Abstract
Short-term starvation (or fasting) protects normal cells, mice, and potentially humans from the harmful side effects of a variety of chemotherapy drugs. Here, we show that treatment with starvation conditions sensitized yeast cells (Saccharomyces cerevisiae) expressing the oncogene-like RAS2val19 to oxidative stress and 15 of 17 mammalian cancer cell lines to chemotherapeutic agents. Cycles of starvation were as effective as chemotherapeutic agents in delaying progression of different tumors and increased the effectiveness of these drugs against melanoma, glioma, and breast cancer cells. In mouse models of neuroblastoma, fasting cycles plus chemotherapy drugs—but not either treatment alone—resulted in long-term cancer-free survival. In 4T1 breast cancer cells, short-term starvation resulted in increased phosphorylation of the stress-sensitizing Akt and S6 kinases, increased oxidative stress, caspase-3 cleavage, DNA damage, and apoptosis. These studies suggest that multiple cycles of fasting promote differential stress sensitization in a wide range of tumors and could potentially replace or augment the efficacy of certain chemotherapy drugs in the treatment of various cancers.
INTRODUCTION
A 20 to 40% reduction in calorie intake or dietary restriction (DR) protects a wide variety of organisms against oxidative stress and aging (1-6). Because of this broad ability to promote stress resistance, DR could in theory be applied in the clinic to protect patients from toxic side effects of chemotherapy. However, DR is not feasible for patients already prone to weight loss from the cancer itself or from the chemotherapy, because, based on animal studies, several months may be necessary for patients undergoing DR to reach a protected state. Thus, in addition to requiring major life-style changes, DR would inevitably also cause chronic weight loss. Also, DR only retards the progression of specific cancers, possibly because of its relatively small effect on glucose and growth factors (7, 8). In humans, DR does not reduce growth-promoting insulin-like growth factor 1 (IGF-1) unless it is combined with protein restriction (9). Finally, it is not known whether DR would also protect cancer cells from chemotherapy.
By contrast, a limited exposure to a severely restricted diet (short-term starvation or fasting) can protect yeast, mammalian cells, mice, and possibly patients from the toxic effects of oxidative and chemotherapeutic agents without causing chronic weight loss (10-14). For example, fasting for 48 to 60 hours protected mice of three different genetic backgrounds from the chemotherapy drug etoposide (12). Fasting apparently protects normal cells by reallocating energy toward maintenance pathways from reproduction and growth processes when nutrients are scarce or absent (2, 10, 13, 15). This switch to a protected mode occurs only in normal cells, not cancer cells, because oncogenes prevent the activation of stress resistance. This feature of cancer cells thus provides a way to enhance cancer treatment by selectively increasing protection only in normal cells [differential stress resistance (DSR)] rather than by the more typical strategy of increasing the toxicity of drugs to cancer cells (10-12). DSR in mice and mammalian cells is mediated in part by the reduction of extracellular glucose and IGF-1 concentration and signaling (10-12, 16). Potentially harnessing DSR for clinical cancer therapy is attractive because fasting for 2 to 3 days before and 24 hours after chemotherapy is well tolerated by cancer patients receiving a variety of toxic treatments and may even reduce the common side effects caused by chemotherapy (12). Further, in mouse models, fasting protects against ischemia-reperfusion injury (17), and deprivation of a single amino acid results in both lower IGF-1 levels and protection against renal and hepatic ischemic injury (18).
The therapeutic potential of fasting would be even greater if it also increased the death of cancer cells. Here, we tested this possibility by studying the effect of fasting on cancer cell survival in the presence or absence of chemotherapeutic agents.
RESULTS
Starvation sensitizes yeast and cancer cells to toxins
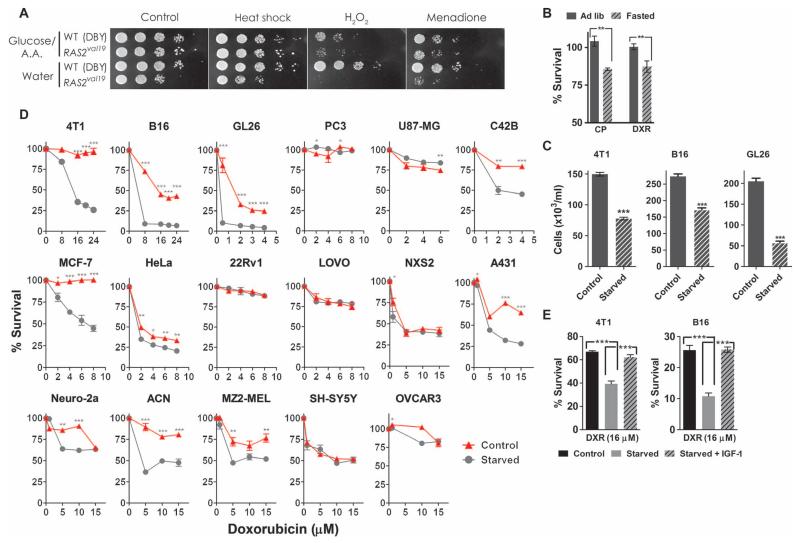
We have previously shown that, unlike wild-type cells, yeast cells expressing an oncogene-like constitutive active form of Ras (RAS2val19) are not protected from oxidative and chemotherapeutic agents by prior starvation (10, 11). We repeated these experiments by switching normal yeast cells from glucose medium containing amino acids and other essential nutrients to water (19, 20) for 24 hours before exposure to various toxic agents. The starvation (water) treatment increased the survival of wild-type cells under oxidative stress induced by hydrogen peroxide or menadione, both of which mimic the oxidation-dependent cytotoxicity of many chemotherapy drugs (Fig. 1A) (10, 11). In contrast, starvation sensitized yeast cells carrying the RAS2val19 mutation so that they were less able to withstand heat shock or oxidative stress (Fig. 1A). These data suggest that, in contrast to the protection afforded to normal cells, starvation increases the susceptibility of yeast cells expressing an oncogene-like protein to stress (7, 21).
Fig. 1.
