Abstract
Polysialic acid (polySia) is a large, cell-surface linear homopolymer composed of α2,8-linked sialic acid residues. Most extensively studied in the nervous system, this unique glycan modulates development by enhancing cell migration and regulating differentiation. PolySia also functions in developing and adult immune systems and is a signature of many cancers. In this study, we demonstrated that human placental trophoblasts, an epithelial lineage, also display this glycan. Cytotrophoblasts and syncytiotrophoblasts expressed polySia in the first trimester and downregulated it during the course of pregnancy. PolySia promoted cytotrophoblast migration in an explant model of chorionic villous growth. Removal of this glycan also reduced cytotrophoblast penetration of basement membranes in an in vitro model of invasion. Finally, we showed that polySia was overexpressed in biopsies from patients with gestational trophoblastic diseases, including benign molar pregnancies and malignant choriocarcinomas. These results demonstrated, for the first time, functional roles for polySia during normal human placental development and implicated these unusual oligosaccharides in the unrestrained invasion of trophoblast tumors.
Keywords: human placenta, polysialic acid, migration, invasion, trophoblast tumors
Introduction
Polysialic acid (polySia) is a cell-surface linear homopolymer composed of α2,8-linked sialic acid residues. This unique glycan regulates cell–cell interactions by two distinct mechanisms enabled by its unusual physical properties, which include impressive size (measuring up to 300+ sialic acid residues) and repeating negative charges. Accordingly, through steric hindrance, polySia modulates the distance between the apposing plasma membranes of neighboring cells, inhibiting the binding of homo- and heterotypic adhesion molecules (Rutishauser 2008). Additionally, polySia binds to small cationic molecules (e.g., growth factors), modulating their ability to signal through receptors (Zhang et al. 2004; Kanato et al. 2008; Bax et al. 2009; Rey-Gallardo et al. 2010, 2011; Li et al. 2011; Ono et al. 2011). PolySia is synthesized in the Golgi apparatus by two independent polysialyltransferases: ST8SiaII (STX), which is largely restricted to the nervous system, and ST8SiaIV (PST1), which is more broadly expressed, for example, in the immune system and the human placenta (Angata et al. 1997). Unlike most glycans, polySia modifications have thus far been identified on a limited number of protein scaffolds including the neural cell adhesion molecule (NCAM) (Hoffman et al. 1982; Finne et al. 1983), neuropilin-2 (NRP2) (Curreli et al. 2007), CD36 (Yabe et al. 2003), the α-subunit of the voltage-sensitive sodium channel (James and Agnew 1987) and SynCAM1 (Galuska et al. 2010). Autocatalytic polysialylation of ST8SiaII and ST8SiaIV has also been reported (Muhlenhoff et al. 1996).
NCAM is by far the best studied of the known scaffolds and the impact of polysialylation on brain development and activity is well understood, in part because of studies in polySia-deficient mice (Hildebrandt et al. 2007). In the brain, polySia influences the migration of neuronal progenitors (Ono et al. 1994; Hu et al. 1996; Seki et al. 2007) and some mature neurons (Murakami et al. 2000). Additionally, the glycan modulates contact-dependent differentiation. For example, removal of polySia can lead to premature neuronal maturation (Petridis et al. 2004; Rockle et al. 2008). Finally, this glycan influences cell signaling by increasing responses to a number of growth factors (Kanato et al. 2008; Ono et al. 2011). The latter effect may also be related to cell viability, as removal of polySia can increase apoptosis without affecting proliferation (Vutskits et al. 2006; Gascon et al. 2007a,b).
PolySia is also expressed outside of the nervous system, most notably in the immune system, where it is carried by NCAM on human natural killer (NK) cells and murine myeloid cells (Drake et al. 2008) and by NRP2 on human and murine dendritic cells (Curreli et al. 2007). In some cases, polySia enhances the migration of these cells. In the mouse, NCAM-polySia is required for the mobilization of hematopoietic progenitors from the bone marrow to the thymus (Drake et al. 2009) and NRP2-polySia enhances CCL21-dependent migration (i.e., chemotaxis) of dendritic cells to secondary lymphoid organs (Bax et al. 2009; Rey-Gallardo et al. 2010, 2011). PolySia is also found on many human tumors, including lung carcinomas, myelomas, neuroblastomas, gliomas and Wilm's tumor (Roth et al. 1988; Hildebrandt et al. 1998). In the context of cancer, polySia may promote invasion (Suzuki et al. 2005), and in some cases, its expression has been correlated with tumor progression (Hildebrandt et al. 1998). PolySia is also transiently expressed in the developing embryo (Rutishauser 2008). With the exceptions noted above, expression of this glycan is lost in most adult organs. Overall, the published evidence suggests that polySia is critical to enhancing cell movement during periods of rapid growth, for example, as progenitors migrate great distances (Ono et al. 1994; Hu et al. 1996; Seki et al. 2007; Drake et al. 2009), and during tumorigenesis and metastasis (Komminoth et al. 1991). In adults, this homopolymer may also regulate plasticity, promoting cell migration to new locations in response to environmental cues (Bax et al. 2009; Rey-Gallardo et al. 2010, 2011).
Here, we provide evidence that polySia modulates human placentation, a developmental process that requires explosive growth, cellular plasticity and invasion. During placentation, trophoblast cells initiate and maintain a physical connection to the mother, setting up a nutrient/waste exchange system that supports embryonic/fetal development (Larsen 2001; Red-Horse et al. 2004). Placental architecture at the histological level reveals how this connection is established (Figure 1J). Two trophoblast cell types, invasive cytotrophoblasts (iCTBs) and syncytiotrophoblasts (STBs), are derived from a common progenitor cytotrophoblast (pCTB). In one developmental pathway, pCTBs fuse to form a multinucleate layer of STBs (i.e., syncytium), which covers the villi and mediates nutrient, oxygen and waste exchange between the maternal and the fetal blood. In the other pathway, pCTBs differentiate into iCTBs, forming columns of cells that migrate away from the placenta and invade the uterine wall. This results in an adhesive anchor between the mother and the embryo/fetus. Some iCTBs go on to remodel maternal uterine arterioles, replacing resident endothelial cells and widening the vessel bore, which increases blood flow to the placenta. Although iCTB penetration of the uterus resembles cancer cell invasion, this phenomenon is tightly regulated during normal pregnancy and iCTBs invade only as far as the inner third of the myometrium. Uncontrolled iCTB invasion can also occur, however, most notably in the form of trophoblast-derived tumors (e.g., choriocarcinomas). During invasion, iCTBs modulate the expression of cell adhesion molecules, downregulating epithelial markers—integrin α6β4 and epithelial cadherin—as they exit the villi, and upregulating endothelial markers—integrins αVβ3 and α1β1, vascular-endothelial cadherin and vascular- and platelet-endothelial cell adhesion molecules (VCAM-1 and PECAM-1)—as they enter the uterus (Red-Horse et al. 2004). Glycosylation is also regulated as a function of differentiation. For example, integrin oligosaccharides (Moss et al. 1994) and L-selectin ligands (Prakobphol et al. 2006) are modulated during iCTB invasion.
