Abstract
Background
ParkWest is a large Norwegian multicenter study of newly diagnosed drug-naïve subjects with Parkinson’s disease (PD). Cognitively normal PD subjects (PDCN) and PD subjects with mild cognitive impairment (PDMCI) from this cohort have significant hippocampal atrophy and ventricular enlargement compared to normal controls. Here we aimed to investigate whether the same structural changes are associated with CSF levels of Aβ38, Aβ40, Aβ42, total tau and phosphorylated tau.
Methods
We performed 3D radial distance analyses of the hippocampi and lateral ventricles using the MRI data from ParkWest subjects who provided CSF at baseline. Our sample consisted of 73 PDCN and 18 PDMCI subjects.
Results
We found significant associations between the levels of all three CSF Aβ analytes and t-tau and lateral ventricular enlargement in the pooled sample. In the PDCN sample all three amyloid analytesshowed significant associations with the radial distance of the occipital and frontal horns of the lateral ventricles. CSF Aβ38 and Aβ42 showed negative associations with enlargement in occipital and frontal horns of the lateral ventricles in the pooled sample, and a negative association with the occipital horns in PDMCI.
Conclusion
CSF Aβ levels in early PD correlate with ventricular enlargement, previously associated with PDD. CSF and MRI markers may therefore help identify PD patients at high risk for developing cognitive decline and dementia in the course of their illness. Contrary to Alzheimer’s disease, we found no associations between CSF t-tau and p-tau and hippocampal atrophy.
Keywords: Parkinson disease, Mild Cognitive Impairment, Magnetic Resonance Imaging, Cerebrospinal Fluid, Hippocampus, Lateral Ventricles
1. Introduction
Mild cognitive impairment (MCI) is often present in Parkinson’s disease (PD) patients at the time of diagnosis1. PD subjects with MCI (PDMCI) are about five times more likely to receive a diagnosis of dementia over the following four years.2. Pathologically, PD is characterized by progressive degeneration of dopaminergic neurons, Lewy bodies and Lewy neurites. Cortical Lewy bodies are characteristic for PD dementia (PDD)3. Diffuse cortical amyloid deposition has been reported post-mortem in PDD 4 suggesting that the interface between PD and Alzheimer’s disease (AD) needs to be more thoroughly investigated. Co-existing AD pathology promotes more rapid cognitive decline and early dementia in PD5.
Amyloid beta 1-42 (Aβ42), total tau (t-tau) and phosphorylated tau (p-tau) are the most established cerebrospinal fluid (CSF) biomarkers of AD. Low CSF Aβ42 and high CSF t-tau and p-tau levels have been reported in AD and in MCI subjects who later converted to AD6. Investigations into the levels of traditional CSF AD biomarkers in PD have been conflicting 7, 8, 9. Yet recent large-scale studies have reported reduced CSF levels of Aβ42 in both cognitively normal and cognitively impaired PD patients10, 11. We recently reported reduced CSF Aβ42, but normal CSF t-tau and p-tau levels in our newly diagnosed drug-naïve ParkWest PD cohort10.
CSF Aβ42, t-tau and p-tau levels have been linked to hippocampal atrophy and ventricular enlargement in AD 12, 13,14. Ventricular enlargement, hippocampal atrophy and white matter hyperintensities (WMH) are commonly seen in PD and have been associated with cognitive decline 15, 16, 17, 18. Previous ParkWest analyses showed larger ventricles but no hippocampal atrophy or greater WMH burden in PDMCI relative to PDCN 15,19,. The associations between white matter changes and CSF markers in the ParkWest sample is currently being investigated. A previously published ParkWest analysis failed to find an association between WMH and cognitive performance 19. However, as other research groups have reported potential associations between cognitive function and CSF markers in PD 20 and that white matter change may contribute to cognitive deficits associated with PD16, we have included WMH as a covariate in our analyses.
