Abstract
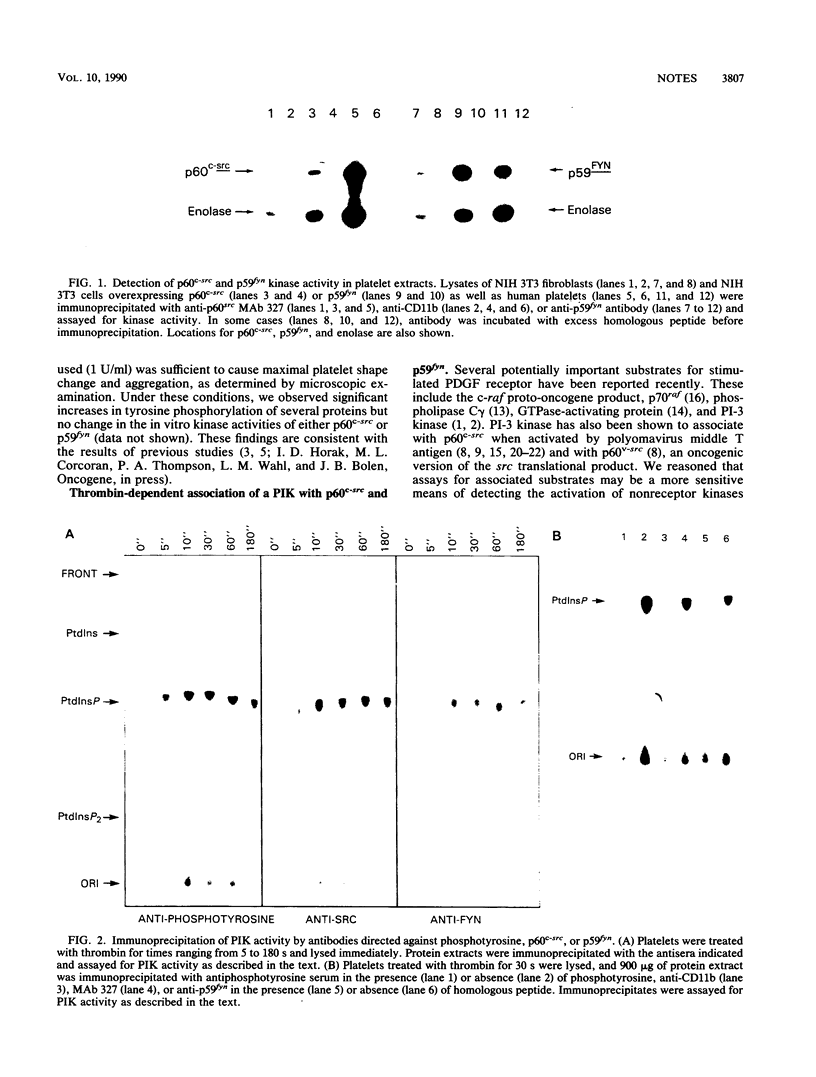
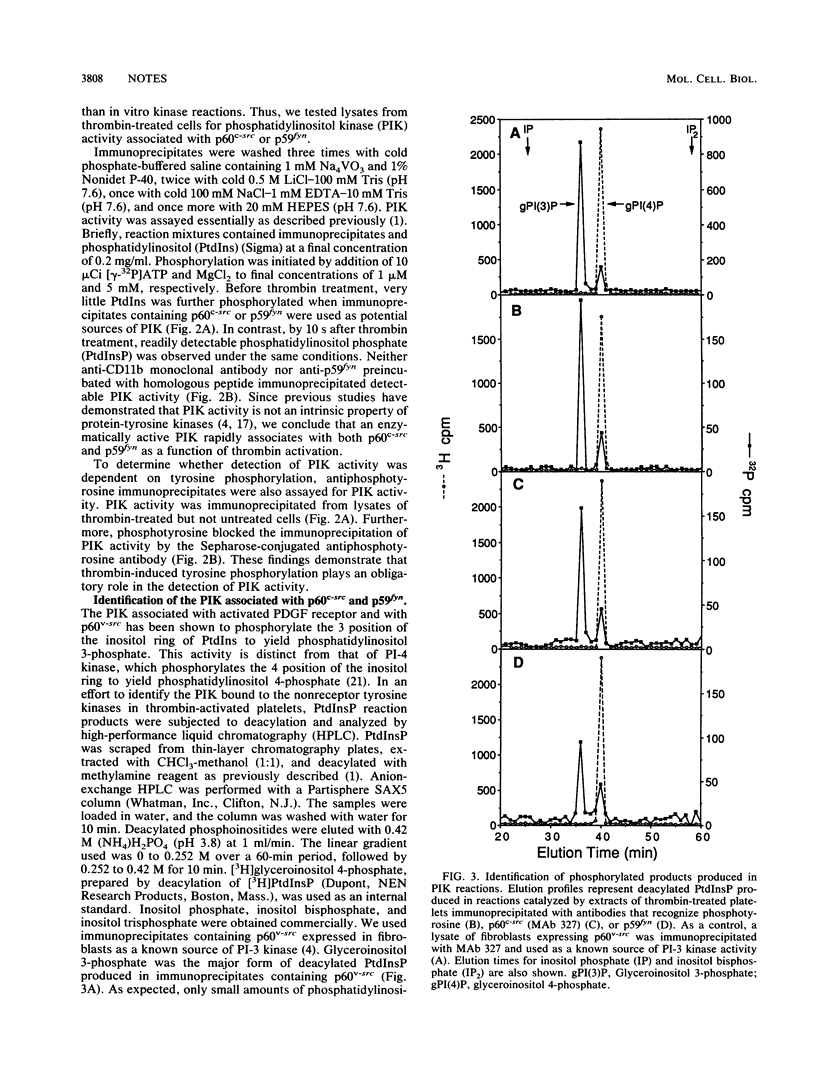
Recent studies have shown that ligand-activated growth factor receptors as well as transforming versions of nonreceptor protein-tyrosine kinases physically associate with phosphatidylinositol-3 kinase (PI-3 kinase). Reasoning that PI-3 kinase might also play a role in the normal functions of nonreceptor kinases, we sought to determine whether association with PI-3 kinase might serve as a measure of nonreceptor protein-tyrosine kinase activation under physiological conditions. We found that p60c-src as well as p59fyn, the product of another member of the src family of proto-oncogenes, physically associated with a PI kinase activity within 5 s after exposure to thrombin. Furthermore, PI kinase reaction products generated in p60v-src, p60c-src or p59fyn containing immunoprecipitates were indistinguishable, demonstrating the identity of the associated enzyme as PI-3 kinase. These findings demonstrate a thrombin-dependent interaction between p60c-src or p59fyn and PI-3 kinase and suggest a role for nonreceptor protein-tyrosine kinases in human platelet signal transduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988 Sep;8(9):3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol Cell Biol. 1989 Apr;9(4):1651–1658. doi: 10.1128/mcb.9.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Robbins K. C. Translocation of the FGR protein-tyrosine kinase as a consequence of neutrophil activation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8783–8787. doi: 10.1073/pnas.86.22.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Raptis L., Garcea R. L., Pallas D., Roberts T. M., Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Kawakami Y., Aaronson S. A., Robbins K. C. Acquisition of transforming properties by FYN, a normal SRC-related human gene. