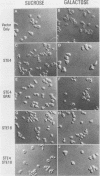
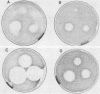
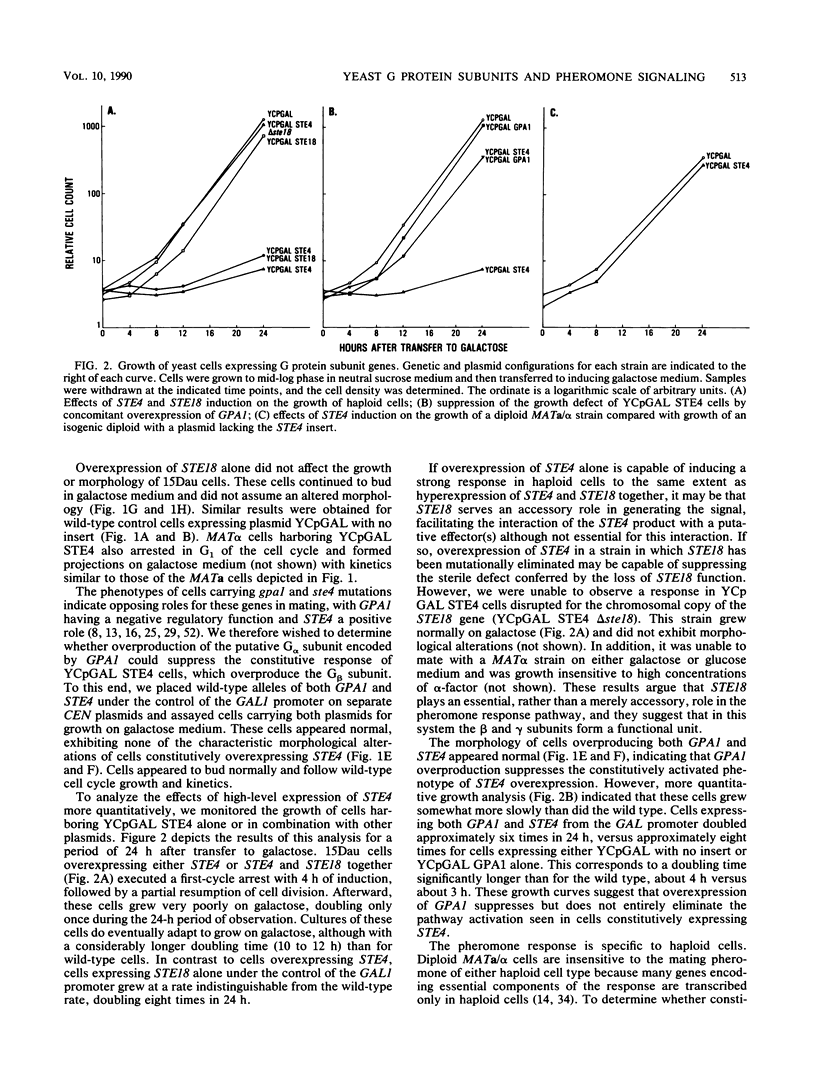
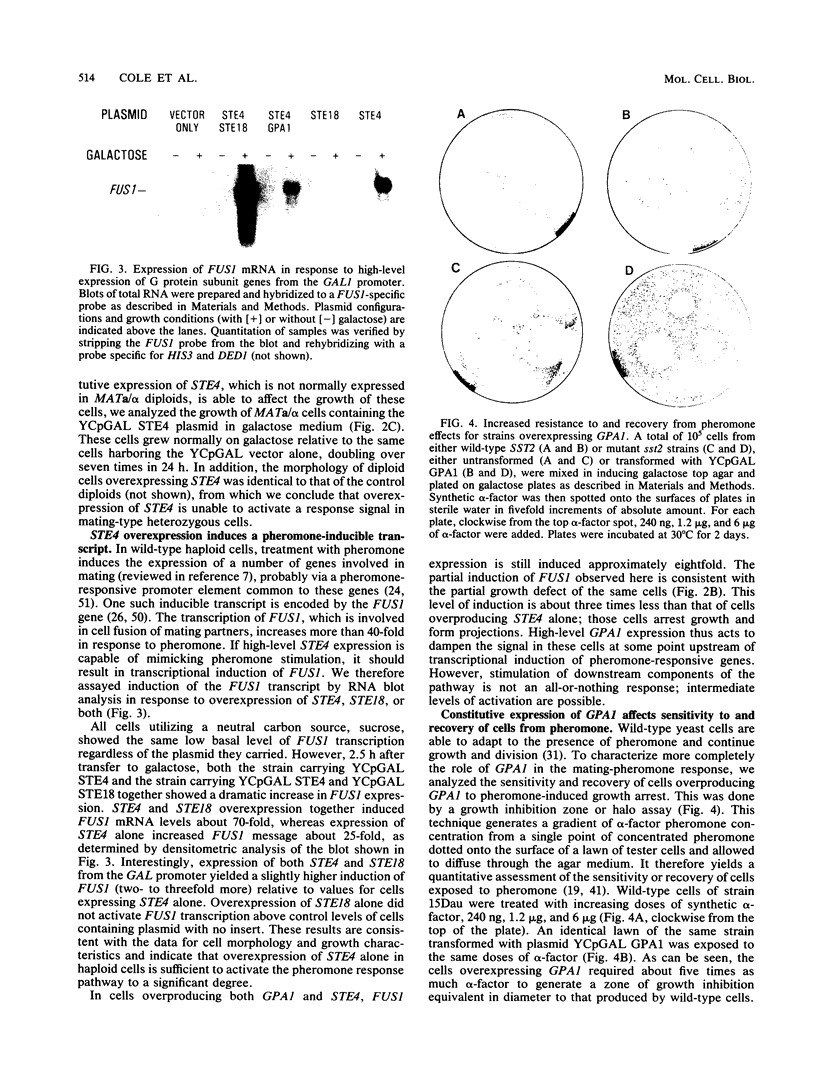
Abstract
The Saccharomyces cerevisiae GPA1, STE4, and STE18 genes encode products homologous to mammalian G-protein alpha, beta, and gamma subunits, respectively. All three genes function in the transduction of the signal generated by mating pheromone in haploid cells. To characterize more completely the role of these genes in mating, we have conditionally overexpressed GPA1, STE4, and STE18, using the galactose-inducible GAL1 promoter. Overexpression of STE4 alone, or STE4 together with STE18, generated a response in haploid cells suggestive of pheromone signal transduction: arrest in G1 of the cell cycle, formation of cellular projections, and induction of the pheromone-inducible transcript FUS1 25- to 70-fold. High-level STE18 expression alone had none of these effects, nor did overexpression of STE4 in a MATa/alpha diploid. However, STE18 was essential for the response, since overexpression of STE4 was unable to activate a response in a ste18 null strain. GPA1 hyperexpression suppressed the phenotype of STE4 overexpression. In addition, cells that overexpressed GPA1 were more resistant to pheromone and recovered more quickly from pheromone than did wild-type cells, which suggests that GPA1 may function in an adaptation response to pheromone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Blinder D., Bouvier S., Jenness D. D. Constitutive mutants in the yeast pheromone response: ordered function of the gene products. Cell. 1989 Feb 10;56(3):479–486. doi: 10.1016/0092-8674(89)90250-x. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Regan J. W., Nakata H., Codina J., Benovic J. L., Gierschik P., Somers R. L., Spiegel A. M., Birnbaumer L., Lefkowitz R. J. Functional reconstitution of the alpha 2-adrenergic receptor with guanine nucleotide regulatory proteins in phospholipid vesicles. J Biol Chem. 1986 Mar 15;261(8):3901–3909. [PubMed] [Google Scholar]
- Chan R. K., Otte C. A. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982 Jan;2(1):21–29. doi: 10.1128/mcb.2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. M., Mortimer R. K. Failure to induce a DNA repair gene, RAD54, in Saccharomyces cerevisiae does not affect DNA repair or recombination phenotypes. Mol Cell Biol. 1989 Aug;9(8):3314–3322. doi: 10.1128/mcb.9.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F., Hartwell L. H., Jackson C., Konopka J. B. Conjugation in Saccharomyces cerevisiae. Annu Rev Cell Biol. 1988;4:429–457. doi: 10.1146/annurev.cb.04.110188.002241. [DOI] [PubMed] [Google Scholar]