Whether CSF Aβ and tau markers are associated with ventricular enlargement and hippocampal atrophy in PD remains to be determined. The aim of this study was to investigate in a 3 dimensional (3D) model whether these MRI measures are associated with CSF levels of Aβ38, Aβ40, Aβ42, t-tau and p-tau in early, untreated PD patients with and without MCI.
2. Methods
2.1 Subjects
All subjects are participants of the ongoing Norwegian ParkWest study21. ParkWest - a population-based multi-center prospective longitudinal cohort study- aiming to improve our understanding of motor and non-motor PD progression and to develop promising biomarkers for PDMCI and PDD. Only newly diagnosed drug-naïve PD subjects meeting the Gelb clinical criteria for PD 22 were eligible to participate.
Diagnostic procedures included evaluation of medical history, standardized clinical, neuropsychiatric and neuropsychological examinations at study entry, radiological examination with 3-dimensional T1-weighted brain magnetic resonance imaging (MRI), dopamine transporter imaging when available (n=31), and repetated clinical assessments to evaluate drug response. Patients with atypical parkinsonism or dementia during the first year of motor onset were excluded, according to the revised DLB criteria by McKeith IG et al.23 The details of the diagnostic ascertainment are reported in Alves et al, 2009 24.
Of the 207 drug-naïve incident PD subjects who agreed to longitudinal participation, 91 provided CSF and MRI data at baseline. Seventy-three were cognitively intact (PDCN) while 18 met criteria for PDMCI. All participants signed informed consent. The study protocol was approved by the Regional Committee for Medical Research Ethics, Western Norway.
2.2 Clinical and neuropsychological examination
Severity of parkinsonian symptoms was assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS) 25 and the modified Hoehn and Yahr (H&Y) scale26. All PD subjects underwent neuropsychological assessments of memory, executive and visuospatial function 21, which consisted of the Mini-Mental State Examination scale (MMSE) 27, verbal memory assessment with the California Verbal Learning Test II 28, visuospatial assessment with the Silhouettes and Cube subtests from the Visual Object and the Space Perception Battery 29 and assessment of attention and executive functioning with a semantic verbal fluency test (animal fluency)30, the serial 7 test from the MMSE 27 and the Stroop test31.
Cognitive data for all PD subjects were converted to z-scores using the mean and standard deviations of an age-matched cognitively intact control group as previously described21,15. PD subjects with cognitive performance more than 1.5 standard deviations below the predicted by an age-, education-, and sex-corrected linear regression model in one or more cognitive domains were classified as having PDMCI.
2.3 Magnetic resonance imaging (MRI)
MRI was performed at four of the five study sites. 1.5 Tesla MRI scanner was used at three centers (Philips Intera, Best, The Netherlands at Stavanger and Haugesund, and Siemens Symphony, Erlangen, Germany, in Bergen). 1.0 Tesla Philips Intera system, (Best, The Netherlands) was used at Arendal.
The following protocols were used:
Stavanger: TR/TE 10.0/4.6 msec, flip angle 30.0 degrees, 1 mm slices with no gap, NEX 2, Matrix 256×256
Haugesund: TR/TE 20.0/4.6msec, flip angle 30.0 degrees, 1 mm slice thickness with no gap, NEX 1, Matrix 256×256
Bergen: TR/TE 2130.0/3.9msec, flip angle 15.0 degrees, 1 mm slice thickness with no gap, NEX 1, Matrix 256×256
Arendal: TR/TE 25/6.9msec, flip angle 30.0 degrees, 2 mm slice thickness with no gap, NEX 1, Matrix 256×256.
All baseline images were inspected by two neuroradialogists. Scans with large vessel cortical infarcts or other structural lesions that could result in parkinsonian symptoms (N=7) were excluded. 12 subjects with scan or movement artifacts and 5 subjects with baseline dementia were also excluded from the study.
