Abstract
Background
Mexican Americans are the fastest aging segment of the U.S. population yet little scientific literature exists regarding the Alzheimer disease (AD) among this segment of the population. The extant literature suggests that biomarkers of AD will vary according to race/ethnicity though no prior work has explicitly studied this possibility. The aim of this study was to create a serum-based biomarker profile of AD among Mexican American.
Methods
Data were analyzed from 363 Mexican American participants (49 AD and 314 normal controls) enrolled in the Texas Alzheimer’s Research & Care Consortium (TARCC). Non-fasting serum samples were analyzed using a luminex-based multi-plex platform. A biomarker profile was generated using random forest analyses.
Results
The biomarker profile of AD among Mexican Americans was different from prior work from non-Hispanic populations with regards to the variable importance plots. In fact, many of the top markers were related to metabolic factors (e.g. FABP, GLP-1, CD40, pancreatic polypeptide, insulin-like-growth factor, and insulin). The biomarker profile was a significant classifier of AD status yielding an area under the receiver operating characteristic curve (AUC), sensitivity (SN) and specificity (SP) of 0.77, 0.92 and 0.64, respectively. Combining biomarkers with clinical variables yielded a better balance of SN and SP.
Conclusion
The biomarker profile for AD among Mexican American cases is significantly different from that previously identified among non-Hispanic cases from many large-scale studies. This is the first study to explicitly examine and provide support for blood-based biomarkers of AD among Mexican Americans. Areas for future research are highlighted.
Keywords: Biomarkers, Mexican American, Alzheimer’s disease, Neuropsychology
Introduction
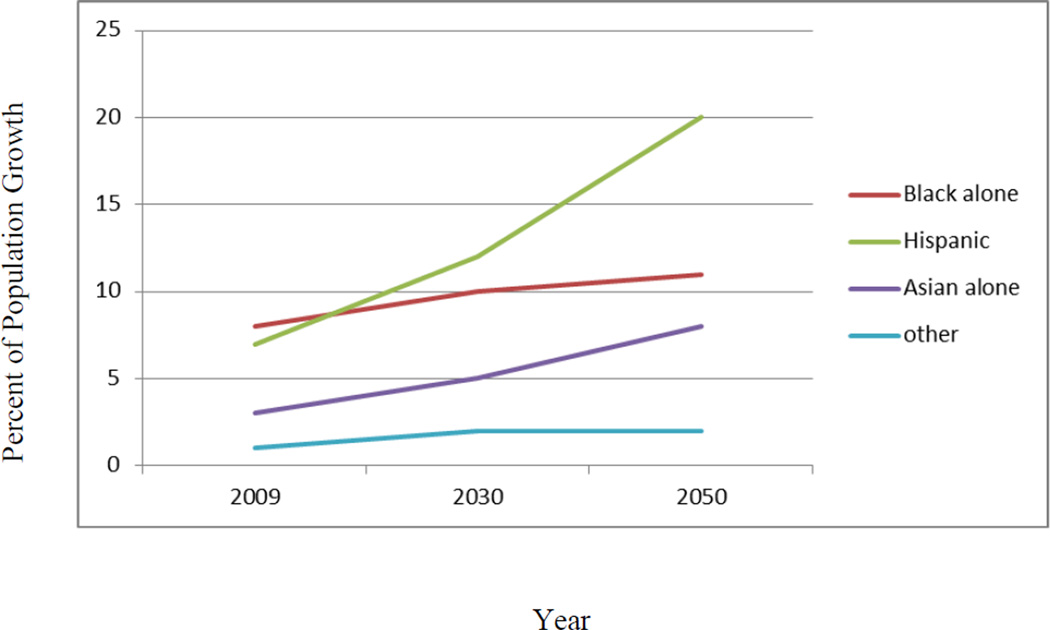
AD is the most common neurodegenerative dementia with over 5.4 million Americans suffering from the disease; every 71 seconds an American develops AD [1]. AD is the 5th leading cause of death for those over 65[1]. As illustrated in Figure 1, the percentage of Hispanics 65 and above will triple by the year 2050[2] (see Figure 1) and rates of AD are expected to grow six-fold among Hispanics[3]. Approximately 65% of Hispanics in the U.S. are Mexican American[4], making them the fastest aging segment of the population. However, there remains a dearth of scientific literature examining AD among Mexican Americans. Research from our group and others suggests that a significant health disparity exists as Mexican Americans may (1) be at increased risk for AD[3], (2) are diagnosed at a more advanced stage of disease progression[5], (3) develop AD at younger ages[5, 6], (4) have a lower frequency of the ApoEε4 allele (the strongest genetic risk for AD among non-Hispanic whites)[6, 7], and (5) suffer from a disproportionate burden of modifiable risk factors for AD (e.g. diabetes)[6, 8]. Therefore, there remains a significant need for research on AD among this underserved ethnic group[3, 5, 6, 9].
Figure 1.
Projected Growth of Minority Elder Population Age 65 and Above in the U.S.
In recent years, there has been a surge in research aimed at the identification of blood-based biomarker of AD that cover a wide range of biological systems[10–15]. Investigators have sought to identify diagnostic biomarkers [16–23], biomarkers of future AD disease risk[24–29], as well as rate of disease progression [30–33]; however, no such work has been demonstrated clinic-ready to date[34]. Despite significant advancements, little work has investigated the impact of race or ethnicity on AD biomarkers, whether blood-based or other modalities. This is also the case for many broader-based AD research questions [35]. Given the rapidly increasing Mexican American population, this underserved community will face a disproportionate burden of AD in the near future and additional research is needed specific to this ethnic group.