Effect of short-term starvation on stress resistance and DXR sensitivity of cancer cell lines. (A) Effect of 24 hours of starvation before treatment on the survival of wild-type (WT) (DBY746) and yeast cells expressing constitutively active Ras (RAS2val19). Starvation was modeled by culturing nondividing yeast cells in water for 24 hours as described (19, 20). Tenfold serial dilutions of cells (from left to right) were spotted on culture plates and incubated at 30°C for 2 to 3 days. For heat shock resistance, cells were incubated at 55°C for 40 min. For oxidative stress resistance assays, cells were diluted to an absorbance at 600 nm of 1 in K-phosphate buffer (pH 6.0) and treated with 100 to 200 mM hydrogen peroxide (H2O2) for 60 min, or cells were treated with 250 mM menadione for 30 min in K-phosphate buffer (pH 7.4). (B) Effect of serum from fasted and ad lib-fed mice on survival of DXR- and CP-treated breast cancer cells (4T1) (n = 3). (C) Effect of starvation (0.5 g/liter, 1% FBS) on cellular proliferation. (D) Effect of starvation on DXR sensitivity of 17 different cancer cells in vitro (n = 3 to 6). Starvation was applied to cells 24 hours before and 24 hours during DXR treatment. Control groups were cultured in glucose (1.0 and 2.0 g/liter, for human and murine cells, respectively), supplemented with 10% FBS. Starved groups were cultured in glucose (0.5 g/liter) supplemented with 1% FBS. Survival was determined by MTT reduction. See the Supplementary Materials for additional data, including the effects of CP. (E) Effect of IGF-1 on starvation-dependent sensitization of cancer cells to DXR. Cells were treated with recombinant human IGF-1 (200 μM) during glucose restriction (0.5 g/liter versus 2.0 g/liter, under 1% FBS), followed by DXR (16 μM) treatment (n = 3). Data are from at least three independent experiments and shown as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test. Murine cells: 4T1, breast cancer; B16, melanoma; GL26, glioma; NXS2 and Neuro-2a, neuroblastoma. Human cells: PC3 and 22Rv1, prostate cancer; MCF-7 and C42B, breast cancer; U87-MG, glioblastoma; HeLa, cervical cancer; LOVO, colon cancer; ACN and SH-SY5Y, neuroblastoma; A431, epidermoid carcinoma; MZ2-MEL, melanoma; OVCAR3, ovarian cancer. See also figs. S1 to S4.
To test whether sensitization by short-term starvation may also occur in mammalian tumor cells, we incubated various cancer cell lines in medium containing serum collected from mice either fed ad lib or fasted for 48 hours. Murine breast cancer cells (4T1) cultured in medium supplemented with serum from fasted mice were more susceptible to the chemotherapeutic drugs doxorubicin (DXR) and cyclophosphamide (CP) compared to cells incubated in serum from mice fed ad lib (Fig. 1B). Because fasting greatly reduces the concentration of serum glucose and growth factors [for example, ~50% reduction in glucose levels and a 75% reduction in IGF-1 (11)], we incubated cells in concentrations of glucose mimicking those of normally fed and fasted mice (fig. S1), supplemented with either 10% or 1% serum, respectively, to also model the reduction in IGF-1 and other growth factors caused by fasting. Restricting glucose and growth factors in this way greatly retarded proliferation (Fig. 1C) and also increased cell death in three different murine cancer cell lines (figs. S2 and S4). The combination of glucose and serum restriction for 24 hours before and 24 hours after chemotherapy drug treatment in culture, to model the fasting and chemotherapy treatment in mice, sensitized 15 of 17 cancer cells lines to DXR and/or CP (Fig. 1D and figs. S2 and S3). These results indicate that short-term starvation can sensitize a broad range of cancer types to chemotherapeutic agents.
Our previous studies suggested that reduced serum levels of IGF-1 are important for the protective effects of fasting and showed that IGF-1 infusion can reverse the fasting-induced protection of mice from the side effects caused by high-dose chemotherapy (11). We therefore tested whether IGF-1, which inhibits apoptosis, can reverse the effects of starvation on the sensitization of 4T1 and B16 cells to DXR. We found that adding IGF-1 to starvation-treated 4T1 cells (incubated in medium containing reduced concentrations of growth factors, including IGF-1) reversed this sensitization (Fig. 1E), suggesting that IGF-1 can protect 4T1 breast cancer cells from chemotherapy. Thus, the decrease in IGF-1 levels caused by fasting may contribute to the sensitization of 4T1 cells to stress.
Fasting retards tumor growth and potentiates chemotherapy in vivo
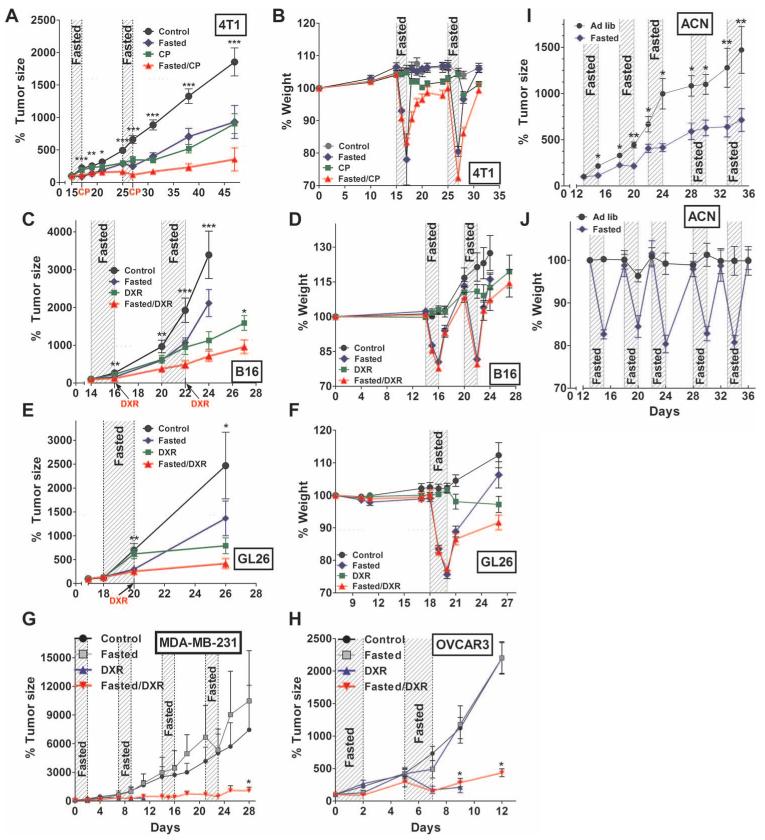
To explore whether our in vitro results apply to mice, we studied subcutaneous allografts of murine breast cancer (4T1), melanoma (B16), glioma (GL26), metastatic neuroblastoma models (NXS2, Neuro-2a), and xenografts of human neuroblastoma (ACN), breast cancer (MDA-MB-231), and ovarian cancer (OVCAR3) cell lines. Fasting was achieved by complete food withdrawal for 48 to 60 hours while allowing mice access to water. In mice bearing subcutaneous breast cancer (4T1), two cycles of fasting alone (48 hours each) were as effective as two cycles of CP treatment (Fig. 2A and fig. S5A), yet the mice were able to regain normal weight within 5 days of refeeding (Fig. 2B). Similar effects were observed in mice bearing subcutaneous melanoma masses (B16cells) treated with DXR (Fig. 2, C and D, and fig. S5B) and also in mice bearing subcutaneous glioma masses (GL26 cells) treated with DXR (Fig. 2, E and F, and fig. S5C), although the growth of melanoma cells was not affected by the second cycle of fasting, indicating that cancer cells may in some cases acquire resistance to fasting alone.
