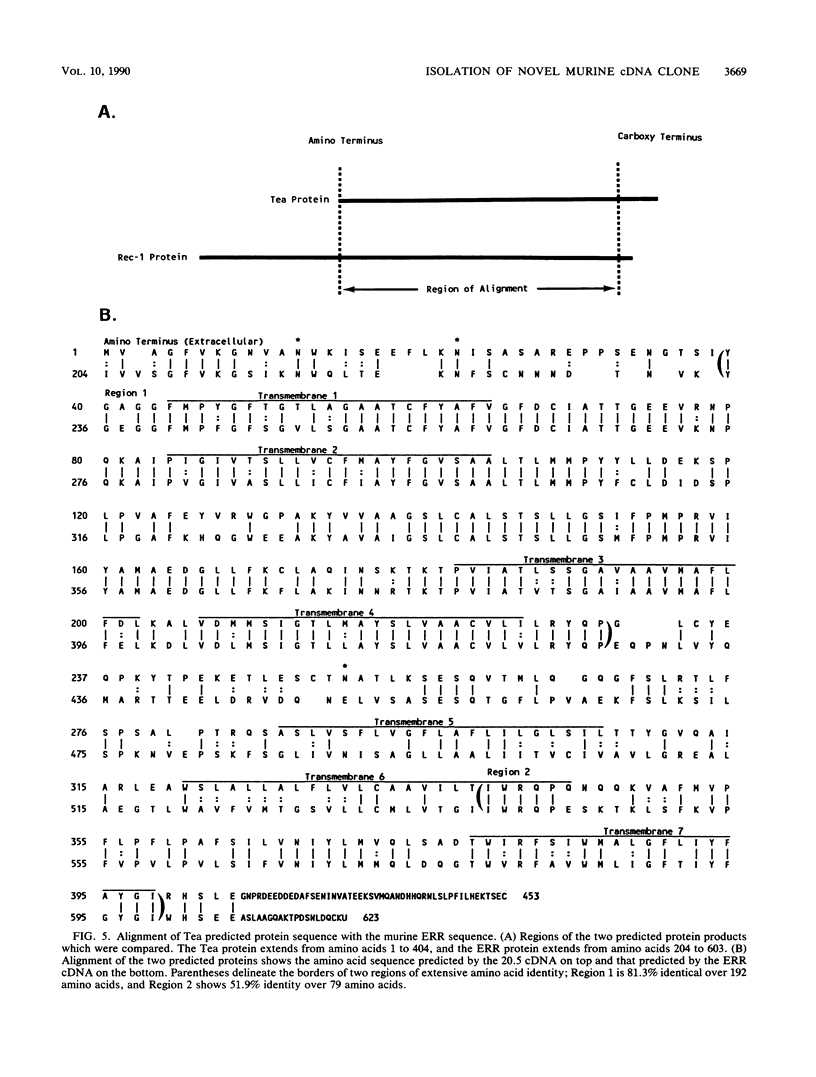
A novel cDNA clone (20.5) which is differentially expressed between two closely related T-lymphoma cell clones was isolated by subtraction-enriched differential screening. SL12.4 cells, from which the cDNA was isolated, have characteristics of thymocytes at an intermediate stage in development. A sister cell clone derived from the same tumor, SL12.3, does not express this mRNA, has a distinct phenotype, and expresses fewer genes required for mature T-cell function. The cDNA sequence predicts a highly hydrophobic protein (approximately 49.5 kilodaltons) which contains seven putative membrane spanning domains. The gene was expressed on concanavalin A-activated T lymphocytes and was designated Tea (T-cell early activation gene). The Tea gene mapped to chromosome 8 and appeared to be conserved among mammalian and avian species. The Tea gene is distinct from, but bears extensive amino acid and DNA sequence similarity with, the murine ecotropic retroviral receptor which is encoded by the Rec-1 gene. Neither gene product displayed significant homology with other known transmembrane-spanning proteins. Thus, the Tea and Rec-1 genes establish a new family encoding multiple membrane-spanning proteins.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Mueller C., Okada C. Y., Reichert R. A., Weissman I. L., Spangrude G. J. Early events in T-cell maturation. Annu Rev Immunol. 1987;5:325–365. doi: 10.1146/annurev.iy.05.040187.001545. [DOI] [PubMed] [Google Scholar]
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- BUONASSISI V., SATO G., COHEN A. I. Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1184–1190. doi: 10.1073/pnas.48.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley T. R., Pierschbacher M., Wetzel G. D., Hays E. F. Induction of T-cell maturation by a cloned line of thymic epithelium (TEPI). Proc Natl Acad Sci U S A. 1983 Oct;80(19):6005–6009. doi: 10.1073/pnas.80.19.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. T. The information content of phase-known matings for ordering genetic loci. Genet Epidemiol. 1985;2(4):349–361. doi: 10.1002/gepi.1370020404. [DOI] [PubMed] [Google Scholar]
- Burd P. R., Freeman G. J., Wilson S. D., Berman M., DeKruyff R., Billings P. R., Dorf M. E. Cloning and characterization of a novel T cell activation gene. J Immunol. 1987 Nov 1;139(9):3126–3131. [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Dorio R. J., Buck C. A. Manipulation of cell-cell and cell-substratum interactions in mouse mammary tumor epithelial cells using broad spectrum antisera. J Cell Biol. 1981 May;89(2):173–184. doi: 10.1083/jcb.89.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J., Church J. G., Buick R. N. Isolation of a cDNA corresponding to a developmentally regulated transcript in rat intestine. Mol Cell Biol. 1988 Oct;8(10):4243–4249. doi: 10.1128/mcb.8.10.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B. J., Pardoll D. M. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. Differentiation-linked leukemogenesis in lymphocytes. Science. 1986 Nov 7;234(4777):697–704. doi: 10.1126/science.3535067. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanley-Hyde J. M., Lynch R. G. The physiology of B cells as studied with tumor models. Annu Rev Immunol. 1986;4:621–649. doi: 10.1146/annurev.iy.04.040186.003201. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hays E. F., Weinroth S. E., MacLeod C. L., Kitada S. Tumorigenicity of T lymphoma/T lymphoma hybrids and T lymphoma/normal cell hybrids. Int J Cancer. 1986 Oct 15;38(4):597–601. doi: 10.1002/ijc.2910380421. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Halden N. F., Buckler C. E., Kozak C. A. Genetic mapping of the mouse c-fms proto-oncogene to chromosome 18. J Virol. 