Despite the lack of research attention, empirical evidence supports the notion that biological markers associated with AD may be different for Hispanics and Mexican Americans. Jun and colleagues[36] recently conducted a meta-analysis of genome-wide allelic association study (GWAS) data from several large-scale cohorts that included over 500 Caribbean Hispanics AD cases. In this study, ApoEε4 genotype was significantly associated with AD status among all ethnic groups, including Hispanics. While CLU (SNP rs11136000), CR1 (SNP rs3818361) and PICALM (SNP rs3851179) were associated with AD status among non-Hispanic whites, these genetic markers were not associated with AD status in any other ethnic group (African American, Israeli-Arab, or Hispanic). Given that ApoEε4 frequency is less common among Mexican Americans [6, 7], additional work is needed to identify other genetic contributors to this disease among this ethnic group. Bertoli Avella et al[37] identified a novel presenilin 1 mutation (L174 M) that was associated with early onset AD among a large Cuban family. Therefore, current work suggests variability in susceptibility genes of AD by race/ethnicity, which suggests that proteins related to the disease will also likely vary.
To date, a scant literature exists on the link between blood-based biomarkers and cognition among Hispanics, or Mexican Americans in particular. A series of studies have examined the relation between blood-based markers and cognition as well as dementia status among the Sacramento Area Latino Study on Aging (SALSA) cohort[38–40]. In this cohort, folate levels were shown to be related to cognitive functioning and dementia diagnosis[38] while homocysteine levels were shown to be associated with neuropsychological functioning[40] as well as risk for incident dementia[39]. However, none of this work has specifically examined blood-based biomarkers of AD among Mexican Americans. We recently analyzed serum C-reactive protein (CRP) data from 471 Mexican Americans (AD n=55, MCI n=91, control n=325) and 595 non-Hispanic whites (AD n=229, MCI n=134, NC n=232) enrolled into the Texas Alzheimer’s Research and Care Consortium (TARCC). In the non-Hispanic portion of this cohort, CRP levels were significantly decreased among AD cases as compared to controls, which is consistent with other work[13, 30, 41]; however, CRP levels were not related to such outcomes among Mexican Americans [42]. The notion of CRP being differentially related to clinical outcomes by ethnicity is consistent with prior work. Veeranna and colleagues[43] conducted archival analysis of data from 6,067 participants (2362 Caucasian, 1601 African Americans, 1353 Hispanics, 751 Chinese) from the Multi-Ethnic Study of Atherosclerosis (MESA) to examine the link between baseline blood-based biomarkers, including CRP and incidence of cardiovascular disease (CVD). CRP levels were higher among Hispanics as compared to Caucasians; however, CRP level was a significant predictor of incident CVD among Caucasians, but not among Hispanics. Therefore, additional work is needed to examine biomarkers of AD among Mexican Americans, as well as other ethnic minorities. The current study sought to (1) generate a serum-based biomarker profile of AD among Mexican Americans, (2) compare the biomarker profile to our prior work with non-Hispanics and (3) evaluate the utility of this preliminary blood-based biomarker profile in discriminating between Mexican American AD cases and normal controls.
Methods
Participants
Data were analyzed from 363 Mexican American participants (AD n=49, control n=314) of the Texas Alzheimer’s Research & Care Consortium (TARCC). Each participant underwent a standardized evaluation at the respective TARCC site, which included an interview (e.g. demographics, family dementia history, NSAID, Vitamin E & anti-dementia medication history, medical history), neuropsychological testing and non-fasting blood draw. Global cognition was assessed via the Mini Mental State Examination (MMSE)[44] and disease severity rated according to the Clinical Dementia Rating scale [45] sum of boxes scores (CDR SB)[46, 47]. An informant interview was conducted for each research participant to obtain information regarding his/her activities of daily living (basic and instrumental). All information was reviewed by consensus committee who diagnosed participants as of NINCDS-ADRDA Probable Alzheimer’s disease[48] or cognitively normal control (NC) if they performed within normal limits on psychometric assessment[49]. Interviews and assessments were conducted in Spanish or English depending on the participant’s preference. Demographic characteristics of the sample are presented in Table 1.
Table 1.
Demographic Characteristics of Mexican Americans in the Cohort
| AD (N=49) | Control (N=314) | P-value | |
|---|---|---|---|
| Gender (male) | 41% | 37% | 0.37 |
| Age | 76.5 (7.6) 61–94 |
65.0 (8.0) 50–87 |
<0.001 |
| Education | 9.2(5.5) 0–20 |
11.2(4.7) 0–20 |
0.008 |
| MMSE | 19.5(4.9) 9–28 |
28.3(2.3) 19–30 |
<0.001 |
| CDR SB | 5.4(3.0) 2–13 |
0.02(0.18) 0–3 |
<0.001 |
| ApoEε4 carrier (yes/no) | 47%* | 13%* | 0.002 |
NOTE:
ApoEε4 genotype only available on 17 of the AD cases and 147 of the controls
Assays
Non-fasting samples were collected with 10mL serum-separating (tiger-top) vacutainers tubes at the time of interview. Samples were allowed to clot at room temperature for 30 minutes in a vertical position before being centrifuged at 1300 × g for 10 minutes. Next, 1mL aliquots were pipetted into polypropylene cryovial tubes and placed in −20° C (non-frost free) or −80° C freezers until shipment to TARCC Biobank. All samples from the current project were shipped in to Myriad Rules Based Medicine (Myriad RBM) in a single batch for assay on the luminex-based HumanMAP 1.0 platform. Over 100 proteins were quantified utilizing fluorescent microspheres with protein-specific antibodies. Information regarding the least detectable dose (LDD), inter-run coefficient of variation, dynamic range, overall spiked standard recovery, and cross-reactivity with other humanMAP analytes can be obtained from Myriad Rules Based Medicine. Buffy coats were extracted from EDTA plasma collection tubes (purple top) for DNA extraction using Puregene® isolation kits. ApoEε4 genotyped was conducted using standard PCR methods[50].