Fig. 2.
Effect of fasting on the sensitivity of allograft and xenograft tumors to chemotherapeutic agents in mice. (A to F) Effect of fasting on tumor progression as percent of initial tumor size (A, C, and E) and body weight (B, D, and F) in breast tumors (4T1; n = 12) (A and B), melanoma (B16; n = 11) (C and D), and glioma (GL26; n = 8) (E and F). Fasting in the glioma model was applied only once due to the rapid tumor growth in the control (ad lib, no chemotherapy) group. (G) Effect of fasting on human breast cancer cells (MDA-MB-231) subcutaneously xenografted into nude mice. Four cycles of fasting (48 hours) and/or DXR were performed. Tumor measurements from mice that were fed ad lib and treated with DXR were terminated at day 11 due to death of all mice from DXR toxicity (n = 5). (H) Effect of fasting on human ovarian cancer cells (OVCAR3) subcutaneously xenografted into nude mice. Two cycles of fasting (48 hours) and/or DXR were performed. Tumor measurements from mice that were fed ad lib and treated with DXR were terminated at day 9 due to death of all mice from DXR toxicity (n = 5). In both xenograft models, fasted mice treated with DXR did not experience death from chemotherapy toxicity. *P < 0.05, Student’s t test, compared to control. (I and J) Effect of fasting alone (48 hours for five cycles) in nude mice on tumor progression of a xenografted human neuroblastoma cell line (ACN; n = 7) (I) and on body weight (J). One-way ANOVA with Tukey’s post test [Student’s t test for (C) day 27)]. *P < 0.05; **P < 0.01; ***P < 0.001. All data are means ± SEM. See also figs. S5 and S6.
The greatest therapeutic effect was observed when fasting was combined with chemotherapy drugs (DXR, 10 mg/kg, or CP, 150 mg/kg) (Fig. 2, A, C, and E). For 4T1 breast cancer, two fasting cycles combined with CP maintained tumor size at less than half of that in the CP treatment alone group, even 20 days after the last treatment (Fig. 2A). Similar effects were observed in subcutaneous melanoma and glioma models (Fig. 2, C and E). Prefasted mice receiving chemotherapy returned to their normal weight soon after the last fasting cycle (Fig. 2, B, D, and F). The growth of subcutaneous human breast cancer (MDA-MB-231) and ovarian cancer (OVCAR3) xenografts was also retarded by fasting but returned to a size similar to that in animals on the control diet after refeeding (Fig. 2, G and H). This post-fasting cancer growth may be the result of overfeeding and weight gain by prefasted mice given an ad lib diet. However, when fasting was combined with DXR, the progression of the human breast cancer tumors was markedly retarded and the tumor did not progress (Fig. 2, G and H). In agreement with our previous results, fasting also allowed multiple treatments with a dose of DXR that was lethal to all normally fed mice by day 14 (fig. S6).
To test the effect of fasting alone in a model of human metastatic cancer, we applied cycles of 48-hour fasting in immunocompromised nude mice subcutaneously injected with human ACN neuroblastoma cells (Fig. 2I). After 34 days and five fasting cycles, tumor size was less than half of that in normally fed mice (Fig. 2I). In agreement with the other tumor mouse models, animals were able to return to the normal weight after each cycle (Fig. 2J).
These results suggest that cycles of fasting can delay the growth of some cancer cell types, in some cases as effectively as chemotherapy drugs, but that the combination of fasting and chemotherapy cycles provides a more effective, consistent, and potent effect on a wide range of tumors.
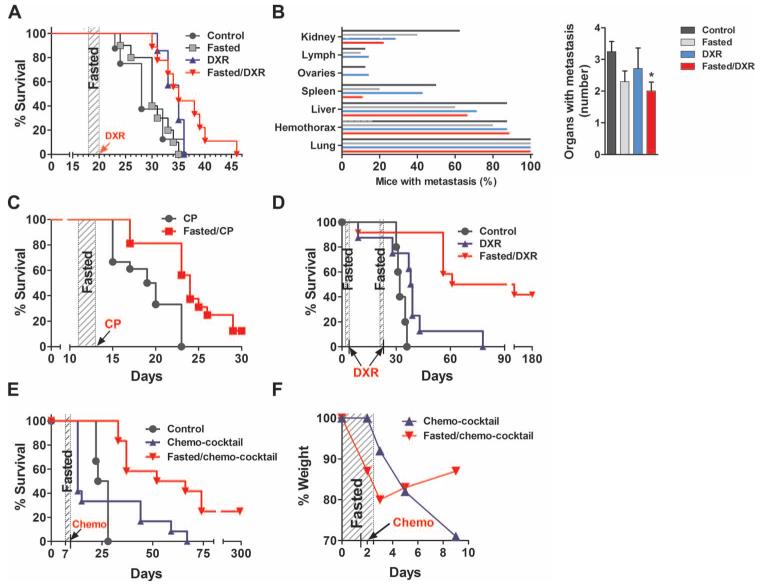
Fasting enhances cancer-free survival of mice with metastatic cancer receiving chemotherapy
Because advanced metastatic tumors are extremely difficult to cure once tumor masses have spread to different organs, we studied whether the combination of multiple fasting cycles and high-dose chemotherapy can increase survival in aggressive metastatic models before large tumor masses can be formed. To perform this experiment, we intravenously injected immunocompetent mice with murine breast cancer cells (4T1), melanoma cells (B16), and two neuroblastoma cell lines (NXS2 and Neuro-2a). Fasting potentiated the effects of chemotherapy and extended the survival of mice injected with each type of cancer cell (Fig. 3, A and C to E). In the model of metastatic melanoma, one cycle of DXR treatment alone was not sufficient to extend survival, and only mice that were both fasted and treated with DXR showed extended maximum survival time (Fig. 3A). The tumor load in these mice was estimated by bioluminescence imaging. By day 28, fasting alone caused a significant reduction in bioluminescence, which was further reduced in mice treated with both fasting and DXR (fig. S7, A and B). Body weight was rapidly recovered upon refeeding (fig. S7C). Fasting alone, but to a greater extent fasting combined with DXR, reduced the metastases of B16 melanoma cells to some organs compared to mice fed the standard diet (Fig. 3B). Lung metastases were detected in all mice, as expected, and served as a control for tumor seeding. Melanoma spleen metastases were found in ~40% of mice treated with DXR but only in 20% of mice that were fasted for a single cycle and 10% of mice that were fasted but also received DXR for a single cycle. In addition, unlike normally fed mice, metastases were not detected in the ovaries or lymph of fasted mice whether they received chemotherapy or not (Fig. 3B). Overall, mice fasted and treated with DXR had a 40% reduction in metastases compared to controls (Fig. 3B). In agreement with the results for melanoma cells, a single fasting cycle potentiated the effects of chemotherapy and extended survival in mice bearing metastatic breast tumor cells (4T1) (Fig. 3C).