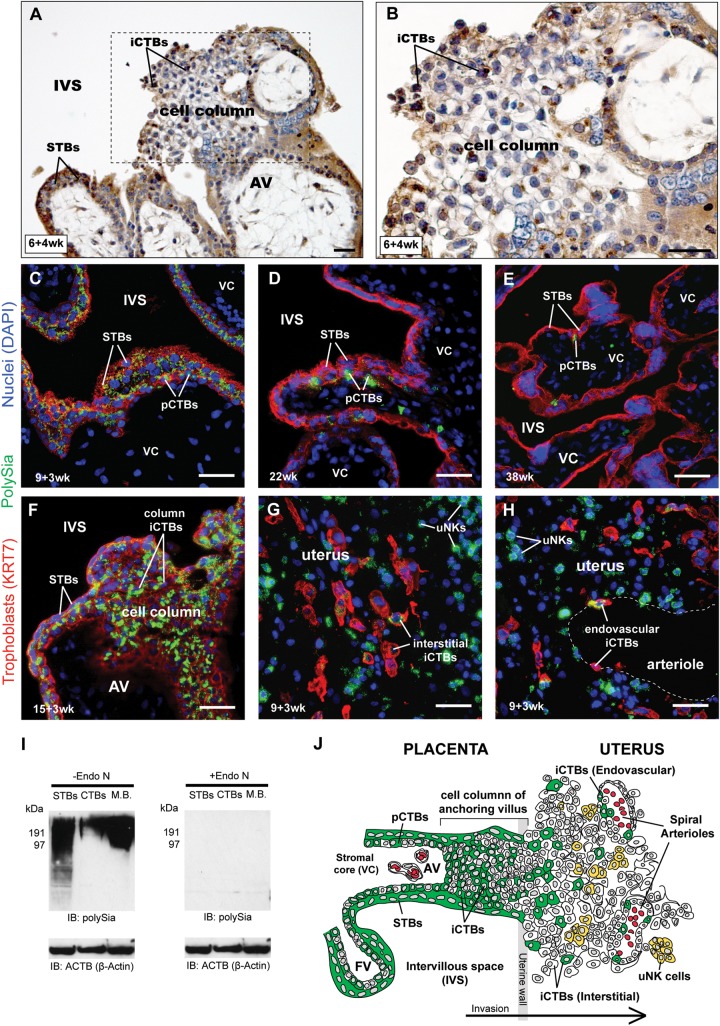
Fig. 1.
STBs and CTBs express polySia and abundance decreases as gestational age advances. (A and B) PolySia was detected with mAb 735 and visualized using (DAB, brown). Sections were counterstained with hematoxylin (blue). (A) In 6-week first-trimester chorionic villi, mAb 735 reacted with STBs that covered the villi, underlying progenitor CTBs (pCTBs), and invasive CTBs (iCTBs) in cell columns. Box enlarged in (B). Cell-surface polySia appeared light brown, while Golgi polySia was punctate and dark brown. (C–H) Placental sections were stained with an anti-cytokeratin 7 (KRT7) mAb (red, to visualize trophoblasts), DAPI (blue, to visualize nuclei) and the anti-polySia mAb 12F8 (green). (C–E) 12F8 reacted with STBs and underlying pCTBs. PolySia signal was highest in first-trimester samples (7-week, C) and declined in second-trimester biopsies (22-week, D) such that the glycan's expression was restricted to pCTBs at term (38-week, D). In cell columns (F), intense immunoreactivity was observed on iCTBs. In the uterus (G and H), staining was detected on some interstitial iCTBs (G) and endovascular iCTBs (H) that lined the uterine arterioles. Uterine natural killer (uNK) cells, which express NCAM, were also reactive with mAb 12F8. (A–H) Scale bars, 40 µM. (I) First-trimester, 7-week STB plasma membrane fractions, CTB lysates and MB preparations were separated by SDS–PAGE and treated with Endo N to remove polySia (right) or buffer alone (left) prior to immunoblotting with mAb 735. An identical blot was probed with anti-actin to demonstrate protein loading (bottom). (J) Diagrammatic representation of the localization pattern of human placental polySia. In floating chorionic villi, expression was restricted to pCTBs, STBs and iCTBs (green). In the uterus, polySia was detected on interstitial and endovascular iCTBs (green) and uNK cells (yellow).
Given polySia's ability to regulate cellular movement and plasticity, we investigated its involvement in the formation of the human maternal-fetal interface. First, we showed that this glycan is exclusively expressed by placental trophoblasts, an epithelial lineage, and that levels of polySia are regulated as a function of gestational age. PolySia is very abundant early in pregnancy and nearly absent at term. As to functional roles, removal of polySia diminished iCTB migration and invasion. Finally, we showed that polySia is overexpressed in biopsies from patients with gestational trophoblastic diseases (GTDs), ranging from benign moles to malignant choriocarcinomas. These novel findings demonstrated functional roles for polySia during human placental development.
Results
PolySia immunolocalized to several trophoblast subtypes and expression was regulated as a function of gestational age
We localized polySia in placental biopsies with two well-characterized monoclonal antibodies (mAbs) that specifically recognize α2-8 linked sialic acid chains: 735 (Frosch et al. 1985) and 12F8. In first-trimester formalin-fixed, paraffin-embedded samples, monoclonal antibodies (mAb) 735 reacted with STBs covering the chorionic villi, pCTBs and iCTBs in cell columns (Figure 1A, cell column at higher magnification in Figure 1B). Occasionally, weaker immunoreactivity was observed in the stromal compartments of villi. The same staining patterns were observed using mAb 12F8 and first-trimester paraformaldehyde-fixed, frozen biopsies (Figure 1C, green). By the end of the second trimester, both anti-polySia mAbs failed to stain syncytium and immunoreactivity was restricted to pCTBs, which are depleted as pregnancy advances (Figure 1D and E, green). In contrast, immunoreactivity associated with extravillous iCTBs was observed beyond 15 weeks. Invasive CTBs in cell columns, which are transiently present during the first and the second trimesters as cells migrate into the uterus, also reacted with anti-polySia (Figure 1F, green). In addition, both mAbs reacted with iCTBs that invaded the uterus; signal was detected in association with some interstitial iCTBs (Figure 1G) and endovascular iCTBs that lined uterine spiral arterioles (Figure 1H). Cytokeratin 7 (KRT7)-negative uterine NK (uNK) cells that expressed NCAM also reacted, a result that is in accord with previous studies demonstrating that human NK cells carry polySia (Drake et al. 2008). Immunoblotting with mAb 735 using mouse brain (MB) as a positive control confirmed that STBs and iCTBs expressed polySia (Figure 1I). Furthermore, expression was restricted to first- and second-trimester samples and not observed in term placentas (Supplementary data, Figure S1A). Treatment with endoneuraminidase N (Endo N), which removes polySia, abrogated immunoreactivity on immunoblots (Figure 1I and Supplementary data, Figure S1A) and tissue sections (Supplementary data, Figure S1B). Together, these results demonstrated that trophoblasts displayed polySia at the early stages of pregnancy and downregulated its expression as gestation advanced (summarized in Figure 1J).
In trophoblasts, ST8SiaIV added polySia to N-linked carbohydrates
To determine whether ST8SiaII or ST8SiaIV synthesized placental polySia, we interrogated gene expression profiling data from our laboratory (Figure 2A). ST8SiaII and ST8SiaIV mRNA levels were measured in CTBs isolated from villi (pCTBs) or from iCTBs that migrated from villous explants. Both populations expressed ST8SiaIV (black bars), but not ST8SiaII (white bars).
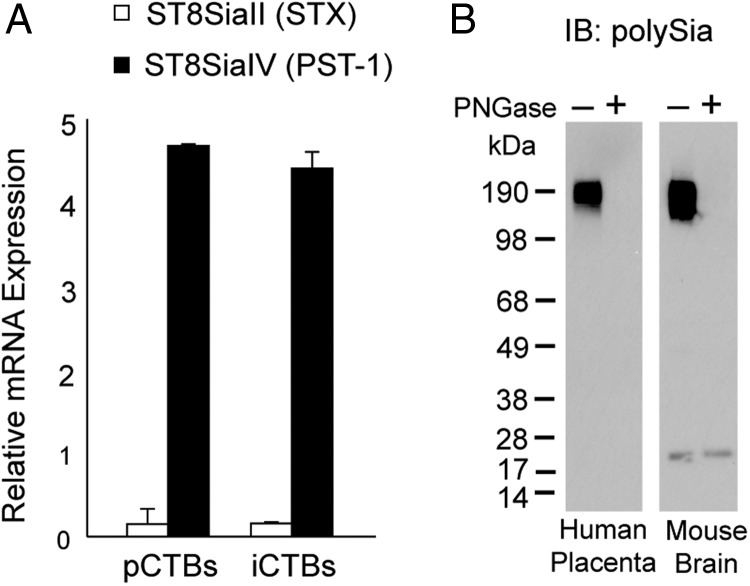
Fig. 2.