Statistical Analyses
Analyses were performed using SPSS 19 (IBM) and R (V 2.10) statistical software[51]. Chi square and t-tests were used to compare case versus controls for categorical variables (ApoEε4 allele frequency, gender) and continuous variables (age and education), respectively. The biomarker data was log transformed and then standardized for each analyte and the sample randomly spilt into a training (AD n=25, control n=157) and test set (AD n=24, control n=157). Using only the training set, a random forest (RF) biomarker profile was generated using R package randomForest (V 4.5)[52], with all software default settings. The RF Gini plot was examined to compare the top 30 markers from Mexican Americans to the top 30 from our prior work with non-Hispanic whites[22]. The RF biomarker risk score was then applied to the test sample to determine the ability of the biomarker profile in discriminating AD cases from normal controls. The ROC (receiver operation characteristic) curves were analyzed using R package AUC (area under the curve) was calculated using R package DiagnosisMed (V 0.2.2.2). ROC curves reflect sensitivity and 1-specificity for all possible scores on an item with AUC reflective of the overall discriminability based on the ROC figure.
Results
Demographic characteristics of the sample can be found in Table 1. When compared to normal controls, the AD cases were significantly older (t=0.53, p<0.001), achieved significantly fewer years of education (t=2.65, p=0.008), obtained significantly lower MMSE scores (t=20.52, p<0.001), and were rated higher on the CDR SB scores (t=31.3, p<0.001).
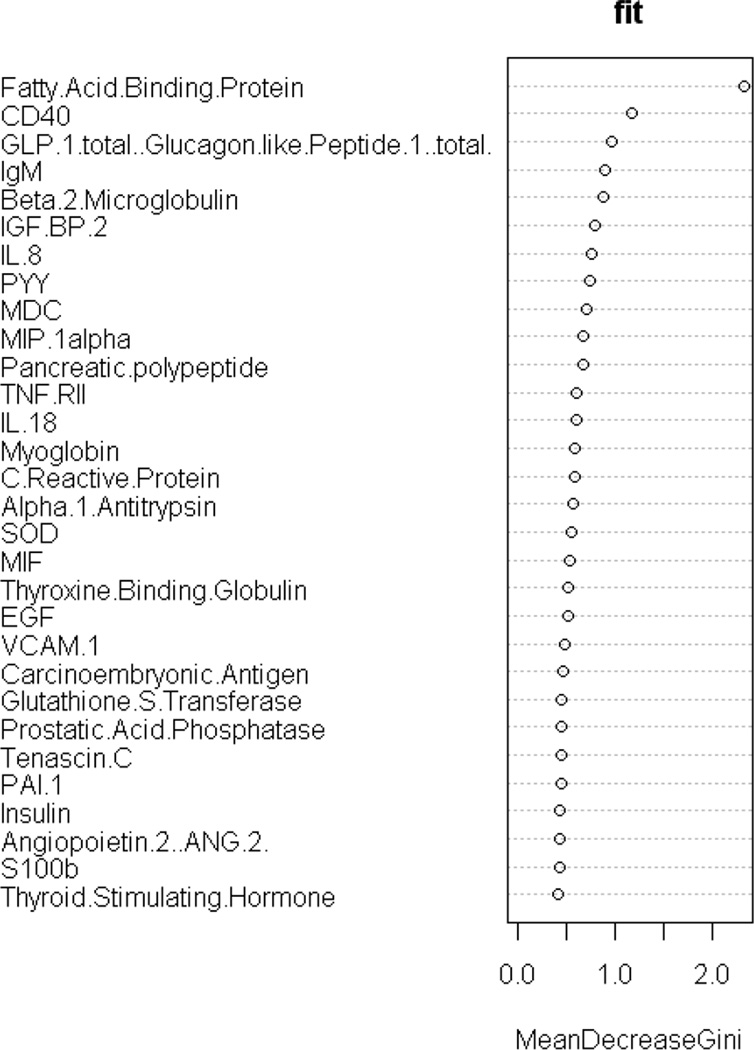
First, the biomarker profile was generated using Random Forest within the training set. As can be seen, among the top 30 markers 22 were overexpressed among AD cases while 8 were under-expressed. The overall biomarker profile was then compared to the profile of the top 30 markers previously identified among non-Hispanics in our prior work [22]. When examining the RF Gini plot, the top 30 serum-based biomarkers related to AD status among Mexican Americans was significantly different from the top markers found in our prior work with biomarkers among non-Hispanics whites (see Figure 2 and Table 2). The fold change for the markers can be found in Table 2.
Figure 2.
Gini Plot of top 30 serum biomarkers of AD among Mexican Americans
Table 2.