2.4 Imaging analyses
The 3D T1-weighted MRI data were subjected to intensity32 and spatial normalization to the International Consortium for Brain Mapping ICBM53 brain atlas using a 9-parameter transformation (3 translations, 3 rotations, 3 scales)33. The hippocampal formations of a randomly selected ParkWest training data set were manually segmented on gapless coronal slices by one experienced rater (MKB) blinded to subjects’ age, sex, and diagnosis following a detailed, well-established protocol. The traces were closely inspected for accuracy by a second experienced hippocampal rater (LGA). The training sample composition was proportionate to the ratio of subject enrolment across the four imaging centers, to prevent as far as possible potential center bias in the statistical sampling. The hippocampal traces included hippocampus proper, dentate gyrus and subiculum.
2.4.1 Hippocampal segmentation
The hippocampi of the full dataset were segmented with our automated machine-learning hippocampal segmentation approach, based on statistical adaptive boosting34. Using the manually traced hippocampal training set, AdaBoost develops statistical rules for classifying each voxel in a new image as belonging to the hippocampus or not. Based on the feature information contained in the positive and negative voxels of the training dataset, AdaBoost developed a set of rules and computed the optimal combination and weighting of these features for accurate segmentation of unknown images34.
2.4.2 Ventricular segmentation
We employed a previously validated semi-automated ventricular segmentation approach (MAFIA)35. Briefly, a human rater (MKB) first traced the lateral ventricles of four subjects. These traces were converted into 3D parametric ventricular mesh models, termed atlases. Using fluid registration, each atlas was separately warped to match and thereby extract the shape of the lateral ventricle of each new subject’s scan. This resulted in four lateral ventricle segmentations per subject, which were then averaged to create one final ventricular model. Averaging four separate segmentations minimizes automated labeling errors that occur when only one atlas is used.
After converting the segmented hippocampi and lateral ventricles into 3D parametric meshes, we computed the medial core (a medial curve threading down the center of each structure) and the radial distance from the medial core to each surface point for each structure in each subject36. Radial distance provides an intuitive measure of the thickness of the structure from its core to each point on its boundary.
Analysis of white matter hyperintense lesion volumes:
Mask of white matter hyperintense lesions (WMH) were prepared using a semiautomated local threshold technique on axial FLAIR images as previously described 19. One single rater created the masks blinded to diagnosis and test results, with excellent reproducibility. Normalized volumes of WMH (to total brain volume) were used in the further analyses.
2.4.3 Inter-Scanner Reliability Analyses
In order to test scanner reliability we conducted human phantom scanning. Three cognitively normal volunteers were imaged twice on the same day on each scanner. The hippocampi were manually traced and volumes obtained as previously described. Whole brain volumes were obtained using Sienax37. We used PASW software (PASW for Windows Release 18.0.1) to obtain Cronbach’s Alpha reliability coefficients on whole brain and hippocampal volumetric measurements between scanning sites. Inter-site Cronbach’s Alpha for whole brain volume was 0.96 and for hippocampal volume was 0.97.
2.5 CSF collection and analyses
All lumbar punctures were performed between 7 and 10 am after overnight fast in order to minimize diurnal variation of the level of CSF Aβ. CSF levels of Aβ38, Aβ40 and Aβ42 were quantified by an Aβ triplex assay (Human Aβ peptide Ultra-Sensitive Kit, Meso Scale Discovery, Gaithersburg, Maryland, USA). CSF levels of t-tau and p-tau were analyzed using a commercial sandwich ELISA (INNOTEST® hTAU-Ag and INNOTEST® PHOSPHO-TAU(181P), Innogenetics, Gent, Belgium). CSF samples were randomized and run in duplicates, blinded for clinical diagnosis, according to the manufacturer’s instructions.
2.6 Apolipoprotein E analysis
DNA was extracted from 200 μl of EDTA-blood using the QIAamp 96 DNA Blood Kit (Qiagen, Hilden, Germany). Apolipoprotein E genotype was determined using the Light Cycler APOE Mutation Detection Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions.