In trophoblasts, ST8SiaIV synthesizes N-linked polySia. (A) Trophoblast-specific expression of the two polysialyltransferase enzymes, ST8SiaII and ST8SiaIV, was determined by relative RNA quantification using the Affymetrix Gene ST 1.0 exon array platform. iCTBs; invasive cytotrophoblasts. pCTBs; progenitor cytotrophoblasts. (B) First-trimester, 7-week human placental lysates were treated with PNGase F, separated by SDS–PAGE and immunoblotted with anti-polySia mAb 735 (left). MB lysates were included as a positive control (right).
Next, we investigated whether polySia was attached to its carrier protein(s) via an N- or O-linked glycan. Placental lysates were treated with peptide N-glycosidase F (PNGase F), which specifically removes N-linked carbohydrates. Control and enzymatically treated 7-week lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose blots that were subsequently probed with mAb 735 to detect polySia (Figure 2B). Immunoreactivity was completely abrogated by PNGase F treatment, suggesting that trophoblast polySia was attached via an N-linkage to its protein carrier (left blot). The mouse brain, which predominately expresses polySia N-linked to NCAM, served as a positive control (right blot). Together, these results provided evidence that ST8SiaIV added polySia to N-linked core carbohydrate structures in the human placenta.
PolySia promoted iCTB migration in a human chorionic villous explant model
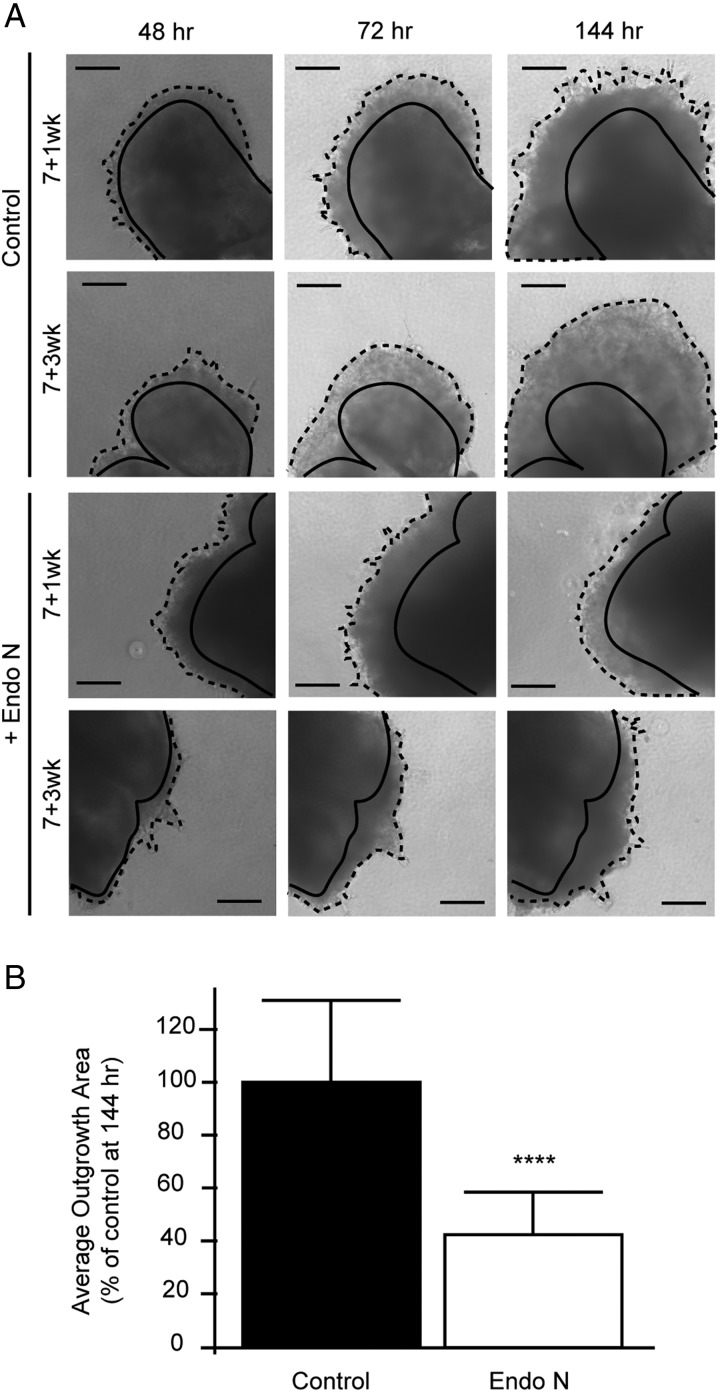
To investigate whether polySia modulated migration, we used an in vitro organ culture system (Genbacev et al. 1992). Anchoring chorionic villi were dissected from 5- to 8-week placentas and cultured on Matrigel-coated wells in the presence or absence of Endo N (Figure 3). Preliminary experiments showed that optimal effects occurred when the enzyme was diluted 1:100 in Matrigel and 1:1000 in medium. Over several days, untreated control villi gave rise to iCTB outgrowths that migrated away from the villi (Figure 3A; top panels), a process that was inhibited by Endo N treatment (Figure 3A; bottom panels). A quantitative analysis of migration (from 48 to 144 h) demonstrated an ∼60% reduction in Endo N-treated cultures compared with untreated controls, P < 0.0001 (Figure 3B). Together, these results demonstrated that polySia promoted iCTB migration.
Fig. 3.
Removal of placental polySia inhibited iCTB migration. (A) Examples of 7-week anchoring villi plated on Matrigel in the absence (top panels) or presence (bottom panels) of Endo N that removes polySia. At ∼48 h, outgrowths of cell columns that gave rise to iCTBs were visible. Villi were cultured for 172 h and imaged every 24 h. Scale bars, 200 µM. (B) The radial migration of explant-derived cells was quantified using a relative area index, which was defined as the area 144–172 h after plating (hatched lines) divided by the area of the initial leading front at 48 h (solid lines). Endo N treatment significantly reduced explant expansion by 60% (white bar) compared with the control (black bar), P < 0.0001 (****). Error bars represent standard deviation (SD).
Removal of polySia reduced iCTB invasion
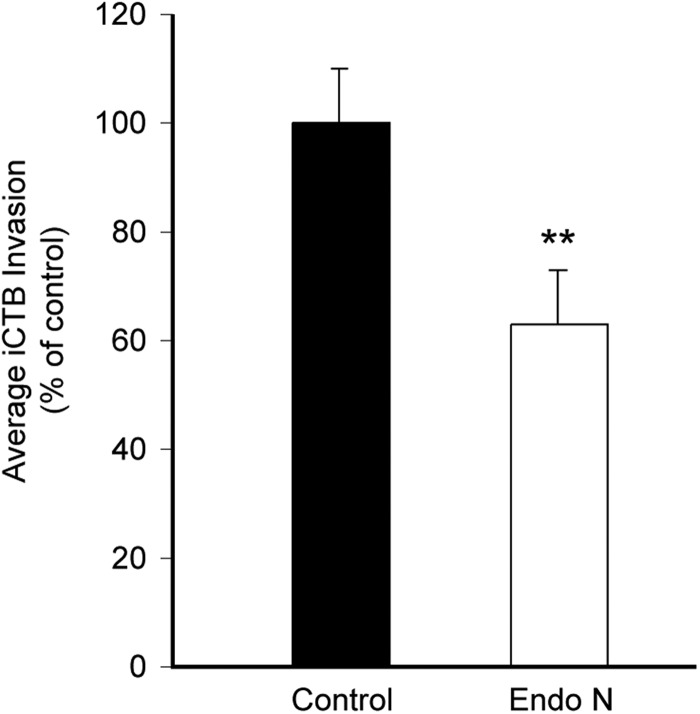
Next, we investigated whether polySia promoted iCTB invasion in an in vitro assay (Hunkapiller and Fisher 2008). Freshly isolated iCTBs were plated in the upper portions of Matrigel-coated transwell inserts ± Endo N. Preliminary experiments showed that optimal effects occurred when the enzyme was diluted 1:50 in Matrigel and 1:500 in medium. After 40 h, the invasion was quantified by counting the number of iCTBs that invaded the Matrigel and reached the underside of the filter (Figure 4). Compared with untreated cultures (black bars), Endo N digestion (white bars) significantly reduced iCTB invasion, P < 0.01.