Top 30 markers associated with AD among Mexican Americans as compared to non-Hispanic whites
| Top 30 biomarkers of AD among Mexican Americans |
Fold Change of Mexican American biomarkers |
Top 30 biomarkers previously identified among non- Hispanic whites22 |
Fold Change among non- Hispanic whites |
|
|---|---|---|---|---|
| 1. | Fatty Acid Binding Protein✓ | 1.70 | Thrombopoietin | 2.18 |
| 2. | CD40* | 1.29 | MIP1α | 0.70 |
| 3. | Glucagon like peptide 1 | 1.21 | Eotaxin 3 | 1.26 |
| 4. | IgM | 0.67 | TNFα | 0.74 |
| 5. | Beta 2 Microglobulin✓* | 1.34 | Creatine kinase | 0.80 |
| 6. | IGF BP2* | 1.61 | Tenascin C | 1.60 |
| 7. | IL8 | 1.09 | FAS | 1.03 |
| 8. | Peptide YY | 1.69 | Fibrinogen | 0.87 |
| 9. | Macrophage-derived chemokine | 1.09 | IL 10 | 0.76 |
| 10. | MIP1α✓* | 1.31 | IL 7 | 1.02 |
| 11. | Pancreatic polypeptide ✓* | 1.50 | Cancer antigen 19 9 | 1.09 |
| 12. | TNF RII* | 1.30 | Prostatic acid phosphatase | 0.78 |
| 13. | IL18✓ | 0 98 | Apolipoprotein CIII | 1.12 |
| 14. | Myoglobin | 1.34 | Fas ligand | 0.85 |
| 15. | CRP✓ | 0.75 | CRP | 0.86 |
| 16. | α1-antitrypsin* | 1.16 | Pancreatic polypeptide | 1.33 |
| 17. | Super oxide dismutase* | 1.24 | TIMP 1 | 0.99 |
| 18. | Migration inhibitory factor | 1.50 | Angiopoietin 2 | 0.95 |
| 19. | Thyroxine binding globulin | 0.95 | Stem cell factor | 0.74 |
| 20. | EGF | 1.06 | IL 5 | 0.92 |
| 21. | VCAM1✓ | 1.17 | Lipoprotein a | 1.07 |
| 22. | Carcinoembryonic antigen* | 1.34 | α2-macroglobulin | 2.45 |
| 23. | Glutathione S transferase | 0.69 | ACE CD143 | 1.01 |
| 24. | Prostatic acid phosphatase✓ | 1.04 | MCP 1 | 0.85 |
| 25. | Tenascin C✓ | 1.23 | Ferritin | 0.97 |
| 26. | PAI1 | 0.95 | PARC | 1.12 |
| 27. | Insulin | 0.79 | Cancer Antigen 125 | 1.11 |
| 28. | Angiopoietin2* | 1.21 | Von Willebrand Factor | 1.29 |
| 29. | S100b | 1.57 | Carcinoembryonic Antigen | 1.10 |
| 30. | TSH | 0.99 | MIF | 1.11 |
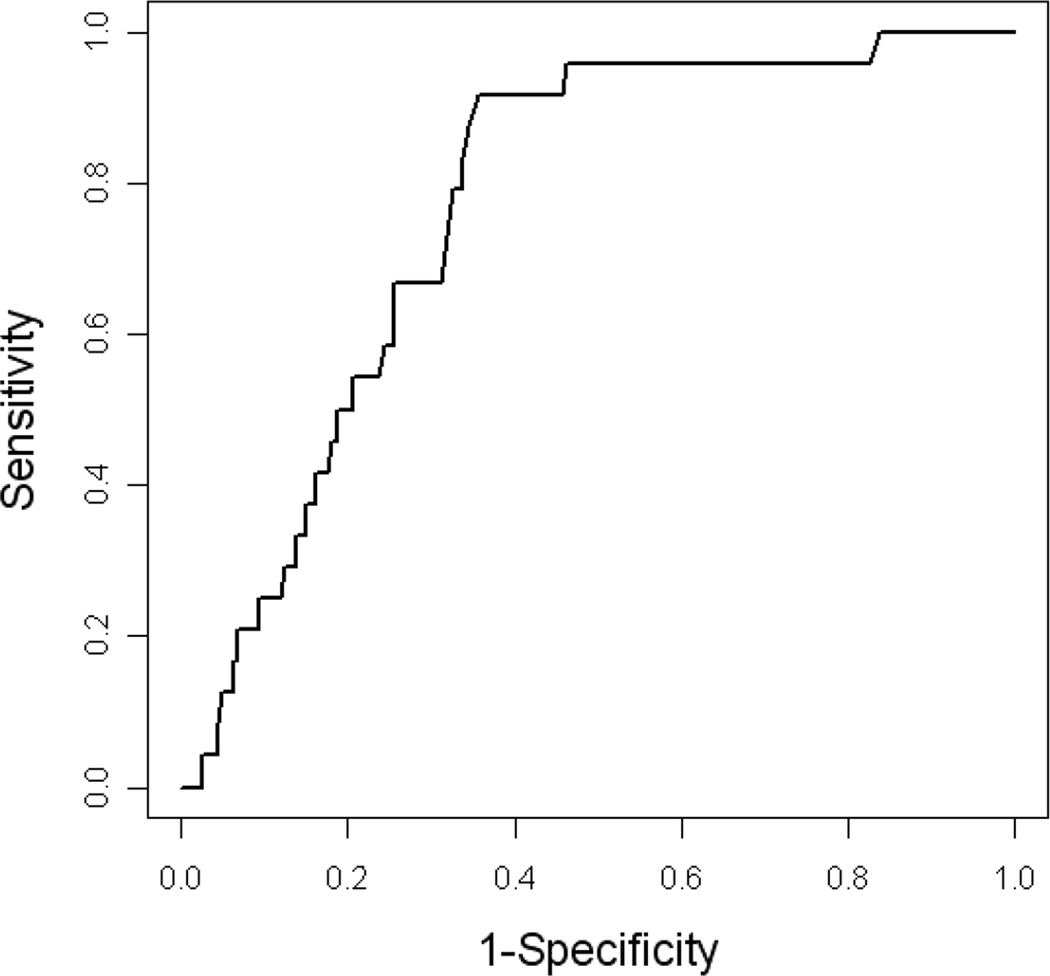
Next, the biomarker risk score was applied to the test set to determine the classification accuracy of the profile. A cut off of 0.126 on the RF risk score yielded an AUC of 0.77 (95% CI = 0.69–0.85) with a sensitivity of 0.92 (95% CI= 0.74–0.98) and specificity of 0.64 (95% CI= 0.57–0.71)(see Figure 3 and Table 3). The biomarker risk score yielded a superior sensitivity to clinical data, but inferior specificity. Clinical variables alone (age, gender, education and ApoEε4 genotype) yielded an AUC of 0.88 (95% CI= 0.81–0.95), sensitivity of 0.83 (95% CI=0.64–0.93) and specificity of 0.78 (95% CI= 0.71–0.84). Of note, only age (p<0.001) and ApoEε4 genotype (p=0.003) were significant contributors among the demographic factors. When the biomarker risk score and demographic factors were combined into a single predictive algorithm, as done in our prior work [21–23], the AUC of 0.88 (95% CI= 0.81–0.96) remained unchanged from the clinical data alone. However, the combined algorithm yielded a better overall balance of sensitivity and specific with an increase in sensitivity to 0.92 (95% CI=0.74–0.98) with a small decrease in specificity (0.73, 95% CI=0.65–79).