2.7 Statistical methods
Demographic and clinical between-group comparisons were conducted with two-sample t-test using PASW software. Differences in sex and apolipoprotein E4 genotype (APOE4) distributions were assessed with a χ2 test. The presence of at least one APOE4 allele was coded as 1, the absence was coded as 0. Three PDCN with missing APOE4 data were coded as 0.5. Variables with non-normal distribution (i.e., MMSE) were analyzed using the Mann-Whitney U test. We used univariate statistics to examine for an effect of age, APOE4, WMH and scanning site on CSF analytes and ventricular volume in our sample.
Main analyses were conducted with linear regression. The models tested the associations between hippocampal and ventricular radial distance (or volume) and CSF variables while adjusting for scanning site, age, WMH and APOE4. Partial correlations between ventricular volumes and CSF variables while adjusting for age, scanning site, WMH and APOE4 were also sought.
For 3D map-wise multiple comparisons correction, we ran 100,000 permutations thresholded at p<0.01. After completion of these random permutations a final corrected p-value is computed indicating in what proportion of the iterations the experimental p-value beat the p-value of the original experiment.
3. Results
CSF Aβ, t-tau and p-tau were available for 91, 84 and 89 subjects respectively. There were no differences in age, education, motor subscale of UPDRS (UPDRSm) or H&Y, APOE4 genotype or MMSE between subjects who agreed or refused to undergo a lumbar puncture. A significantly greater proportion of men agreed to a lumbar puncture compared to women (68 % vs. 43%; p=0.015).
PDMCI subjects were on average older and had lower mean MMSE score than PDCN. There were no significant differences in CSF analyte levels between the diagnostic groups or between APOE4 carriers and noncarriers (Table 1). PDMCI subjects had significantly larger left and trend larger right ventricular volumes (left p=0.027, right p= 0.083) but comparable hippocampal volumes compared to PDCN (Table 1).
Table 1.
Comparison of demographic, clinical and CSF variables in PD controls and PDMCI (top) as well as ApoE4 carriers and noncarriers (bottom). Significant p-values (in bold) show group differences.
| Variable | PDMCI (N=18) | PDCN (N=73) | P-value |
|---|---|---|---|
| Sex (m/f) | 10/8 | 50/23 | 0.41 |
| Age, years | 71.7 (6.4) | 65.1(9.9) | 0.008 |
| Education mean, years | 11.4 (3.4) | 11.4(3.5) | 0.94 |
| APOE4 allele present, N (%) | 4 (22%) | 28 (38.4%) | 0.25 |
| PD side, R/L/both | 14/10/7 | 61/53/13 | 0.17 |
| UPDRS – motor score | 20.8 (9.0) | 20.7(10.3) | 0.72 |
| H&Y on state score | 1.9 (0.4) | 1.8(0.6) | 0.36 |
| MMSE | 26.7 (3.0) | 28.3 (1.8) | 0.035 |
| CSF Aβ38 pg/ml | 417.2 (262.9) | 467.5(277.3) | 0.43 |
| CSF Aβ40 pg/ml | 5545.9 (1422.6) | 5820.2(2214.0) | 0.84 |
| CSF Aβ42 pg/ml | 363.0 (169.4) | 363.3(208.2) | 0.7 |
| CSF t- tau pg/ml | 227.3 (84.6) | 221.2(127.5) | 0.26 |
| CSF p- tau pg/ml | 53.1(24.6) | 58.3(37.4) | 0.86 |
| Left hippocampus, mm3 | 4603 (909) | 4897 (866) | 0.21 |
| Right hippocampus, mm3 | 4654 (992) | 4868 (791) | 0.33 |
| Left frontal horn, mm3 | 15461 (1217) | 14939 (1316) | 0.13 |
| Left temporal horn, mm3 | 265 (63) | 237 (45) | 0.18 |
| Left occipital horn, mm3 | 6251 (954) | 5371 (1348) | 0.011 |
| Left whole ventricle, mm3 | 21976 (1588) | 20546 (2341) | 0.016 |
| Right frontal horn, mm3 | 17553 (2666) | 15821 (2989) | 0.027 |
| Right temporal horn, mm3 | 404 (66) | 392 (62) | 0.48 |
| Right occipital horn, mm3 | 3696 (1169) | 4089 (1050) | 0.17 |
| Right whole ventricle, mm3 | 21653 (2967) | 20302 (2919) | 0.083 |
| APOE4 carriers (N=32) | APOE4 noncarriers (N=59) | P-value | |
| CSF Aβ38 pg/ml | 483.5(255.5) | 433.3(267.8) | 0.240 |
| CSF Aβ40 pg/ml | 6082.1(2018.4) | 5550.3(2036.9) | 0.391 |
| CSF Aβ42 pg/ml | 350.1(195.7) | 358.4(181.8) | 0.842 |
| CSF t- tau pg/ml | 231.5(111.4) | 211.8(118.1) | 0.386 |
| CSF p- tau pg/ml | 61.4(33.6) | 54.6(36.4) | 0.275 |
Values denote means (SD). P values computed using two-sample t-test for continuous variables and a χ2 test for sex. For tests with categorical variables or non-normal distribution a Kruskal Wallis test with a Mann Whitney test was used.