Fig. 3.
Effect of fasting on survival and tumor load in metastatic mouse models of cancer treated with chemotherapeutic agents. (A and B) Effect of 48 hours of fasting on survival of DXR-treated mice with metastatic murine melanoma (B16; n = 9 to 10; P < 0.05) (A) and metastasis (left) and number of organs with metastases (right). (*P < 0.05 compared to control, Student’s t test; mean ± SEM) (B). (C) Effect of 48 hours of fasting on survival of CP-treated mice with metastatic murine breast cancer (4T1) (n = 16 to 18; P < 0.001). (D) Effect of 48 hours of fasting on survival of DXR-treated mice with metastatic murine neuroblastoma NXS2 (n = 5 to 12; P < 0.001). (E and F) Effect of 48 hours of fasting on survival (E) and weight loss (F) of mice with metastatic murine neuroblastoma Neuro-2a treated with a chemotherapy cocktail (n = 6 to 12; P = 0.005). Statistical significance for all survival curves was determined by log-rank test. See also fig. S7.
To more closely mimic cancer treatment in humans and to determine whether the combination of fasting cycles and chemotherapy has the potential to cure subjects with aggressive metastatic malignancies, we tested the effect of multiple cycles of fasting cycles in combination with chemotherapy drugs on a schedule that more closely reflects the therapy regimen administered to children with neuroblastoma. We monitored the survival of two mouse models of metastatic neuroblastoma. Long-term survival (more than 180 days) was achieved in 42% of murine neuroblastoma (NXS2)–bearing mice, which underwent two cycles of fasting with high-dose DXR (16 mg/kg) treatment (Fasted/DXR) (Fig. 3D), comparedtothe100% mortality in the group receiving chemotherapy under a normal diet (DXR). To test the effect of fasting on another model of metastatic neuroblastoma, we combined fasting with a cocktail of two chemotherapy drugs administered only once [DXR (10 mg/kg) + cisplatin (8 mg/kg)]. We injected murine neuroblastoma cells (Neuro-2a) intravenously into mice and allowed the tumor cells to spread for 9 days before initiating chemotherapy (Fig. 3E). Whereas all mice treated with the chemotherapy cocktail combined with an ad lib diet died by day 75, ~25% of the mice that were fasted in addition to receiving the chemotherapy cocktail achieved long-term survival while maintaining a normal weight (Fig. 3, E and F). At 300 days, the mice remained cancer-free.
These results suggest that fasting cycles potently sensitize a wide variety of tumor cell types to several of the most widely used chemotherapy drugs. This fasting-dependent potentiation of chemotherapeutic action significantly extended overall survival time and allowed long-term cancer-free survival, even in mice with a large number of dispersed cancer cells.
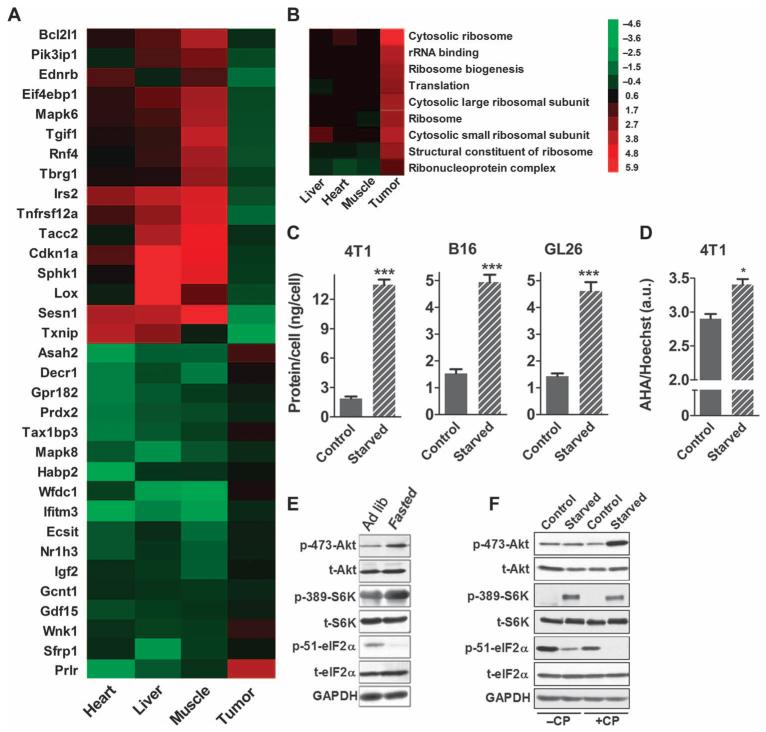
Fasting differentially regulates translation and proliferation genes
To identify the gene expression changes that may be regulating the differential resistance to chemotherapeutics in normal and cancer cells in response to fasting, we performed genome-wide microarray analyses on the liver, heart, skeletal muscle, and subcutaneous 4T1 breast tumor masses removed from mice that were either fasted for 48 hours or fed an ad lib diet. Fasting differentially regulated, in normal and cancer cells, many genes involved in cellular proliferation such as the insulin signaling adaptor (Irs2) and the mitogenic hormone prolactin receptor (Prlr) (Fig. 4A). In many cases, proliferation-associated genes were down-regulated in normal tissues but upregulated or unaffected in cancer cells. Further, the expression of translation and ribosome biogenesis/assembly genes such as elongation factor 1γ (Eef1g) and components of the 60S and 40S ribosomal proteins were significantly increased in response to fasting in breast cancer allografts (4T1), whereas in normal tissues they were either repressed or minimally affected (Fig. 4B and fig. S8). In agreement with these results, 4T1 cells cultured under starvation conditions for 48 hours displayed a major increase in cellular protein concentration (Fig. 4C) and in translation, as estimated by incorporation of the methionine analog azidohomoalanine (AHA) (Fig. 4D). In addition, the phosphorylation of Akt and S6 kinase (S6K), which regulate translation and proliferation, was elevated, and that of eukaryotic initiation factor 2α (eIF2α), which impairs protein synthesis, was reduced in tumors from fasted mice (Fig. 4E). Similar results were observed in 4T1 cells undergoing starvation in vitro, although Akt phosphorylation was not induced by starvation conditions alone but only by starvation plus CP treatment (Fig. 4F). Despite this starvation-dependent activation of translation control pathways, cancer cell number was greatly reduced in vitro by starvation conditions (Fig. 1C), a finding consistent with the retardation of tumor progression by fasting in vivo (Fig. 2, A, C, E, and G, and fig. S5).