Figure 3.
ROC figure for biomarker profile of AD among Mexican Americans
Table 3.
Classification Accuracy of Biomarker Profile among Mexican Americans
| AUC | Sensitivity | Specificity | |
|---|---|---|---|
| Biomarker alone | 0 77 [0 69 – 0 85] | 0 92 [0 74 – 0 98] | 0 64 [0 57 – 0 71] |
| Clinical alone | 0 88 [0 81 – 0 95] | 0 83 [0 64 – 0 93] | 0 78 [0 71 – 0 84] |
| Biomarker + Clinical | 0 88 [0 81 – 0 96] | 0 92 [0 74 – 0 98] | 0 73 [0 65 – 0 79] |
NOTE: AUC = area under the receiver operating characteristic, sensitivity = proportion of true positives correctly identified by the test; specificity = proportion of true negatives that are correctly identified by the test
Discussion
There are several important findings from the current study. First, our prior methods can be utilized to generate a biomarker profile of AD among Mexican Americans [21–23]. However, when using the same biomarker multiplex platform containing over 100 proteins, the biomarker profile of AD among Mexican Americans was substantially different from that previously observed among non-Hispanic whites. Second, a significant classifier of AD can be generated for Mexican Americans though the blood-based algorithm is less accurate than what has been identified among non-Hispanic whites leaving room for improvement. This latter finding is likely due to the small sample size in the current study and the current algorithm is preliminary in nature. To our knowledge, this is the first project to explicitly search for blood-based biomarkers of AD among Mexican Americans.
The differential biomarker profile of AD among Mexican Americans is not surprising. First, as pointed out previously, the genetic markers associated with AD appear to be different when non-Hispanics and Hispanics are compared [36, 37] and ApoEε4 is less common among Mexican Americans [6, 7]. Additionally, Crean and colleagues [53] recently demonstrated that ApoEε4 prevalence rates fluctuate globally, which supports this notion. If the genetics of the disease vary by ethnicity, the proteins associated with the disease must also vary. Second, many of the protein markers that have been previously linked to AD have also been linked to other health conditions, such as diabetes, obesity, insulin resistance and the metabolic syndrome, which are more common among Mexican Americans [6, 54–57]. Given this increased prevalence of these metabolic conditions among Mexican Americans, it is possible that a metabolic phenotype of AD is more important in this population than among non-Hispanic whites. In fact, in the current study, the number one marker in the risk score was fatty acid binding protein (FABP). Fatty acid binding proteins are small intracellular cytoplasmic proteins involved in the binding, transport and metabolism of long-chain free fatty acids [58, 59] that have been implicated in diabetes, obesity, insulin resistance and the metabolic syndrome. Interestingly, genetic analyses of FABPs have also shown ethnic variation and many haplotypes were only related to clinical outcomes among non-Hispanic whites [59]. For example, the Ala54Thr FABP2 polymorphism was specifically associated with type 2 diabetes (T2DM) among Hispanic Americans [58], and a new SNP of FABP5 (rs454550) specifically associated with T2DM among non-Hispanic whites and African Americans [58]. Other markers in the biomarker profile of AD among Mexican Americans have also been associated with metabolic conditions including CD40 [60], glucagon-like peptide 1[61, 62], pancreatic polypeptide [63], β2-microglobulin [64], insulin-like-growth factor [65], peptide YY [66], insulin, and TSH [67]. Therefore, the current findings may point towards a metabolic endophenotype of AD among Mexican Americans and this possibility requires further attention. The notion of metabolic endophenotypes related to neurological and neuropsychiatric conditions has been proposed for both Autism [68] and schizophrenia [69], which should provide the groundwork for such investigations in AD. The biomarker profile also included markers related to inflammation, infection, protease inhibition, iron- and oxygen binding, and oxidative stress.
Several of the markers in the current Mexican American AD profile overlap with those identified using the same biomarker assay platform among different cohorts, all of which were primarily non-Hispanic white. In the current sample, five of the top 30 markers (pancreatic polypeptide CRP, tenacin C, MIP1α, and prostatic acid phosphatase) overlapped with our prior serum-only based work among non-Hispanics from TARCC with two of these (pancreatic polypeptide and CRP) overlapping with recent work examining biomarkers of AD across Penn and Wash U cohorts [41]. An additional four markers (fatty acid binding protein, β2-microglobulin, IL18 and VCAM1) overlapped with our prior work examining AD biomarkers across serum and plasma examining data from TARCC and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [23]. Several markers overlapped with those recently published by the Australian Imaging Biomarker and Lifestyle study (AIBL) [70], including insulin-like-growth factor binding protein 2, pancreatic polypeptide, MIP1α, carcinoembryonic antigen, tumor necrosis factor receptorlike2, angiopoietin 2, VCAM1, superoxide dismutase, α1-antitrypsin, β2-microglobulin, and CD40. Together these findings are supportive of overlap across AD blood-based biomarkers among Mexican Americans and non-Hispanics, but also point to (1) different markers and (2) different overall profiles (i.e. relative importance) of the biomarkers themselves (see Table 2).
There are several weaknesses to the current study. First, the sample size of AD cases is small and the accuracy of the biomarker algorithm, therefore, should be viewed as preliminary. Our group is currently recruiting larger numbers of Mexican Americans with and without AD with future studies planned to cross-validate and expand the current findings (including into Mexican Americans with Mild Cognitive Impairment). Second, while the biomarker platform contained a large number of markers (over 100), there are likely additional markers not investigated that will be of importance among Mexican Americans. Third, detailed information regarding metabolic conditions is not available among the TARCC database and therefore, additional analyses into a metabolic endophenotype of AD among Mexican Americans will be conducted utilizing a different cohort currently being established. Despite these limitations, the current project is the first to explicitly examine blood-based biomarkers of AD among Mexican Americans. The results suggest that the biomarker profile of AD among this underserved ethnic group is different from that of non-Hispanic whites. This hypothesis is supported by other work related to the genetics and biomarker analyses of AD and associated risk factors (e.g. diabetes, metabolic syndrome, obesity, insulin resistance) among Mexican Americans. These findings point towards the need for additional work aimed at understanding AD among Mexican Americans.