Fig. 4.
Removal of polySia reduces CTB invasion. iCTBs (10–12 week gestational age) were monitored for their ability to invade Matrigel. Removal of polySia with Endo N (white) significantly reduced invasion compared with an untreated control (black), **P < 0.01. Error bars represent SD.
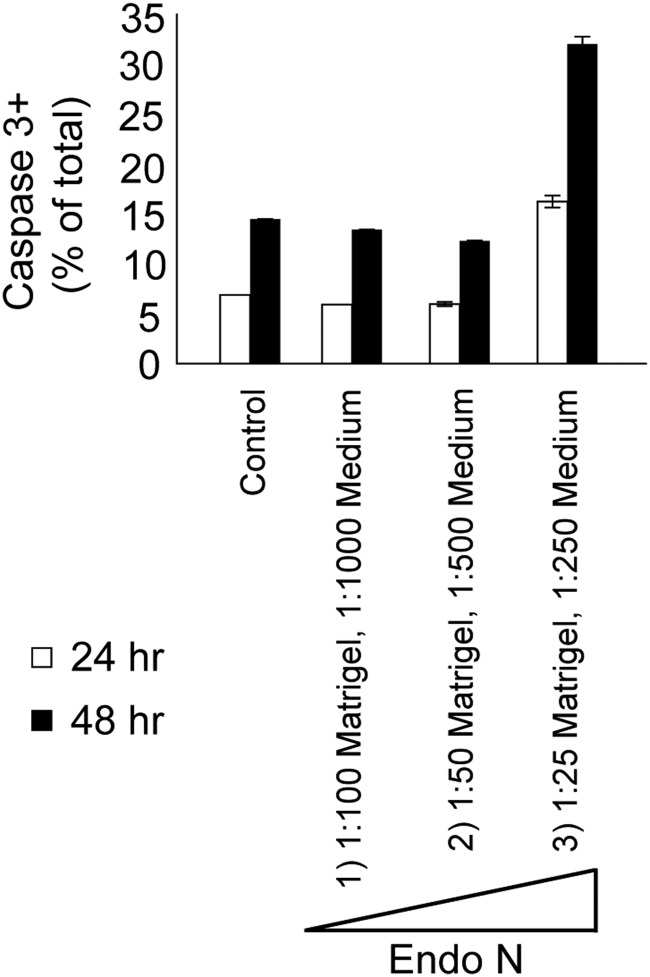
PolySia also promotes the viability of newly generated neurons and removal of polySia can induce apoptosis (Gascon et al. 2007a,b). To ensure that the reduced migration and invasion were not attributable to apoptosis, we cultured iCTBs ± Endo N for 24 or 48 h and immunostained the cells for activated caspase 3 (CASP3) (Figure 5). Enzyme levels that were used in the migration (1:100 Matrigel, 1:1000 medium) and invasion (1:50 Matrigel, 1:500 medium) assays did not induce apoptosis; however, some staining was observed in cultures that were treated with 2- to 4-fold higher concentrations (1:25 Matrigel, 1:250 medium). Together, these results demonstrated that removal of polySia diminished iCTB migration and invasion and that the observed reductions were not the result of cell death.
Fig. 5.
PolySia removal does not trigger apoptosis under the conditions that were used to quantify CTB migration and invasion. Primary iCTBs were seeded on Matrigel and exposed to different concentrations of Endo N or, as controls, were left untreated. Cells were grown for 24 or 48 h, paraformaldehyde-fixed and stained with anti-ACTIVE CASP3 Ab. The number of CASP3-positive/total cells was quantified. Endo N concentrations were as follows: (1) 1:100 in Matrigel, 1:1000 in medium (the concentration used in the migration experiments), (2) 1:50 in Matrigel, 1:500 in medium (the concentration used in the invasion experiments) and (3) 1:25 in Matrigel, 1:250 in medium.
PolySia was overexpressed in biopsies from patients with GTDs
We hypothesized that polySia levels would be increased in placental pathologies that are characterized by uncontrolled trophoblast invasion (e.g., placental tumors). To address this question, we investigated polySia expression in the trophoblast-derived choriocarcinoma cell lines BeWo, JEG-3 and Jar (Ganapathy et al. 1999). BeWo and JEG-3 were isolated from cerebral metastases, while Jar was established from a placental site tumor. Immunostaining with the polySia-specific mAb 12F8 showed that these cell lines stained brightly for polySia (Supplementary data, Figure S2).
Next, we investigated whether choriocarcinomas and other gestational trophoblastic tumors also stained for polySia. This diverse group of disorders arises from trophoblasts at various stages during the differentiation process. Some are benign (e.g., placental site nodules), while others present as aggressive cancers (e.g., choriocarcinomas, as well as placental site and epithelioid trophoblastic tumors). Some of these pathologies arise from hydatidiform moles, abnormal trophoblast growths that contain two paternal X-chromosomes. We immunostained tissue microarrays of tumor biopsies and compared the results with the staining patterns of first-trimester placentas (Figure 6A). The intensity of polySia immunoreactivity was scored in a blinded analysis, using a scale of 0 (no signal) to 3 (intense staining). The mean score for trophoblasts in biopsies of normal first-trimester placental tissue was ∼1. All the cells in the gestational trophoblastic tumors stained more intensely, averaging ∼1.5–2 (Figure 6B). Staining was not seen in necrotic regions. These data revealed that trophoblast transformation and uncontrolled invasion were associated with a significant increase in polySia levels (P < 0.001–0.01).
Fig. 6.