CSF = cerebrospinal fluid; MMSE = Mini-Mental State Examination; UPDRS = Unified Parkinson’s disease Rating Scale. H&Y = Hoehn and Yahr score. Significant p-values are in bold.
3D univariate age, center, APOE4 and WMH statistical maps are provided in Figure 1. Only age and WMH showed significant association with ventricular radial distance (age left pcorrected=0.04, right pcorrected=0.0006; WMH left pcorrected=0.045, right pcorrected=0.053). Age was also correlated with hippocampal volume (left r=−0.17, p=0.019; right r=−0.36, p<0.001), Aβ40 (r=0.25, p=0.016), t-tau (r=0.44, p<0.001) and p-tau (r=0.23, p=0.03). WMH volume was correlated with hippocampal volume (left r=−0.38, p<0.001; right r=−0.24, p=0.03) and t-tau (r=0.4, p<0.001). One-way ANOVA showed no significant differences in mean CSF analyte levels between centers.
Figure 1.
3D significance (left panel) and correlation maps (right panel) showing the associations of ventricular radial distance with age, center, APOE4 and WMH in the pooled sample. Areas in red and white in the statistical maps show statistical significance (p<0.01).
It is worth noting that our results were not driven by outliers, that the regression standardized residuals had a normal distribution and that we excluded the possibility of multi-colinearity using the colinearity diagnostics option procedure recommended by Belsey and Kuh 38.
Multivariate analyses
3D regression analyses were conducted in the pooled sample and then separately in PDCN and PDMCI while controlling for age, APOE4 status, WMH and scanning site. We found no significant associations between any of our CSF analytes and hippocampal radial distance (maps not shown).
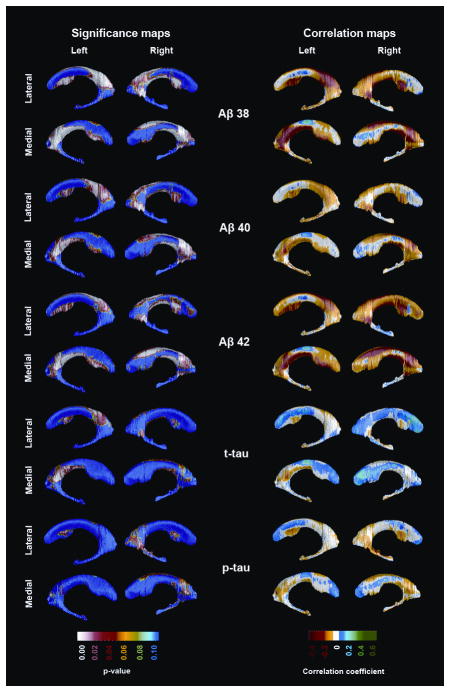
In contrast, we found significant associations with ventricular radial distance. In the pooled sample (Figure 2), all three amyloid measures showed significant negative associations with the occipital (Aβ38: left pcorrected=0.0023, right pcorrected=0.0034; Aβ40: left pcorrected=0.025, right pcorrected=0.043; Aβ42: left pcorrected=0.022, right pcorrected=0.01) and frontal horns (Aβ38: left pcorrected =0.0022, right pcorrected=0.0027; Aβ40: left pcorrected=0.016, right pcorrected=0.01; Aβ42: left pcorrected=0.0054, right pcorrected=0.0045). CSF t-tau showed significant negative associations with the left occipital (pcorrected=0.046) and frontal horns (pcorrected=0.034).