Acknowledgement
This study was made possible by the Texas Alzheimer’s Research and Care Consortium (TARCC) funded by the state of Texas through the Texas Council on Alzheimer’s Disease and Related Disorders. Research reported in this publication was supported by the National Institute on Aging (NIA) and National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health under Award Numbers R01AG039389, P30AG12300, and L60MD001849. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank all of the participants of the TARCC along with the incredible support staff that make this study possible.
References
- 1.Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2008;4:110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen LA, Kent M, Lee M, Mather M. America's aging population. Population Bulletin. 2011;66 [Google Scholar]
- 3.Novak K, Riggs JA. Hispanics/Latinos and Alzheimer's disease, Alzheimer’s Association, Chicago: 2004. [Google Scholar]
- 4.US Census Bureau. American Fact Finder. 2005 http://www.census.gov/.
- 5.O'Bryant SE, Humphreys JD, Schiffer RB, Sutker PB. Presentation of Mexican Americans to a memory disorder clinic. J Psychopathol Behav Assess. 2007;29:137–140. [Google Scholar]
- 6.O'Bryant SE, Johnson L, Balldin V, Edwards M, Barber RC, Williams B, Devous M, Sushing B, Knebl J, Hall J. Characterization of Mexican Americans with Mild Cognitive Impairment and Alzheimer's disease. J Alzheimers Dis. 2012 doi: 10.3233/JAD-2012-121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 8.Sundquist J, Winkleby MA. Cardiovascular risk factors in Mexican American adults: a transcultural analysis of NHANES III, 1988–1994. Am J Public Health. 1999;89:723–730. doi: 10.2105/ajph.89.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitten LJ, Ortiz F, Ponton M. Frequency of Alzheimer's disease and other dementias in a community outreach sample of Hispanics. J Am Geriatr Soc. 2001;49:1301–1308. doi: 10.1046/j.1532-5415.2001.49257.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM, Diaz-Arrastia R for the Texas Alzheimer's Research Consortium. Brain-derived neurotrophic factor levels in Alzheimer's disease. J Alzheimers Dis. 2009;17:337–341. doi: 10.3233/JAD-2009-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Bryant SE, Hobson VL, Hall JR, Barber RC, Zhang S, Johnson L, Diaz-Arrastia R for the Texas Alzheimer's Research Consortium. Serum Brain-Derived Neurotrophic Factor Levels Are Specifically Associated with Memory Performance among Alzheimer's Disease Cases. Dement Geriatr Cogn Disord. 2010;31:31–36. doi: 10.1159/000321980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G, Schott K. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm. 2006;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- 13.O'Bryant SE, Waring SC, Hobson V, Hall JR, Moore CB, Bottiglieri T, Massman P, Diaz-Arrastia R. Decreased C-reactive protein levels in Alzheimer disease. J Geriatr Psychiatry Neurol. 2010;23:49–53. doi: 10.1177/0891988709351832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teunissen CE, de Vente J, Steinbusch HW, De Bruijn C. Biochemical markers related to Alzheimer's dementia in serum and cerebrospinal fluid. Neurobiol Aging. 2002;23:485–508. doi: 10.1016/s0197-4580(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 15.Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, Trojanowski JQ, Blennow K. Biological markers of amyloid β-related mechanisms in Alzheimer's disease. Exp Neurol. 2010;223:334–346. doi: 10.1016/j.expneurol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 17.Buerger K, Ernst A, Ewers M, Uspenskaya O, Omerovic M, Morgenthaler NG, Knauer K, Bergmann A, Hampel H. Blood-Based Microcirculation Markers in Alzheimer's Disease-Diagnostic Value of Midregional Pro-atrial Natriuretic Peptide/C-terminal Endothelin-1 Precursor Fragment Ratio. Biol Psychiatry. 2009;65:979–984. doi: 10.1016/j.biopsych.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 18.Laske C, Leyhe T, Stransky E, Hoffmann N, Fallgatter AJ, Dietzsch J. Identification of a blood-based biomarker panel for classification of Alzheimer's disease. Int J Neuropsychopharmacol. 2011;14:1147–1155. doi: 10.1017/S1461145711000459. [DOI] [PubMed] [Google Scholar]
- 19.Nagele E, Han M, DeMarshall C, Belinka B, Nagele R. Diagnosis of Alzheimer's disease based on disease-specific autoantibody profiles in human sera. PLoS One. 2011;6:e23112. doi: 10.1371/journal.pone.0023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy MM, Wilson R, Wilson J, Connell S, Gocke A, Hynan L, German D, Kodadek T. Identification of candidate IgG biomarkers for Alzheimer's disease via combinatorial library screening. Cell. 2011;144:132–142. doi: 10.1016/j.cell.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Bryant SE, Xiao G, Barber R, Reisch J, Doody R, Fairchild T, Adams P, Waring S, Diaz-Arrastia R for the Texas Alzheimer's Research Consortium. A serum protein-based algorithm for the detection of Alzheimer disease. Arch Neurol. 2010;67:1077–1081. doi: 10.1001/archneurol.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Bryant SE, Xiao G, Barber R, Reisch J, Hall J, Cullum CM, Doody R, Fairchild T, Adams P, Wilhelmsen K, Diaz-Arrastia R for the Texas Alzheimer's Research and Care Consortium. A blood-based algorithm for the detection of Alzheimer's disease. Dement Geriatr Cogn Disord. 2011;32:55–62. doi: 10.1159/000330750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Bryant SE, Xiao G, Barber R, Huebinger R, Wilhelmsen K, Edwards M, Graff-Radford N, Doody R, Diaz-Arrastia R for the Texas Alzheimer's Research & Care Constortium and the Alzheimer's Disease Neuroimaging Initiative. A Blood-Based Screening Tool for Alzheimer's Disease That Spans Serum and Plasma: Findings from TARC and ADNI. PLoS One. 2011;6:e28092. doi: 10.1371/journal.pone.0028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, Younkin LH, Kuller L, Ayonayon HN, Ding J, Harris TB. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305:261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet. 2006;5:735–740. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 27.van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36:2637–2641. doi: 10.1161/01.STR.0000189721.31432.26. [DOI] [PubMed] [Google Scholar]