1988 Mar;62(3):1055–1056. doi: 10.1128/jvi.62.3.1055-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Albritton L. M., Cunningham J. Genetic mapping of a cloned sequence responsible for susceptibility to ecotropic murine leukemia viruses. J Virol. 1990 Jun;64(6):3119–3121. doi: 10.1128/jvi.64.6.3119-3121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A. Genetic mapping of a mouse chromosomal locus required for mink cell focus-forming virus replication. J Virol. 1983 Oct;48(1):300–303. doi: 10.1128/jvi.48.1.300-303.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kuziel W. A., Tucker P. W. Determination of vector: insert junctions in lambda gt10 cDNAs that do not recut with EcoRI. Nucleotide sequence of the lambda imm434 HindIII-EcoRI DNA fragment encoding part of the cI protein. Nucleic Acids Res. 1987 Apr 10;15(7):3181–3181. doi: 10.1093/nar/15.7.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Kim G. S., Prystowsky M. B., Lancki D. W., Sabath D. E., Pan J. L., Weissman S. M. Isolation and initial characterization of multiple species of T-lymphocyte subset cDNA clones. Proc Natl Acad Sci U S A. 1987 May;84(9):2896–2900. doi: 10.1073/pnas.84.9.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Adrenergic receptors. Models for the study of receptors coupled to guanine nucleotide regulatory proteins. J Biol Chem. 1988 Apr 15;263(11):4993–4996. [PubMed] [Google Scholar]
- MacLeod C. L., Fong A. M., Seal B. S., Walls L., Wilkinson M. F. Isolation of novel complementary DNA clones from T lymphoma cells: one encodes a putative multiple membrane-spanning protein. Cell Growth Differ. 1990 Jun;1(6):271–279. [PubMed] [Google Scholar]
- MacLeod C. L., Hays E. F., Hyman R., Bourgeois S. A new murine model system for the in vitro development of thymoma cell heterogeneity. Cancer Res. 1984 May;44(5):1784–1790. [PubMed] [Google Scholar]
- MacLeod C. L., Weinroth S. E., Streifinger C., Glaser S. M., Hays E. F. SL12 murine T-lymphoma: a new model for tumor cell heterogeneity. J Natl Cancer Inst. 1985 Apr;74(4):875–882. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Ott D., Friedrich R., Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J Virol. 1990 Feb;64(2):757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. B., Speers W. C. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 1988 Apr 15;48(8):1996–2004. [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984 Jul 15;136(1):144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Lugo J. P. Differentiation and cell division in the mammalian thymus. Dev Biol. 1985 Nov;112(1):1–17. doi: 10.1016/0012-1606(85)90114-9. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Siegel J. N., Turner C. A., Klinman D. M., Wilkinson M., Steinberg A. D., MacLeod C. L., Paul W. E., Davis M. M., Cohen D. I. Sequence analysis and expression of an X-linked, lymphocyte-regulated gene family (XLR). J Exp Med. 1987 Dec 1;166(6):1702–1715. doi: 10.1084/jem.166.6.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. F., Georgopoulos K., Terhorst C., MacLeod C. L. The CD3 delta gene encodes multiple transcripts regulated by transcriptional and post-transcriptional mechanisms. Eur J Immunol. 1989 Dec;19(12):2355–2360. doi: 10.1002/eji.1830191226. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. F., MacLeod C. L. Induction of T-cell receptor-alpha and -beta mRNA in SL12 cells can occur by transcriptional and post-transcriptional mechanisms. EMBO J. 1988 Jan;7(1):101–109. doi: 10.1002/j.1460-2075.1988.tb02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. K., Slaughter C., Ruoho A. E., Ross E. M. The catecholamine binding site of the beta-adrenergic receptor is formed by juxtaposed membrane-spanning domains. J Biol Chem. 1988 Jun 15;263(17):7925–7928. [PubMed] [Google Scholar]