- Dietzel C., Kurjan J. Pheromonal regulation and sequence of the Saccharomyces cerevisiae SST2 gene: a model for desensitization to pheromone. Mol Cell Biol. 1987 Dec;7(12):4169–4177. doi: 10.1128/mcb.7.12.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel C., Kurjan J. The yeast SCG1 gene: a G alpha-like protein implicated in the a- and alpha-factor response pathway. Cell. 1987 Sep 25;50(7):1001–1010. doi: 10.1016/0092-8674(87)90166-8. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher G., Perry K., Thorner J. Cell-cell recognition in Saccharomyces cerevisiae: regulation of mating-specific adhesion. J Bacteriol. 1978 Jun;134(3):893–901. doi: 10.1128/jb.134.3.893-901.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating hormone. J Cell Biol. 1980 Jun;85(3):811–822. doi: 10.1083/jcb.85.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng K. Y., Ferguson J., Reed S. I. Mutations in a gene encoding the alpha subunit of a Saccharomyces cerevisiae G protein indicate a role in mating pheromone signaling. Mol Cell Biol. 1988 Jun;8(6):2484–2493. doi: 10.1128/mcb.8.6.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness D. D., Burkholder A. C., Hartwell L. H. Binding of alpha-factor pheromone to Saccharomyces cerevisiae a cells: dissociation constant and number of binding sites. Mol Cell Biol. 1986 Jan;6(1):318–320. doi: 10.1128/mcb.6.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983 Mar;32(3):839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Katada T., Kusakabe K., Oinuma M., Ui M. A novel mechanism for the inhibition of adenylate cyclase via inhibitory GTP-binding proteins. Calmodulin-dependent inhibition of the cyclase catalyst by the beta gamma-subunits of GTP-binding proteins. J Biol Chem. 1987 Sep 5;262(25):11897–11900. [PubMed] [Google Scholar]
- Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984 Mar 25;259(6):3578–3585. [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Mechanisms for inhibition of the catalytic activity of adenylate cyclase by the guanine nucleotide-binding proteins serving as the substrate of islet-activating protein, pertussis toxin. J Biol Chem. 1986 Apr 15;261(11):5215–5221. [PubMed] [Google Scholar]