Fig. 4.
Effect of fasting on genes involved in growth and proliferation. (A and B) Microarray analysis on the liver, heart, skeletal muscle, and subcutaneous breast tumors (4T1) from normally fed or fasted (48 hours) mice of cellular proliferation pathways (A) and translational machinery including ribosome assembly/biogenesis (B). (C) Effect of starvation on protein concentration in 4T1, B16, and GL26 cells (n = 3). (D) Effect of starvation on the rate of AHA (methionine analog) incorporation in 4T1 cells (n = 3). Data are means ± SEM [(C) and (D)]. (E and F) Effect of fasting on Akt, S6K, and eIF2α phosphorylation in murine breast cancer cells (4T1) in vivo (E) and in vitro (F). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test. See also fig. S8.
In agreement with our previous studies (10-12), these results indicate that fasting promotes differential regulation of pro-growth and pro-translation genes in normal and cancer cells.
Short-term starvation synergistically increases chemotherapy-induced DNA damage
To determine the mechanisms by which short-term starvation cycles reduce tumor progression, we tested the hypothesis that lower concentrations of extracellular glucose and growth factors may increase DNA damage in cancer cells. In both cultured 4T1 breast cancer cells (Fig. 5A) and B16 melanoma cells (Fig. 5B), the reduction of glucose concentrations from those in ad lib–fed mice (2.0 g/liter) to a concentration similar to that reached after fasting (0.5 g/liter), in combination with low serum [1% fetal bovine serum (FBS)] to mimic the fasting-dependent reduction in blood growth factors and proteins, increased DNA damage more than chemotherapy alone. Indeed, the combination of low glucose concentrations and treatment with a chemotherapeutic agent promoted a seemingly synergistic, 20-fold increase in DNA damage in both breast cancer and melanoma cells. In contrast, in GL26 glioma cells, the effect of reduced glucose concentrations on DNA damage was additive with that of the drug DXR (Fig. 5C).
Fig. 5.
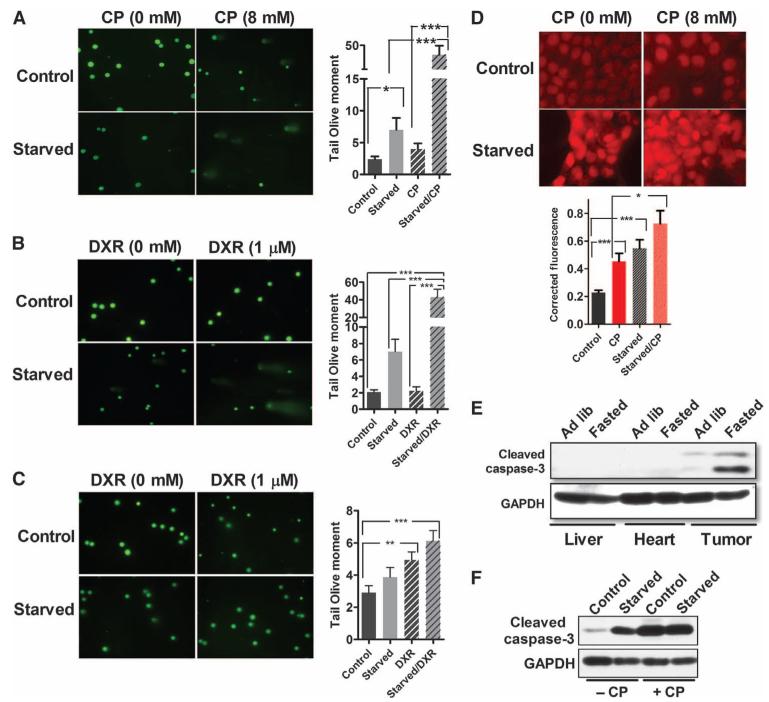
Effect of fasting on chemotherapy-induced DNA damage. (A to C) Effect of starvation alone and when combined with CP in breast cancer (4T1) (A) and with DXR in melanoma (B16) (B), and with DXR in glioma (GL26) (C) cells as determined by Comet assay (n = 6). The green signal represents intact and fractured DNA. Cells in both groups were cultured in normal glucose (2.0 g/liter) or low glucose (0.5 g/liter), respectively, supplemented with 1% FBS. Drugs were selected to match those in Fig. 2, A, C, and E. *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test. Data are from at least three independent experiments and shown as means ± SEM. (D) Effect of starvation on intracellular oxidative stress as estimated by a superoxide marker (DHE) in vitro. Corrected total cell fluorescence was quantified with ImageJ (NIH) and corrected for background fluorescence. *P < 0.05; ***P < 0.001, Student’s t test. (E and F) Effect of fasting on caspase-3 cleavage in allografted 4T1 breast tumors (E) and in 4T1 breast cancer cells cultured under starvation, that is, restricted glucose (0.5 g/liter) and growth factor (1% FBS) concentration (F). See also fig. S9.
These results indicate that starvation conditions and chemotherapy drugs can act in an additive or synergistic manner to promote DNA breaks in cancer cells. These effects may contribute to the chemotherapy-potentiating effects that we observed in vivo (Figs. 2 and 3).