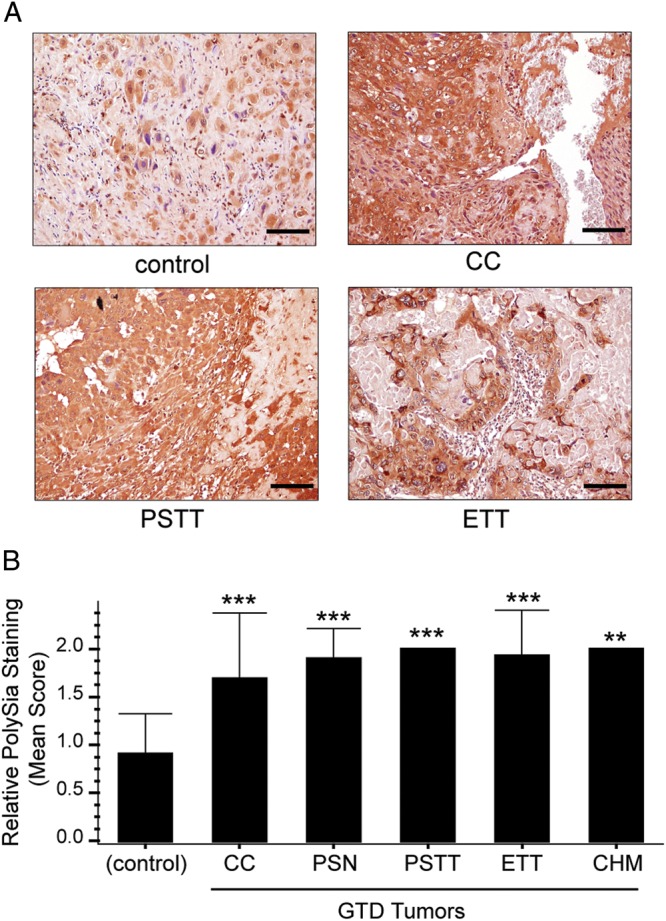
PolySia is overexpressed in biopsies of gestational trophoblastic disease (GTD) tumors. Biopsies of trophoblastic tumors and first-trimester normal placentas (controls) were paraffin-embedded and arranged into tissue microarrays. PolySia was detected with mAb 735 and visualized using DAB (brown). (A) Malignant tumors (choriocarcinomas [CC], placental site trophoblastic tumors [PSTT] and epithelioid trophoblastic tumors [ETT]) stained brightly for polySia compared with controls (normal first-trimester placental biopsies). Staining was not observed in necrotic regions. Scale bars, 40 µM. (B) The mean intensity of polySia immunoreactivity was significantly higher in biopsies from patients with GTD compared with controls, ***P < 0.001 or **P < 0.01. Error bars represent SD.
Discussion
This is the first description of trophoblast polySia and our results suggested that it functions during processes that are critical to human placentation. Immunolocalization experiments showed that iCTBs expressed polySia as they differentiated, migrated and invaded the uterus during the first half of pregnancy (Figure 1). The results of our in vitro experiments suggested that polySia facilitates the en masse migration of iCTBs into cell columns (Figure 3) and that this glycan promotes iCTB invasion (Figure 4) but does not affect apoptosis (Figure 5). Together, these findings resonate with a large body of literature demonstrating that polySia enhances the migration of neural (Ono et al. 1994; Hu et al. 1996; Seki et al. 2007) and immune (Drake et al. 2009) progenitors.
We also found that STBs, which cover the chorionic villi and respond to maternal cues via receptors for growth factors— e.g., epidermal growth factor (Richards et al. 1983)—expressed polySia during the early stages of placentation. Although we do not know the role of polySia on these cells, it is intriguing to speculate that STB-associated polySia might act as a web to capture small molecules (e.g., growth factors, hormones and cytokines), which are abundant at the maternal–fetal interface. Alternatively, polySia might facilitate pCTB detachment from the trophoblast basement membrane. Depending on the location, this could promote STB formation or pCTB transmigration through the STB layer, thereby promoting cell column formation.
This study adds to a body of literature describing polySia expression by cancer cells. Here, we screened a library of biopsies from gestational trophoblastic tumors in a tissue microarray format. Our results demonstrated that polySia was significantly overexpressed in malignant and benign tumors compared with biopsies of normal first-trimester placental bed sites (Figure 6). These findings agree with previous reports that breast cancers and leukemia cells carried a high molecular weight glycoprotein bearing polySia (Martersteck et al. 1996). Our data suggest that these glycans could promote migration and invasion of tumor cells.
Our work addresses aspects of the synthesis and the structure of polySia glycans carried by human trophoblasts. For example, we showed that ST8Sia4 was the relevant transferase (Figure 2A). We also provided evidence that polySia is added to N-linked carbohydrate structures (Figure 2B). Previously, we showed that trophoblast proteins carry other unusual glycans that have been implicated in tumorigenesis such as polylactosamine structures (McMaster et al. 1995; Lau et al. 2007). However, the protein backbone(s) that displays polySia on trophoblasts is unknown. NCAM, the best-described scaffold in other locations, is expressed by endovascular iCTBs that line uterine arterioles (Proll et al. 1996), but is not found on trophoblasts that reside in the villi (pCTBs and STBs) or iCTBs in cell columns, an observation confirmed by our microarray results. Interrogation of these data also failed to detect the expression of CD36, but showed low levels of mRNAs encoding the SynCAM isoforms. As part of ongoing analyses of VEGF effects on CTBs, immunostaining showed that these cells expressed NRP2 (data not shown). Since NRP2 (and also CD36) have been shown to contain polySia on O-linked glycans (Yabe et al. 2003; Curreli et al. 2007), the available data suggest that the scaffold may be a SynCAM isoform or a novel molecule.
In summary, our findings suggested important roles for polySia during human placentation. During the early stages of pregnancy, these glycans may serve as sinks for growth factors that promote trophoblast growth and differentiation. We also propose that under normal conditions placental polySia enhances iCTB migration and invasion through steric hindrance. Once the cells have reached their destination within the uterine wall, the glycan is downregulated. However, in pathological conditions involving unrestrained invasion, polySia expression is maintained and magnified. Thus, downregulation of polySia expression might be one of the elusive “stop” signals that circumscribe the uterine boundaries of CTB invasion. In this way, trophoblast polySia may regulate formation of the maternal–fetal interface.
Materials and methods
Placental collection
The University of California San Francisco Committee on Human Research approved this study and informed written consent was obtained from all participants. Normal placentas were donated by patients undergoing elective terminations of pregnancy (5–22 weeks) or from women who had uncomplicated deliveries at the University of California, San Francisco.
Immunolocalization
For histochemical detection, biopsies were fixed in 10% formalin for 24 h, transferred to 70% ethanol, paraffin-embedded and sectioned (5 µm). Sections were deparaffinized in xylene and rehydrated in a series of graded ethanol solutions. Endogenous peroxidase activity was blocked by incubation in 0.3% H2O2 for 30 min at room temperature (RT). Signals from maternal IgG bound to fetal Fc receptors were blocked by incubation with goat or donkey anti-human IgG (1.0 µg/mL; Jackson ImmunoResearch; 1 h at RT). To block binding of immune IgG to unoccupied Fc receptors, samples were incubated in 1.5% serum from the species in which the secondary Ab was produced. Sections were incubated overnight (O/N) at 4°C with an anti-polySia mAb (735; 20 μg/mL; generously provided by Dr. Rita Gerardy-Schahn), which was detected with a species-specific, biotin-conjugated, secondary Ab (Jackson ImmunoResearch) and ABC-peroxidase (Vector Laboratories, Burlingame, CA). The reaction was visualized using 3,3-diaminobenzidine (DAB, Vector Laboratories) and sections were counterstained with hematoxylin. For controls, mouse IgG2a (BD Pharmingen) or phosphate buffered saline (PBS) was substituted for the primary antibody (Ab).
For fluorescence detection, biopsies were fixed in 3% paraformaldehyde, embedded in optimal cutting temperature (OCT) medium and frozen as previously described (Zhou et al. 2007). Then, they were cryosectioned (5 µm) and permeabilized with ice-cold methanol/acetone (2:1) for 5 min. Nonspecific reactivity was inhibited by incubating for 1 h in blocking buffer (1% bovine serum albumin [BSA], 0.1% fish gelatin, 0.1% Triton-X-100 and 0.05% Tween-20). Then the tissue sections were incubated O/N at 4°C with the following mAbs (single or in combinations) diluted in blocking buffer: rat anti-polySia (12F8; 5 μg/mL; BD Pharmingen) or mouse anti-KRT7 (OV-TL 12/30; 1:100; Dako). Binding of primary Abs was detected with fluorescein isothiocyanate- or tetramethylrhodamine isothiocyanate-conjugated, species-specific, secondary Abs (Jackson ImmunoResearch). Some sections were preincubated with Endo N O/N at 37°C (1:500–1:1000 in PBS, pH 8.0; generously provided by Dr. Rita Gerardy-Schahn), an enzyme that specifically cleaves the α2-8 linkages of polySia. As controls, irrelevant rat IgM (BD Pharmingen), mouse IgG1 (BioLegend) or PBS was substituted for the primary Ab. Sections were mounted with 4′,6-diamidino-2-phenylindole (DAPI)-containing Vectashield (Vector Laboratories) and imaged with a Leica CTR5000 upright microscope.