- 28.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: The Framingham study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 29.van Exel E, Eikelenboom P, Comijs H, Frolich M, Smit JH, Stek ML, Scheltens P, Eefsting JE, Westendorp RG. Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch Gen Psychiatry. 2009;66:1263–1270. doi: 10.1001/archgenpsychiatry.2009.146. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson K, Gustafson L, Hultberg B. C-reactive protein level is decreased in patients with Alzheimer's disease and related to cognitive function and survival time. Clin Biochem. 2011;44:1205–1208. doi: 10.1016/j.clinbiochem.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Laske C, Sopova K, Gkotsis C, Eschweiler GW, Straten G, Gawaz M, Leyhe T, Stellos K. Amyloid-Beta peptides in plasma and cognitive decline after 1 year follow-up in Alzheimer`s disease patients. J Alzheimers Dis. 2010;21:1263–1269. doi: 10.3233/jad-2010-100510. [DOI] [PubMed] [Google Scholar]
- 32.Laske C, Stellos K, Hoffmann N, Stransky E, Straten G, Eschweiler GW, Leyhe T. Higher BDNF serum levels predict slower cognitive decline in Alzheimer´s disease patients. Int J Neuropsychopharmacol. 2011;14:399–404. doi: 10.1017/S1461145710001008. [DOI] [PubMed] [Google Scholar]
- 33.Stellos K, Panagiota V, Kogel A, Leyhe T, Gawaz M, Laske C. Predictive value of platelet activation for the rate of cognitive decline in patients with dementia. J Cereb Blood Flow Metab. 2010;30:1817–1820. doi: 10.1038/jcbfm.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Bryant S. Using blood markers for Alzheimer's disease in clinical practice? Neurology. 2012;79:846–847. doi: 10.1212/WNL.0b013e318266fd21. [DOI] [PubMed] [Google Scholar]
- 35.Mayeux R, Reitz C, Brickman AM, Hann MN, Manly JJ, Glymour MM, Weiss CC, Yaffe K, Middleton L, Hendrie HC, Warren LH, Kayden KM, Welsh-Bohmer KA, Breitner JC, Morris JC. Operationalizing diagnostic criteria for Alzheimer's disease and other age-related cognitive impairment - Part 1. Alzheimers Dement. 2011;7:15–34. doi: 10.1016/j.jalz.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, Buxbaum JD, Ertekin-Taner N, Fallin MD, Friedland R, Inzelberg R, Kramer P, Rogaeva E, St George-Hyslop P, Cantewell LB, Dombroski BA, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Lunetta KL, Martin ER, Montine TJ, Goate AM, Blacker D, Tsuang DW, Beekly D, Cupples LA, Hakonarson H, Kukull W, Foroud TM, Haines J, Mayeux R, Farrer LA, Pericak-Vance MA, Schellenberg GD. Meta-analysis confirms CR1, CLU, and PICALM as Alzheimer disease risk loci and reveals interactions with ApoEε4 genotypes. Arch Neurol. 2010;67:1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertoli Avella AM, Marcheco Teruel B, Llibre Rodriguez JJ, Gomex Viera N, Borrajero Martinez I, Severijnen EA, Joosse M, van Duijn CM, Heredero Baute L, Heutink P. A novel presenilin 1 mutation (L174 M) in a large Cuban family with early onset Alzheimer disease. Neurogenetics. 2002;4:97–104. doi: 10.1007/s10048-002-0136-6. [DOI] [PubMed] [Google Scholar]
- 38.Ramos MI, Allen LH, Mungas DM, Jaqust WJ, Haan MN, Green R, Miller JW. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2005;82:1346–1352. doi: 10.1093/ajcn/82.6.1346. [DOI] [PubMed] [Google Scholar]
- 39.Haan MN, Miller JW, Aiello AE, Whitmer RA, Jaqust WJ, Mungas DM, Allen LH, Green R. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: Results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2007;85:511–517. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller JW, Green R, Ramos MI, Allen LH, Mungas DM, Jaqust WJ, Hann MN. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2003;78:441–447. doi: 10.1093/ajcn/78.3.441. [DOI] [PubMed] [Google Scholar]
- 41.Hu WT, Holtzman DM, Fagan AM, Shaw LM, Perrin R, Arnold SE, Grossman M, Xiong C, Craig-Schapiro R, Clark CM, Pickering E, Kuhn M, Chen Y, Van Deerlin VM, McCluskey L, Elman L, Karlawish J, Chen-Plotkin A, Hurtig HI, Siderowf A, Swenson F, Lee VM, Morris JC, Trojanowski JQ, Soares H for the Alzheimer's Disease Nueroimaing Initiative. Plasma multi-analyte profiling in mild cognitive impairment and Alzheimer's disease. Neurology. 2012;79:897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Bryant SE, Johnson L, Edwards M, Soares H, Devous MD, Ross S, Hall J for the Texas Alzheimer’s Research & Care Consortium. The link between C-Reactive protein and Alzheimer’s disease among Mexican Americans. Journal of Alzheimer’s Disease. doi: 10.3233/JAD-122071. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veeranna V, Zalawadiya SK, Niraj A, Kumar A, Ference B, Afonso L. Association of novel biomarkers with future cardiovascular events is influenced by ethnicity: Results from a multi-ethnic cohort. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 44.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 46.O'Bryant SE, Waring SC, Cullum CM, hall J, Lacritz L, Massman PJ, Lupo PJ, Reisch JS, Doody R for the Texas Alzheimer's Research Constorium. Staging dementia using clinical dementia rating scale sum of boxes scores: A Texas Alzheimer's research consortium study. Arch Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Bryant SE, Lacritz LH, Hall J, Waring SC, Chan W, Khodr ZG, Massman PJ, Jobson V, Cullum CM. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the National Alzheimer's Coordinating Center database. Arch Neurol. 2010;67:746–749. doi: 10.1001/archneurol.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivnik R, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurkland LT. Mayo's Older Americans Normative Studies: WAIS-R norms for age 56 to 97. Clin Neuropsychol. 1992;6:1–30. [Google Scholar]
- 50.Koch W, Ehrenhaft A, Griesser K, Pfeufer A, Muller J, Schomig A, Kastrati A. TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40:1123–1131. doi: 10.1515/CCLM.2002.197. [DOI] [PubMed] [Google Scholar]
- 51.R Development Core Team. R: A language and environment for statistical computing. 2009 http://www.R-project.org.