Fasting-induced sensitization of 4T1 cells is associated with oxidative stress and caspase-3 activation
Because various chemotherapy drugs, including CP, damage DNA and cause cell death in part by promoting oxidative damage, we measured the level of reactive oxygen species (ROS), as indicated by dihydroethidium (DHE) oxidation, in 4T1 cells under standard and starvation conditions after treatment with CP. We detected more DHE oxidation in the cells after starvation, suggesting that starvation conditions promoted oxidative stress and possibly increased superoxide levels (Fig. 5D and fig. S9). CP alone increased DHE oxidation (2-fold), and treatment of the cells with both starvation and CP resulted in even higher DHE oxidation levels (3.2-fold) (Fig. 5D) compared to control. This increase in ROS may have contributed to the synergistic DNA damage caused by fasting and chemotherapy (Fig. 5, A to C). Because oxidative stress is a central promoter of apoptosis, we measured the effect of fasting on the activation of the proapoptotic enzyme caspase-3 both in vivo and in vitro. Cleaved caspase-3 was increased in allografted tumors but not in normal organs in response to fasting in mice (Fig. 5E) and also in cancer cells undergoing starvation in vitro, an effect that was potentiated by treatment with CP (Fig. 5F). Because caspase-3 was activated during fasting only in the transplanted tumors (Fig. 5, E and F), and because starvation conditions increased DNA damage, particularly in the presence of CP (Fig. 5A), we conclude that the retarded tumor growth by fasting (Figs. 1C and 2A) and the cell death caused by the combination of starvation and chemotherapy drugs may be at least partially attributed to increased apoptosis.
DISCUSSION
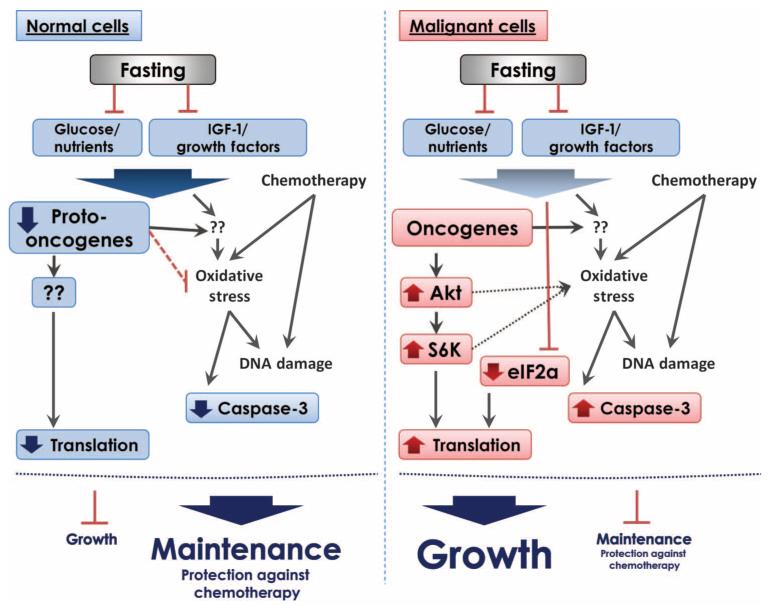
Most cancer drugs are being developed for specific tumor types, and in many cases, they are effective for only a subset of patients with a specific tumor. Furthermore, the development of each of these targeted cancer drugs is extremely costly, and assembling sufficient data for Food and Drug Administration approval requires years of clinical testing. Although there are undoubtedly targeted drugs that will be effective in the treatment of specific cancers, there would be advantages to therapies that could be available sooner, at a lower cost, and that could be effective for a wide range of cancers. Here, we have taken advantage of cancer cells’ relative independence from growth signals and unresponsiveness to anti-growth signals (22) to show that this inability of cancer cells to properly respond to extreme environments renders them unable to cope effectively with the markedly altered concentrations of glucose, growth factors, and other molecules caused by fasting (Fig. 6).
Fig. 6.
A model for fasting-dependent sensitization of tumor cells to chemotherapy. In response to fasting, glucose, IGF-1, and other pro-growth proteins/factors (including oncogenes) are reduced in the serum. Malignant cells respond to this reduction by activating Akt/S6K. Notably, S6K can also be activated independently of Akt via energy-sensing pathways such as AMPK-mTORC1 (34). These changes lead to an increase in oxidative stress, an increase in DNA damage, activation of caspase-3, and eventually cell death, particularly in the presence of chemotherapy.
Mutations that increase cellular protection and extend longevity often cause reduced growth and fertility (2). In cancer cells, mutations and epigenetic modifications that increase growth and promote insensitivity to anti-growth signals also impose a trade-off: the loss of the appropriate responses to rapidly adapt to a variety of extreme environments including starvation. For example, in our study, 4T1 tumor cells that were fasted appear to attempt to compensate for the lack of nutrients and growth factors by increasing translation and, as a result, may consume even more energy, eventually promoting oxidative stress and cell death. Translation is closely coupled with cell cycle progression and cell growth (23) and is a costly process that can consume up to 75% of the cellular energy in rapidly dividing cells (24, 25). It will be important to determine whether this increase in translation is associated with protein unfolding and endoplasmic reticulum stress.
Another factor responsible for the effect of fasting on the sensitization of 4T1 cells may be the increase in ROS, possibly caused in part by the hyperactivation of the Akt and target of rapamycin (TOR)-S6K pathways. In both yeast and higher eukaryotes, the activation of Akt and TOR-S6K pathways promotes oxidative stress and sensitizes cells to oxidative damage, in part by inactivating stress resistance transcription factors (26, 27). These pro-oxidation conditions caused by starvation may in turn promote the observed increase in DNA damage and apoptosis.
In addition to our recent case-series study indicating reduced side effects in cancer patients who have voluntarily fasted for 4 or more days in combination with chemotherapy (12), there are ongoing clinical trials testing the effects of shorter fasting periods (2 to 3 days) in combination with chemotherapy in cancer patients (NCT00936364, NCT01175837, and NCT01304251). Notably, both in one of the ongoing clinical trials and in the case studies, patients fasted (water only) for 48 to 72 hours before and also for 24 or more hours after chemotherapy. A period of fasting after chemotherapy treatment may be particularly important because chemotherapy drugs can cause DNA damage that could lead to secondary tumors and other toxicities. The combination of refeeding after fasting and treatment with potent carcinogens can enhance the growth of aberrant foci in liver, colon, and rectum (28, 29), probably because prolonged fasting can cause cell death and atrophy in organs such as the liver, and the cell proliferation induced by refeeding (to reestablish normal organ size) can lead to DNA damage if high concentrations of toxins are still present. Although chemotherapy drugs are not potent carcinogens and toxicity to multiple organs after fasting is reduced, not increased, even when refeeding is initiated immediately after chemotherapy (10-12), the length of the time period of fasting after chemotherapy should be based on the half-life of the chemotherapy drug to minimize toxicity to normal cells when refeeding begins. Further animal and clinical studies to investigate the effect of post-chemotherapy fasting are necessary.