Immunoblotting
Mouse brain lysates were prepared as previously described (Drake et al. 2008). Lysates of chorionic villi were prepared by homogenizing 2 mm3 pieces of tissue in medium salt lysis buffer (20 mM Tris-Cl pH 8.0, 140 mM NaCl, 10% glycerol, 1% Nonidet P-40 (NP-40), 2 mM ethylenediaminetetraacetic acid (EDTA), 10 mM NaF, 0.1% SDS and proteinase inhibitor cocktail [Pierce]), followed by periodic vortexing on ice for 1 h. STB plasma membrane preparations were isolated as previously described (Smith et al. 1977) with minor modifications. Briefly, placental chorionic villi were manually dissected into 5–10 mm pieces, resuspended in ice-cold PBS containing a proteinase inhibitor cocktail (Pierce) and stirred for 1 h. The samples were passed through a 70 µm filter and the membrane fraction was isolated by a series of centrifugation steps: 1000 × g (10 min), 14,000 × g (20 min) and 100,000 × g (1 h). Pellets were resuspended in PBS and solubilized by repeated aspiration through a 26-gauge needle. iCTB lysates were prepared on ice with medium salt lysis buffer. Protein concentrations were determined using the Bradford assay (Bio-Rad, Hercules, CA). The samples were resuspended in NuPAGE lithium dodecyl sulfate (LDS) sample buffer (pH 8.4; Invitrogen) with NuPAGE reducing agent (Invitrogen), which contains 500 mM dithiothreitol (DTT), and then heated at 70°C for 10 min. The lysates were separated (40 µg/lane) on 4–12% Bis–Tris gradient gels in running buffer containing 3-(N-morpholino)propanesulfonic acid (MOPS) and sodium dodecyl sulfate (SDS) (Invitrogen) and transferred to nitrocellulose (Bio-Rad). In some cases, the membranes were preincubated with Endo N (1:500–1:1000 in PBS pH 8.0) O/N on a shaker at 37°C. Nonspecific reactivity was blocked by incubating the membranes for 1 h in PBS containing 0.05% Tween 20 (PBST) and 5% nonfat dried milk (blocking buffer). Then the blots were incubated O/N at 4°C with anti-polySia mAb 735 (1.4 µg/mL) or anti-β-actin (C4) (polyclonal; 1:1000; Santa Cruz Biotechnology) diluted in blocking buffer. After washing three times for 5 min in PBST, the transfers were incubated for 1 h at RT with the appropriate peroxidase-conjugated, species-specific, secondary Abs (1:2500; Jackson ImmunoResearch). Finally, they were washed three times for 5 min in PBST and Ab reactivity was detected with erythrina cristagalli lectin (ECL) Plus (GE Healthcare). In some cases, lysates were treated with PNGase F (NEB; McMaster et al. 1995).
Cell isolation and culturing
CTBs were isolated from first- or second-trimester human placentas by published methods (Hunkapiller and Fisher 2008). Briefly, placentas were subjected to a series of enzymatic digests to remove STBs and to detach pCTBs from the stromal cores of the chorionic villi. Then, the cells were purified over a Percoll gradient and resuspended in serum-free medium containing ITS: Dulbecco's modified Eagle's medium (DMEM), 4.5 g/L glucose (Sigma-Aldrich) with 2% Nutridoma (Boehringer Mannheim Biochemicals), 1% penicillin/streptomycin, 1% sodium pyruvate, 1% HEPES and 1% gentamicin (UCSF Cell Culture Facility). Isolated CTBs (2.5 × 105 cells in 250 µL serum-free medium) or choriocarcinoma cells (1 × 105 cells in 250 µL MEM supplemented with 10% fetal calf serum) were cultured on Matrigel-coated (BD Biosciences) tissue culture plates or cover slips for 24–48 h.
Villous explant culture as a measure of CTB migration
Using methods developed in our laboratory (Genbacev et al. 1992; Hunkapiller and Fisher 2008), human placental villous explants were cultured on undiluted Matrigel (BD Biosciences) in the presence or absence of Endo N (1:100 [vol/vol] in Matrigel, 1:1000 [vol/vol] in medium). Briefly, cell columns were isolated by manual dissection and plated on the Matrigel substrates. The medium was refreshed daily and phase contrast photographs were taken at regular intervals using a Nikon Eclipse TE2000-S microscope. Explants were maintained for a minimum of 1 week. Outgrowths were quantified using the Adobe Illustrator software with an area index defined as the area 144 or 168 h after plating divided by the initial area of the leading front at 48 h. Area index values were expressed in relative units and the mean control area index was set to 100%. Each experiment was performed with two to three explants per condition, and repeated with five placentas between the gestational ages of 5 and 8 weeks. The data were analyzed with an unpaired Student's t-test using the InStat statistical software.
Quantifying cytotrophoblast invasion
Transwell inserts (8 µm pore size, Corning Costar) were coated by incubating with 8 µL of diluted Matrigel (2:1 v/v in serum-free medium BD Biosciences) for 30 min at 37°C. CTBs (2.5 × 105 cells in 250 µL serum-free media) were added to the upper compartments and the inserts were placed in 24-well plates that contained 600 µL medium ± Endo N (1:50 [vol/vol] in Matrigel, 1:500 [vol/vol] in medium). The cells were incubated for 40 h at 37°C, washed in PBS and fixed with 3% paraformaldehyde. Invasion was quantified by microscopy; iCTBs that reached the underside of the filter were labeled with anti-KRT7 mAb as previously described, and counted in five randomly chosen fields. Invasion was expressed as a percentage of control values set to 100%. Each experiment was performed with four replicates per condition, and repeated with CTBs isolated from four placentas. One-way analysis of variance (ANOVA) followed by Tukey's posthoc comparison tests was performed using the InStat statistical software.
Quantifying apoptosis
Freshly isolated iCTBs cultured on Matrigel, primary placental fibroblasts propagated on gelatin and A549 lung adenocarcinoma cells grown on tissue culture-treated plates were exposed to Endo N or medium alone (n = 2 CTB preparations from different placentas/treatment group). Primary placental fibroblasts and the A549 cell line, which do not express polySia, were used to control for apoptosis. Endo N concentrations (vol/vol) were as follows: (1) 1:100 in Matrigel, 1:1000 in medium, (2) 1:50 in Matrigel, 1:500 in medium and (3) 1:25 in Matrigel, 1:250 in medium. Cells were cultured for 24 or 48 h and fixed in 3% paraformaldehyde. Immunofluorescence was performed as previously described with rabbit anti-ACTIVE CASP3 (polyclonal; 1:250; Promega) and the number of positive/total cells was quantified. One-way ANOVA followed by Tukey's posthoc comparisons tests was performed using the InStat statistical software.
Tissue microarrays for analyzing polySia expression by GTD tumors
Tissue microarrays were prepared as previously described (Ueda et al. 2009). Briefly, paraffin-embedded biopsies of tumors and normal placentas were retrieved from the archival files in the Department of Pathology at the Johns Hopkins Hospital and arranged into microarrays. Three representative cores (1.5 mm diameter) from each biopsy were included. All cases were anonymous and the use of archival tissues was approved by the Institutional Review Board. Immunohistochemistry with mAb 735 or a mouse IgG2a isotype control Ab was performed as previously described. Staining intensity was scored by Dr. Ie-Ming Shih in a subset of the biopsies (n = 4–30/group) by light microscopy according to the following system: 0-detectable, 0.5-very weak, 1-weak, 2-moderate and 3-bright. The data were analyzed by one-way ANOVA followed by Tukey's post hoc comparisons tests performed using the InStat statistical software.