- 52.Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 53.Crean S, Ward A, Mercaldi CJ, Collins JM, Cook MN, Baker NL, Arrighi HM. Apolipoprotein E e4 prevalence in Alzheimer’s disease patients varies across global populations: A systematic literature review and meta-analysis. Dement Geriatr Cogn Disord. 2011;31:20–30. doi: 10.1159/000321984. [DOI] [PubMed] [Google Scholar]
- 54.Sundquist J, Winkleby MA, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988–1994. Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2001;49:109–116. doi: 10.1046/j.1532-5415.2001.49030.x. [DOI] [PubMed] [Google Scholar]
- 55.Black SA, Ray LA, Markides KS. The prevalence and health burden of self-reported diabetes in older Mexican Americans: findings from the Hispanic established populations for epidemiologic studies of the elderly. Am J Public Health. 1999;89:546–552. doi: 10.2105/ajph.89.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otiniano ME, Du XL, Maldonado MR, Ray L, Markides K. Effect of metabolic syndrome on heart attack and mortality in Mexican-American elderly persons: findings of 7-year follow-up from the Hispanic established population for the epidemiological study of the elderly. J Gerontol A Biol Sci Med Sci. 2005;60:466–470. doi: 10.1093/gerona/60.4.466. [DOI] [PubMed] [Google Scholar]
- 57.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 58.Bu L, Salto LM, De Leon KJ, De Leon M. Polymorphisms in fatty acid binding protein 5 show association with type 2 diabetes. Diabetes Res Clin Pract. 2011;92:82–91. doi: 10.1016/j.diabres.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damcott CM, Feingold E, Moffett SP, Barmada MM, Marshall JA, Hamman RF, Ferrell RE. Variation in the FABP2 promoter alters transcriptional activity and is associated with body composition and plasma lipid levels. Hum Genet. 2003;112:610–616. doi: 10.1007/s00439-003-0937-1. [DOI] [PubMed] [Google Scholar]
- 60.Vaidya D, Szklo M, Cushman M, Holvoet P, Polak J, Bahrami H, Jenny NS, Ouyang P. Association of endothelial and oxidative stress with metabolic syndrome and subclinical atherosclerosis: Multi-ethnic study of atherosclerosis. Eur J Clin Nutr. 2011;65:818–825. doi: 10.1038/ejcn.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho YM, Merchant CE, Kieffer TJ. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol Ther. 2012;135:247–278. doi: 10.1016/j.pharmthera.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Roth JD, Erickson MR, Chen S, Parkes DG. GLP-1R and amylin agonism in metabolic disease: Complementary mechanisms and future opportunities. Br J Pharmacol. 2012;166:121–136. doi: 10.1111/j.1476-5381.2011.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banerjee A, Onyuksel H. Human pancreatic polypeptide in a phospholipid-based micellar formulation. Pharm Res. 2012;29:1698–1711. doi: 10.1007/s11095-012-0718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman KE, Derdak J, Bernstein D, Reynolds JC, Avila NA, Gerber L, Steinberg SM, Chrousos G, Mackall CL, Mansky PJ. Metabolic syndrome traits in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer. 2008;50:341–346. doi: 10.1002/pbc.21363. [DOI] [PubMed] [Google Scholar]
- 65.Touskova V, Trachta P, Kavalkova P, Drapalova J, Haluzikova D, Mraz M, Lacinova Z, Marek J, Haluzik M. Serum concentrations and tissue expression of components of insulin-like growth factor-axis in females with type 2 diabetes mellitus and obesity: The influence of very-low-calorie diet. Mol Cell Endocrinol. 2012;361:172–178. doi: 10.1016/j.mce.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Pimentel GD, Micheletti TO, Pace F, Rosa JC, Santos RV, Lira FS. Gut-central nervous system axis is a target for nutritional therapies. Nutr J. 2012;11:22. doi: 10.1186/1475-2891-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azizi F, Moazezi Z, Hedayati M, Shirkhani Z. Glucose intolerance in subclinical hyperthyroid patients. Iran J Endocrinol Metab. 2012;14:4. [Google Scholar]
- 68.James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, Cutler P, Bock K, Boris M, Bradstreet JJ, Baker SM, Gaylor DW. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braff DL, Light GA. The use of neurophysiological endophenotypes to understand the genetic basis of schizophrenia. Dialogues Clin Neurosci. 2005;7:125–135. doi: 10.31887/DCNS.2005.7.2/dlbraff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doecke J, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, Mondal A, Bedo J, Busy AI, Brown B, De Ruyck K, Ellis KA, Fowler C, Gupta VB, Head R, Macaulay L, Pertile K, Rowe CC, Rembach A, Rodrigues M, Rumble R, Szoeke C, Taddei K, Taddei T, Trounson B, Aimes D, Masters CL, Martins RN. Blood-based protein biomarkers for the diagnosis of Alzheimer's disease. Arch Neurol. 2012;69:1–8. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]