In summary, the previously described fasting-dependent DSR of normal and cancer cells (10-12) and the tumor cell-specific sensitization to chemotherapeutic agents presented here (DSS) suggest that short-term starvation conditions or modified diets that promote similar changes has the potential to enhance standard cancer therapies. If confirmed in clinical trials, cycles of fasting could also provide an alternative to chemotherapy for early-stage cancer patients who may not be sufficiently at risk to receive chemotherapy or for patients with more advanced malignancies who receive chemotherapy and are at high risk for recurrence. In addition, fasting cycles in combination with chemotherapy could extend the survival of advanced-stage cancer patients by both retarding tumor progression and reducing side effects.
MATERIALS AND METHODS
Cell culture
4T1-luc murine breast cancer cells were purchased from SibTech. B16-fluc murine melanoma cells were provided by N. Craft [University of California, Los Angeles (UCLA)]. GL26 murine glioma and U87-MG human glioblastoma cells were provided by T. Chen [University of Southern California (USC)]. PC3 and 22Rv1 human prostate cancer cells were provided by P. Cohen (UCLA). MCF-7 and C42B human breast cancer cells and HeLa human cervical cancer cells were provided by A. Lee (USC). LOVO human colon cancer cells were provided by D. Shibata (USC). NXS2 and Neuro-2a murine neuroblastoma, ACN and SH-SY5Y human neuroblastoma, OVCAR3 human ovarian carcinoma, MZ2-MEL human melanoma, A431 human epidermoid carcinoma, and MDA-MB-231 human breast cancer cells were routinely cultured in the Laboratory of Oncology of Gaslini Institute. All cells were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) and 10% FBS at 37°C and 5% CO2.
Yeast strains used in this study are derivatives of the DBY746 (MATα leu2-3,112, his3Δ1 trp1-289a ura3-52 GAL+). The strain overexpressing RAS2val19 was generated by transforming wild-type DBY746 cells with pMW101 (pRS416 vector carrying the Cla I–ras2val19–Hind III fragment form pMF100, a gift from J. Broach, Princeton). Medium and growth conditions were as described (14).
Chemotherapy
DXR (Bedford Laboratories), CP (Sigma and Baxter), and cisplatin (APP Pharmaceuticals) were used in vitro and/or in vivo. In vitro chemotherapy was performed by treating cells in medium containing chemotherapy for 24 hours. Optimum drug doses were determined for each individual cell line. For in vivo studies, DXR was injected intravenously via lateral tail veins, and CP was injected intraperitoneally.
Mouse cancer models
All animal experiments were performed according to procedures approved by USC’s Institutional Animal Care and Use Committee, the licensing and ethical committee of the National Cancer Research Institute, Genoa, Italy, and the Italian Ministry of Health. To establish a subcutaneous cancer mouse model, we injected 12-week-old female BALB/c, 12-week-old female and male C57BL/6 mice, and 7-week-old nude mice with 4T1 breast cancer cells, B16 melanoma, and GL26 glioma cells, respectively. Five- to 7-week-old nude mice were injected with ACN human neuroblastoma cells, MDA-MB-231 human breast cancer cells, or OVCAR3 human ovarian carcinoma cells.
For metastatic mouse models of cancer, 12-week-old female BALB/c and 12-week-old female and male C57BL/6 mice were injected intravenously via lateral tail veins with 2 × 105 4T1 or B16 cells, respectively, and 6-week-old female A/J mice were injected via lateral tail veins with 2 × 105 NXS2 and 1 × 106 Neuro-2a cells. Before injection, cells in log phase of growth were harvested and suspended in phosphate-buffered saline (PBS) at 2 × 106 cells/ml, and 100 μl (2 × 105 cells per mouse) was injected subcutaneously in the lower back or intravenously via the lateral tail veins. ACN and Neuro-2a cells were suspended in PBS at a density of 5 × 107 and 1 × 107cells/ml, and 100 μl (5 × 106 ACN cells per mouse and 1 × 106 Neuro-2a cells per mouse) was injected subcutaneously in the lower back or intravenously via the lateral tail veins, respectively. All mice were shaved before subcutaneous tumor injection and were gently warmed before intravenous injections to dilate the veins. Body weights were determined periodically, and tumor size was measured with a digital vernier caliper. Tumor volume was calculated with the following equation: tumor volume (mm3) = (length × width × height) × π/6, where the length, width, and height are in millimeters.
In vitro starvation (short-term starvation)
Cellular fasting was done by glucose and/or serum restriction to achieve blood glucose levels typical of fasted and normally fed mice; the lower level approximated to 0.5 g/liter and the upper level to 2.0 g/liter. For human cell lines, normal glucose was considered to be 1.0 g/liter. Serum (FBS) was supplemented at 1% for starvation conditions. Cells were washed twice with PBS before changing to fasting medium.
In vivo fasting
Animals were fasted for a total of 48 to 60 hours by complete deprivation of food but with free access to water. Mice were individually housed in a clean new cage to reduce cannibalism, coprophagy, and residual chow. Body weight was measured immediately before and after fasting.
In vitro assays
Cytotoxicity was measured by the ability to reduce methylthiazolyldiphenyltetrazolium bromide (MTT). Briefly, MTT was prepared at 5 mg/ml in PBS, diluted to a final concentration of 0.5 mg/ml for assays, and incubated for 3 to 4 hours at 37°C. Formazan crystals were dissolved overnight (16 hours) at 37°C with 100 μl of lysis buffer [15% (w/v) SDS, 50% (v/v) dimethylformamide, pH 4.7]. Survival was presented as percentage of MTT reduction level of treated cells to control cells. Absorbance was read at 570 nm with the microplate reader SpectraMax 250 (Molecular Devices) and SoftMax Pro 3.0 software (Molecular Devices). To determine cellular proliferation, we seeded 50,000 cells and, immediately upon attachment, switched medium to starvation (0.5 g/liter, 1% FBS) or control (2.0 g/liter, 10% FBS) conditions. Forty-eight hours later, cell number was assessed by trypan blue exclusion.
Superoxide levels were estimated by oxidation of the fluorescent dye DHE (Invitrogen). Cells were cultured on slides, treated, and washed twice with PBS before incubation with DHE (10 μM; in 0.1% dimethyl sulfoxide) for 30 min. Total cell fluorescence was quantified with ImageJ [National Institutes of Health (NIH)]. Corrected fluorescence was calculated with the following equation: integrated density × (area of selected cell × mean fluorescence of background readings).
AHA incorporation was measured and normalized against Hoechst 33342 values with the Click-iT AHA Alexa Fluor 488 Protein Synthesis HCS Assay (Invitrogen) following the manufacturer’s instructions.