Supplementary data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported by the National Institutes of Health [1R21AI079329-01A1 to S.J.F.]. B.S.H was supported by a fellowship from the National Science Foundation.
Conflict of interest
None declared.
Abbreviations
Ab, antibody; ANOVA, analysis of variance; CASP3, caspase 3; CC, choriocarcinoma; DAB, aminobenzidine; DMEM, Dulbecco's modified Eagle's medium; DTT, dithiothreitol; ECL, erythrina cristagalli lectin; EDTA, Ethylenediaminetetraacetic acid; Endo N, endoneuraminidase N; ETT, epithelioid trophoblastic tumor; GTD, gestational trophoblastic disease; iCTBs, invasive cytotrophoblasts; KRT7, Cytokeratin 7; LDS, lithium dodecyl sulfate; mAb, monoclonal antibody; mAbs, monoclonal antibodies; MB, mouse brain; NCAM, neural cell adhesion molecule; NK, natural killer; NP-40, Nonidet P-40; NRP2, neuropilin-2; OCT, optimal cutting temperature; PBS, phosphate buffered saline; O/N, overnight; pCTB, progenitor cytotrophoblast; PECAM, platelet endothelial cell adhesion molecule; PNGase F, N-glycosidase F; PolySia, polysialic acid; PSTT, placental site trophoblastic tumor; RT, room temperature; SD, standard deviation; SDS–PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; ST8SiaII, polysialyltransferase II; ST8SiaIV, polysialyltransferase IV; STBs, syncytiotrophoblasts; uNK, uterine natural killer; VCAM, vascular cell adhesion molecule.
Acknowledgements
We are grateful to the women who participated in this study. We thank Mr. Jake Scott for excellent assistance in collecting placentas. We are indebted to Dr. Rita Gerardy-Schahn for critical advice and for generously providing mAb 735 and Endo N. We are grateful to Ms. Linda Prentice for tissue embedding and thank Dr. Steven Rosen for thoughtful discussions.
References
- Angata K, Nakayama J, Fredette B, Chong K, Ranscht B, Fukuda M. Human STX polysialyltransferase forms the embryonic form of the neural cell adhesion molecule. Tissue-specific expression, neurite outgrowth, and chromosomal localization in comparison with another polysialyltransferase, PST. J Biol Chem. 1997;272:7182–7190. doi: 10.1074/jbc.272.11.7182. doi:10.1074/jbc.272.11.7182. [DOI] [PubMed] [Google Scholar]
- Bax M, van Vliet S, Litjens M, García-Vallejo J, van Kooyk Y. Interaction of polysialic acid with CCL21 regulates the migratory capacity of human dndritic cells. PLoS ONE. 2009;4:e6987. doi: 10.1371/journal.pone.0006987. doi:10.1371/journal.pone.0006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos N. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282:30346–30356. doi: 10.1074/jbc.M702965200. doi:10.1074/jbc.M702965200. [DOI] [PubMed] [Google Scholar]
- Drake P, Nathan J, Stock C, Chang P, Muench M, Nakata D, Reader J, Gip P, Golden K, Weinhold B, et al. Polysialic acid, a glycan with highly restricted expression, is found on human and murine leukocytes and modulates immune responses. J Immunol. 2008;181:6850–6858. doi: 10.4049/jimmunol.181.10.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake P, Stock C, Nathan J, Gip P, Golden K, Weinhold B, Gerardy-Schahn R, Bertozzi C. Polysialic acid governs T-cell development by regulating progenitor access to the thymus. Proc Natl Acad Sci USA. 2009;106:11995–12000. doi: 10.1073/pnas.0905188106. doi:10.1073/pnas.0905188106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J, Finne U, Deagostini-Bazin H, Goridis C. Occurrence of alpha 2–8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983;112:482–487. doi: 10.1016/0006-291x(83)91490-0. doi:10.1016/0006-291X(83)91490-0. [DOI] [PubMed] [Google Scholar]
- Frosch M, Gorgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: Isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA. 1985;82:1194–1198. doi: 10.1073/pnas.82.4.1194. doi:10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuska SP, Rollenhagen M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt H, Geyer R, et al. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA. 2010;107:10250–10255. doi: 10.1073/pnas.0912103107. doi:10.1073/pnas.0912103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Prasad PD, Leibach FH. Choriocarcinoma. In: Masters J, Palsson B, editors. Human Cell Culture. Kluwer Academic Publishers; 1999. pp. 141–147. [Google Scholar]
- Gascon E, Vutskits L, Jenny B, Durbec P, Kiss JZ. PSA-NCAM in postnatally generated immature neurons of the olfactory bulb: A crucial role in regulating p75 expression and cell survival. Development. 2007a;134:1181–1190. doi: 10.1242/dev.02808. doi:10.1242/dev.02808. [DOI] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Kiss JZ. Polysialic acid-neural cell adhesion molecule in brain plasticity: From synapses to integration of new neurons. Brain Res Rev. 2007b;56:101–118. doi: 10.1016/j.brainresrev.2007.05.014. doi:10.1016/j.brainresrev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Schubach SA, Miller RK. Villous culture of first trimester human placenta—model to study extravillous trophoblast (EVT) differentiation. Placenta. 1992;13:439–461. doi: 10.1016/0143-4004(92)90051-t. doi:10.1016/0143-4004(92)90051-T. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Becker C, Glüer S, Rösner H, Gerardy-Schahn R, Rahmann H. Polysialic acid on the neural cell adhesion molecule correlates with expression of polysialyltransferases and promotes neuroblastoma cell growth. Cancer Res. 1998;58:779–784. [PubMed] [Google Scholar]
- Hildebrandt H, Muhlenhoff M, Weinhold B, Gerardy-Schahn R. Dissecting polysialic acid and NCAM functions in brain development. J Neurochem. 2007;103(Suppl. 1):56–64. doi: 10.1111/j.1471-4159.2007.04716.x. doi:10.1111/j.1471-4159.2007.04716.x. [DOI] [PubMed] [Google Scholar]
- Hoffman S, Sorkin BC, White PC, Brackenbury R, Mailhammer R, Rutishauser U, Cunningham BA, Edelman GM. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982;257:7720–7729. [PubMed] [Google Scholar]
- Hu H, Tomasiewicz H, Magnuson T, Rutishauser U. The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron. 1996;16:735–743. doi: 10.1016/s0896-6273(00)80094-x. doi:10.1016/S0896-6273(00)80094-X. [DOI] [PubMed] [Google Scholar]
- Hunkapiller N, Fisher S. Chapter 12 placental remodeling of the uterine vasculature. Methods Enzymol. 2008:281–302. doi: 10.1016/S0076-6879(08)03012-7. doi:10.1016/S0076-6879(08)03012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WM, Agnew WS. Multiple oligosaccharide chains in the voltage-sensitive Na channel from electrophorus electricus: Evidence for alpha-2,8-linked polysialic acid. Biochem Biophys Res Commun. 1987;148:817–826. doi: 10.1016/0006-291x(87)90949-1. doi:10.1016/0006-291X(87)90949-1. [DOI] [PubMed] [Google Scholar]
- Kanato Y, Kitajima K, Sato C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology. 2008;18:1044–1053. doi: 10.1093/glycob/cwn084. doi:10.1093/glycob/cwn084. [DOI] [PubMed] [Google Scholar]
- Komminoth P, Roth J, Lackie PM, Bitter-Suermann D, Heitz PU. Polysialic acid of the neural cell adhesion molecule distinguishes small cell lung carcinoma from carcinoids. Am J Pathol. 1991;139:297–304. [PMC free article] [PubMed] [Google Scholar]