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3870–3874. doi: 10.1073/pnas.85.11.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Furue M., Kawakami T. Identification of fyn-encoded proteins in normal human blood cells. Oncogene. 1989 Mar;4(3):389–391. [PubMed] [Google Scholar]
- Ley T. J., Connolly N. L., Katamine S., Cheah M. S., Senior R. M., Robbins K. C. Tissue-specific expression and developmental regulation of the human fgr proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):92–99. doi: 10.1128/mcb.9.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Morgan W. C., Kaplan D. R., Pallas D. C., Roberts T. M. Recombinant retroviruses that transduce middle T antigen cDNAs derived from polyomavirus mutants: separation of focus formation and soft-agar growth in transformation assays and correlations with kinase activities in vitro. J Virol. 1988 Sep;62(9):3407–3414. doi: 10.1128/jvi.62.9.3407-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Nolan R. D., Lapetina E. G. Thrombin stimulates the production of a novel polyphosphoinositide in human platelets. J Biol Chem. 1990 Feb 15;265(5):2441–2445. [PubMed] [Google Scholar]
- Piwnica-Worms H., Kaplan D. R., Whitman M., Roberts T. M. Retrovirus shuttle vector for study of kinase activities of pp60c-src synthesized in vitro and overproduced in vivo. Mol Cell Biol. 1986 Jun;6(6):2033–2040. doi: 10.1128/mcb.6.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M. Expression of proto-oncogenes in neural tissues. Brain Res. 1988 Dec;472(4):391–403. doi: 10.1016/0006-8993(88)91228-0. [DOI] [PubMed] [Google Scholar]
- Talmage D. A., Freund R., Young A. T., Dahl J., Dawe C. J., Benjamin T. L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989 Oct 6;59(1):55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]