Immunoblotting assay
Cells were rinsed once in ice-cold PBS and harvested in radioimmunoprecipitation assay (RIPA) lysis buffer containing protease inhibitors (Roche) and a cocktail of phosphatase inhibitors (Sigma). Tumor tissues were homogenized in RIPA lysis buffer supplemented with the same protease and phosphatase inhibitors. Proteins from total lysates were resolved by 8 to 12% SDS–polyacrylamide gel electrophoresis and analyzed by immunoblotting with antibodies for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Akt and phospho-Ser473 Akt, p70 S6K and phospho-Thr389 p70 S6K, and eIF2α and phospho-Ser51 eIF2α (1:1000 to 1:2000, Cell Signaling Technology).
Comet assay protocol
Cells were diluted to 105/ml in culture medium (DMEM/F12 with 10% FBS) and treated with 50 μM DXR for 1 hour at 37°C. Cells were then washed once with ice-cold PBS and subjected to CometAssay (Trevigen) according to the manufacturer’s recommended procedure. Comet images were acquired with a Nikon Eclipse TE300 fluorescence microscope and analyzed with the Comet Score software (TriTek Corp., version 1.5). Cells (100 to 300) were scored for each genotype per treatment group.
Blood collection and glucose measurements
Mice were anesthetized with 2% inhalant isoflurane, and blood was collected by left ventricular cardiac puncture. Blood was collected in tubes coated with K2-EDTA to process serum (BD). Blood glucose was measured with the Precision Xtra blood glucose monitoring system (Abbott Laboratories).
Microarray analysis
RNA from tissues was isolated according to the procedures described by the manufacturer with the RNeasy kit (Qiagen). Then, RNA was hybridized to BD-202-0202 chips from Illumina Beadchips. Raw data were subjected to Z normalization as described (30). Briefly, for each pathway under each pair of conditions, a Z score was computed as [Z (pathway) = (sm mu)*pow(m,0.5)/delta], where mu = mean Z score of all gene symbols on the microarray, delta = SD of Z scores of all gene symbols on the microarray, sm = mean Z score of gene symbols comprising one pathway present on the microarray, and m = number of gene symbols in a pathway present on the microarray. For each Z (pathway), a P value was also computed in JMP 6.0 to test for the significance of the Z score obtained. These tools are part of DIANE 1.0 (NIH). Parameterized significant analysis is finished according to the SAM protocol (31) with analysis of variance (ANOVA) filtering (ANOVA P < 0.05). Significant genes are selected for each pairwise comparison. Gene set enrichment was tested with the PAGE method as previously described (32). Figures were selected on the basis of the names and descriptions provided by Gene Ontology Database and Pathway Data Set (33). Further gene regulatory relation and canonic pathway analysis is done by the Ingenuity Pathway Analysis System (Ingenuity Systems). All raw data are available in the Gene Expression Omnibus database.
Supplementary Material
Acknowledgments
We thank P. Cohen (UCLA) for providing PC3 and 22Rv1 cells; A. Lee (USC) for providing MCF-7, HeLa, and C42B cells; T. Chen (USC) for providing GL26 and U87 cells; N. Craft (UCLA) for providing B16 cells; D. Shibata (USC) for providing LOVO cells; and W. Wood, E. Lehrmann, and Y. Zhang for microarray assistance. Funding: This study was funded in part by NIH/National Institute on Aging grants AG20642, AG025135, and P01 AG034906; Ted Bakewell (The Bakewell Foundation); the V Foundation for Cancer Research; and a USC Norris Cancer Center pilot grant to V.D.L. L.R. is a recipient of a My First Associazione Italiana per la Ricerca sul Cancro grant, and G.B. is a recipient of a Fondazione Italiana Ricerca sul Cancro fellowship.
Footnotes
Author contributions: C.L., L.R., and V.D.L. designed all experiments. C.L., L.R., S.B., F.M.S., A.M.-M., M.W., S.H., G.B., A.M., and L.E. performed the experiments. C.L., L.R., S.B., F.M.S., A.M., V.P., R.d.C., and V.D.L. analyzed the data. C.L., L.R., and V.D.L. wrote the paper.
Competing interests: V.D.L. has equity interest in L-Nutra, a company that develops medical food for use by cancer patients. The other authors declare that they have no conflicts of interest.
REFERENCES
- 1.Longo VD, Fontana L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol. Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longo VD, Finch CE. Evolutionary medicine: From dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 3.Walford RL, Harris SB, Weindruch R. Dietary restriction and aging: Historical phases, mechanisms and current directions. J. Nutr. 1987;117:1650–1654. doi: 10.1093/jn/117.10.1650. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: Implications for vascular aging. Circ. Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: From model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 8.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: A case series report. Aging. 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell Biol. 1997;137:1581–1588. doi: 10.1083/jcb.137.7.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Müller C, de Jong M, van Ijcken W, Ijzermans JN, Hoeijmakers JH, de Bruin RW. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci. Transl. Med. 2012;4:118ra11. doi: 10.1126/scitranslmed.3002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabrizio P, Hoon S, Shamalnasab M, Galbani A, Wei M, Giaever G, Nislow C, Longo VD. Genome-wide screen in Saccharomyces cerevisiae identifies vacuolar protein sorting, autophagy, biosynthetic, and tRNA methylation genes involved in life span regulation. PLoS Genet. 2010;6:e1001024. doi: 10.1371/journal.pgen.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 21.Fontana L, Partridge L, Longo VD. Extending healthy life span—From yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Meric F, Hunt KK. Translation initiation in cancer: A novel target for therapy. Mol. Cancer Ther. 2002;1:971–979. [PubMed] [Google Scholar]
- 24.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 25.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 26.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 27.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Premoselli F, Sesca E, Binasco V, Caderni G, Tessitore L. Fasting/re-feeding before initiation enhances the growth of aberrant crypt foci induced by azoxymethane in rat colon and rectum. Int. J. Cancer. 1998;77:286–294. doi: 10.1002/(sici)1097-0215(19980717)77:2<286::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Laconi E, Tessitore L, Milia G, Yusuf A, Sarma DS, Todde P, Pani P. The enhancing effect of fasting/refeeding on the growth of nodules selectable by the resistant hepatocyte model in rat liver. Carcinogenesis. 1995;16:1865–1869. doi: 10.1093/carcin/16.8.1865. [DOI] [PubMed] [Google Scholar]
- 30.Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using Z score transformation. J. Mol. Diagn. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SY, Volsky DJ. PAGE: Parametric analysis of gene set enrichment. BMC Bioinformatics. 2005;6:144. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Guan KL. Amino acid signaling in TOR activation. Annu. Rev. Biochem. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.