- Konopka J. B., Jenness D. D., Hartwell L. H. The C-terminus of the S. cerevisiae alpha-pheromone receptor mediates an adaptive response to pheromone. Cell. 1988 Aug 26;54(5):609–620. doi: 10.1016/s0092-8674(88)80005-9. [DOI] [PubMed] [Google Scholar]
- Kronstad J. W., Holly J. A., MacKay V. L. A yeast operator overlaps an upstream activation site. Cell. 1987 Jul 31;50(3):369–377. doi: 10.1016/0092-8674(87)90491-0. [DOI] [PubMed] [Google Scholar]
- Mackay V., Manney T. R. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. I. Isolation and phenotypic characterization of nonmating mutants. Genetics. 1974 Feb;76(2):255–271. doi: 10.1093/genetics/76.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G., Clay F. J., Kelsay K., Sprague G. F., Jr Identification and regulation of a gene required for cell fusion during mating of the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 Aug;7(8):2680–2690. doi: 10.1128/mcb.7.8.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall M. D., Richardson H. E., Reed S. I. Dominant negative protein kinase mutations that confer a G1 arrest phenotype. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4426–4430. doi: 10.1073/pnas.85.12.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima I., Arai K., Matsumoto K. GPA1Val-50 mutation in the mating-factor signaling pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Jun;9(6):2289–2297. doi: 10.1128/mcb.9.6.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima I., Nakafuku M., Nakayama N., Brenner C., Miyajima A., Kaibuchi K., Arai K., Kaziro Y., Matsumoto K. GPA1, a haploid-specific essential gene, encodes a yeast homolog of mammalian G protein which may be involved in mating factor signal transduction. Cell. 1987 Sep 25;50(7):1011–1019. doi: 10.1016/0092-8674(87)90167-x. [DOI] [PubMed] [Google Scholar]
- Moore S. A. Comparison of dose-response curves for alpha factor-induced cell division arrest, agglutination, and projection formation of yeast cells. Implication for the mechanism of alpha factor action. J Biol Chem. 1983 Nov 25;258(22):13849–13856. [PubMed] [Google Scholar]
- Moore S. A. Yeast cells recover from mating pheromone alpha factor-induced division arrest by desensitization in the absence of alpha factor destruction. J Biol Chem. 1984 Jan 25;259(2):1004–1010. [PubMed] [Google Scholar]