- Larsen W. Fetal development and the fetus as patient. In: Sherman LP, Potter SS, Scott WJ, editors. Human Embryology. Churchill Livingstone; 2001. [Google Scholar]
- Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. doi:10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Li J, Dai G, Cheng YB, Qi X, Geng MY. Polysialylation promotes neural cell adhesion molecule-mediated cell migration in a fibroblast growth factor receptor-dependent manner, but independent of adhesion capability. Glycobiology. 2011;21:1010–1018. doi: 10.1093/glycob/cwr020. doi:10.1093/glycob/cwr020. [DOI] [PubMed] [Google Scholar]
- Martersteck CM, Kedersha NL, Drapp DA, Tsui TG, Colley KJ. Unique alpha 2, 8-polysialylated glycoproteins in breast cancer and leukemia cells. Glycobiology. 1996;6:289–301. doi: 10.1093/glycob/6.3.289. doi:10.1093/glycob/6.3.289. [DOI] [PubMed] [Google Scholar]
- McMaster MT, Librach CL, Zhou Y, Lim KH, Janatpour MJ, DeMars R, Kovats S, Damsky C, Fisher SJ. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154:3771–3778. [PubMed] [Google Scholar]
- Moss L, Prakobphol A, Wiedmann T, Fisher S, Damsky C. Glycosylation of human trophoblast integrins is stage and cell-type specific. Glycobiology. 1994;4:567–575. doi: 10.1093/glycob/4.5.567. doi:10.1093/glycob/4.5.567. [DOI] [PubMed] [Google Scholar]
- Muhlenhoff M, Eckhardt M, Bethe A, Frosch M, Gerardy-Schahn R. Autocatalytic polysialylation of polysialyltransferase-1. J. 1996;15:6943–6950. [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Seki T, Rutishauser U, Arai Y. Enzymatic removal of polysialic acid from neural cell adhesion molecule perturbs the migration route of luteinizing hormone-releasing hormone neurons in the developing chick forebrain. J Comp Neurol. 2000;420:171–181. doi: 10.1002/(sici)1096-9861(20000501)420:2<171::aid-cne2>3.3.co;2-0. doi:10.1002/(SICI)1096-9861(20000501)420:2<171::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ono S, Hane M, Kitajima K, Sato C. Novel regulation of fibroblast growth factor 2 (FGF2)-mediated cell growth by polysialic acid. J Biol Chem. 2011;287:3710–3722. doi: 10.1074/jbc.M111.276618. doi:10.1074/jbc.M111.276618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Tomasiewicz H, Magnuson T, Rutishauser U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron. 1994;13:595–609. doi: 10.1016/0896-6273(94)90028-0. doi:10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Petridis AK, El-Maarouf A, Rutishauser U. Polysialic acid regulates cell contact-dependent neuronal differentiation of progenitor cells from the subventricular zone. Dev Dyn. 2004;230:675–684. doi: 10.1002/dvdy.20094. doi:10.1002/dvdy.20094. [DOI] [PubMed] [Google Scholar]
- Prakobphol A, Genbacev O, Gormley M, Kapidzic M, Fisher SJ. A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev Biol. 2006;298:107–117. doi: 10.1016/j.ydbio.2006.06.020. doi:10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Proll J, Blaschitz A, Hartmann M, Thalhamer J, Dohr G. Human first-trimester placenta intra-arterial trophoblast cells express the neural cell adhesion molecule. Early Pregnancy. 1996;2:271–275. [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher S. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Gallardo A, Delgado-Martin C, Gerardy-Schahn R, Rodriguez-Fernandez JL, Vega MA. Polysialic acid is required for neuropilin-2a/b-mediated control of CCL21-driven chemotaxis of mature dendritic cells and for their migration in vivo. Glycobiology. 2011;21:655–662. doi: 10.1093/glycob/cwq216. doi:10.1093/glycob/cwq216. [DOI] [PubMed] [Google Scholar]
- Rey-Gallardo A, Escribano C, Delgado-Martín C, Rodriguez-Fernández JL, Gerardy-Schahn R, Rutishauser U, Corbi AL, Vega MA. Polysialylated neuropilin-2 enhances human dendritic cell migration through the basic C-terminal region of CCL21. Glycobiology. 2010 doi: 10.1093/glycob/cwq078. [DOI] [PubMed] [Google Scholar]
- Richards RC, Beardmore JM, Brown PJ, Molloy CM, Johnson PM. Epidermal growth factor receptors on isolated human placental syncytiotrophoblast plasma membrane. Placenta. 1983;4:133–138. doi: 10.1016/s0143-4004(83)80026-5. doi:10.1016/S0143-4004(83)80026-5. [DOI] [PubMed] [Google Scholar]
- Rockle I, Seidenfaden R, Weinhold B, Muhlenhoff M, Gerardy-Schahn R, Hildebrandt H. Polysialic acid controls NCAM-induced differentiation of neuronal precursors into calretinin-positive olfactory bulb interneurons. Dev Neurobiol. 2008;68:1170–1184. doi: 10.1002/dneu.20649. doi:10.1002/dneu.20649. [DOI] [PubMed] [Google Scholar]
- Roth J, Zuber C, Wagner P, Taatjes DJ, Weisgerber C, Heitz PU, Goridis C, Bitter-Suermann D. Reexpression of poly(sialic acid) units of the neural cell adhesion molecule in Wilms tumor. Proc Natl Acad Sci USA. 1988;85:2999–3003. doi: 10.1073/pnas.85.9.2999. doi:10.1073/pnas.85.9.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. doi:10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Seki T, Namba T, Mochizuki H, Onodera M. Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. J Comp Neurol. 2007;502:275–290. doi: 10.1002/cne.21301. doi:10.1002/cne.21301. [DOI] [PubMed] [Google Scholar]
- Smith CH, Nelson DM, King BF, Donohue TM, Ruzycki S, Kelley LK. Characterization of a microvillous membrane preparation from human placental syncytiotrophoblast: A morphologic, biochemical, and physiologic, study. Am J Obstet Gynecol. 1977;128:190–196. doi: 10.1016/0002-9378(77)90686-x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nakayama J, Suzuki A, Angata K, Chen S, Sakai K, Hagihara K, Yamaguchi Y, Fukuda M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology. 2005;15:887–894. doi: 10.1093/glycob/cwi071. doi:10.1093/glycob/cwi071. [DOI] [PubMed] [Google Scholar]
- Ueda SM, Mao TL, Kuhajda FP, Vasoontara C, Giuntoli RL, Bristow RE, Kurman RJ, Shih Ie M. Trophoblastic neoplasms express fatty acid synthase, which may be a therapeutic target via its inhibitor C93. Am J Pathol. 2009;175:2618–2624. doi: 10.2353/ajpath.2009.081162. doi:10.2353/ajpath.2009.081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vutskits L, Gascon E, Zgraggen E, Kiss J. The polysialylated neural cell adhesion molecule promotes neurogenesis in vitro. Neurochem Res. 2006;31:215–225. doi: 10.1007/s11064-005-9021-7. doi:10.1007/s11064-005-9021-7. [DOI] [PubMed] [Google Scholar]
- Yabe U, Sato C, Matsuda T, Kitajima K. Polysialic acid in human milk. CD36 is a new member of mammalian polysialic acid-containing glycoprotein. J Biol Chem. 2003;278:13875–13880. doi: 10.1074/jbc.M300458200. doi:10.1074/jbc.M300458200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Calaora V, Durbec P, Kiss JZ. A role for the polysialic acid-neural cell adhesion molecule in PDGF-induced chemotaxis of oligodendrocyte precursor cells. J Cell Sci. 2004;117:93–103. doi: 10.1242/jcs.00827. doi:10.1242/jcs.00827. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bianco K, Huang L, Nien J, McMaster M, Romero R, Fisher S. Comparative analysis of maternal-fetal interface in preeclampsia and preterm labor. Cell Tissue Res. 2007 doi: 10.1007/s00441-007-0428-0. [DOI] [PubMed] [Google Scholar]