- Nakafuku M., Itoh H., Nakamura S., Kaziro Y. Occurrence in Saccharomyces cerevisiae of a gene homologous to the cDNA coding for the alpha subunit of mammalian G proteins. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2140–2144. doi: 10.1073/pnas.84.8.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N., Kaziro Y., Arai K., Matsumoto K. Role of STE genes in the mating factor signaling pathway mediated by GPA1 in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Sep;8(9):3777–3783. doi: 10.1128/mcb.8.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K. The structure of transposable yeast mating type loci. Cell. 1980 Mar;19(3):753–764. doi: 10.1016/s0092-8674(80)80051-1. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Shore D. Transcriptional regulation in the yeast life cycle. Science. 1987 Sep 4;237(4819):1162–1170. doi: 10.1126/science.3306917. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Wolf L. G., Gill D. M. The stimulatory guanine-nucleotide regulatory unit of adenylate cyclase from bovine cerebral cortex. ADP-ribosylation and purification. Biochem J. 1987 Jan 15;241(2):325–336. doi: 10.1042/bj2410325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution, activity, and properties of the 35,000-dalton (beta) subunit. J Biol Chem. 1983 Sep 25;258(18):11361–11368. [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneke J. E., Blumer K. J., Courchesne W. E., Thorner J. The carboxy-terminal segment of the yeast alpha-factor receptor is a regulatory domain. Cell. 1988 Oct 21;55(2):221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Jensen R., Zoller M. J., Burke J., Errede B., Smith M., Herskowitz I. Structure of the Saccharomyces cerevisiae HO gene and analysis of its upstream regulatory region. Mol Cell Biol. 1986 Dec;6(12):4281–4294. doi: 10.1128/mcb.6.12.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Struhl K. Nucleotide sequence and transcriptional mapping of the yeast pet56-his3-ded1 gene region. Nucleic Acids Res. 1985 Dec 9;13(23):8587–8601. doi: 10.1093/nar/13.23.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Trueheart J., Boeke J. D., Fink G. R. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol Cell Biol. 1987 Jul;7(7):2316–2328. doi: 10.1128/mcb.7.7.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Arsdell S. W., Stetler G. L., Thorner J. The yeast repeated element sigma contains a hormone-inducible promoter. Mol Cell Biol. 1987 Feb;7(2):749–759. doi: 10.1128/mcb.7.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway M., Hougan L., Dignard D., Thomas D. Y., Bell L., Saari G. C., Grant F. J., O'Hara P., MacKay V. L. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell. 1989 Feb 10;56(3):467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]