Figure 2.
3D significance (left panel) and correlation maps (right panel) of the associations between CSF Aβ and tau species and ventricular radial distance in the pooled sample. Areas in red and white in the statistical maps show statistical significance (p<0.01). All results are corrected for center, age and APOE.
In PDMCI only Aβ40 showed a trend level significant negative association with the right occipital horn enlargement (right pcorrected=0.063), (see Supplementary Figure 3).
In PDCN all three amyloid measuresshowed significant associations with the radial distance of the occipital and frontal horns of the lateral ventricles (occipital horn Aβ38: left pcorrected=0.01, right pcorrected=0.0088, Aβ40: left pcorrected=0.07, right pcorrected=0.029, Aβ42: left pcorrected=0.057, right pcorrected=0.046; frontal horn Aβ38: left pcorrected=0.0027, right pcorrected=0.0033, Aβ40: left pcorrected=0.059, right pcorrected=0.018, Aβ42: left pcorrected=0.015, right pcorrected=0.0052,(see Supplementary Figure 4). T-tau and p-tau showed no significant associations with the morphology of the lateral ventricles in PDCN.
We repeated the linear regression analyses with ventricular volumes as the dependent variable. In all cases the direction of the observed associations remained unchanged yet some of the effects became trend level or not significant (Supplementary Table 1). We also reversed the regression models taking CSF analytes as the dependent variables. Mean hippocampal volume was not a significant predictor of any CSF measure. Mean ventricular volume was significant predictor of CSF Aβ38 (p=0.03), Aβ40 (p=0.016) and Aβ42 (p=0.007). Finally, we repeated the analyses after removing all subjects scanned on the 1T MRI instrument. The results remained largely unchanged (Supplementary Table 2, Supplementary Figures 3–5).
4. Discussion
Using the baseline data from a large population-based prospective study of incident PD we found significant associations between several CSF Aβ species and lateral ventricular enlargement in PDCN and in the pooled sample of PD subjects. No significant correlation was detected between ventricular radial distance and CSF p-tau. T-tau showed significant associations with ventricular size only in the pooled sample. There were no significant associations between any CSF analyte and hippocampal radial distance.
Aβ42, Aβ40 and Aβ38 are the three most common Aβ peptides generated in the processing of amyloid precursor protein (APP)39. Aβ42 is particularly prone to aggregate and sequester in the form amyloid plaques in the human brain 40. Low CSF Aβ is reported in both AD and PD10. The significance of the other two abundant Aβ species - Aβ40 and Aβ38, on cognitive impairment in PD remains poorly understood. A recent study reported that CSF Aβ42/Aβ38 ratio as the measure that best discriminates between dementia with Lewy bodies and AD41. Ventricular expansion predicts future cognitive decline 42 and often coincides with low levels of CSF Aβ42 in cognitively normal persons13, 43. All three Aβ species were associated with memory impairment in our research cohort10. Taken together these data suggest a role of these CSF biomarkers for cognitive decline in PD.
We found a significant negative association between CSF t-tau with the left occipital and frontal horns in the pooled sample. CSF tau - a marker of axonal neuronal degeneration44, has been reported to be normal 10 or decreased in PDCN11. Yet increased CSF tau levels were evident once cognitive impairment was clinically manifest 9. As we follow the ParkWest cohort we will be able to investigate the development of late-occurring CSF tau changes and their relation to structural brain changes.
To our knowledge, this is the first study to investigate associations between hippocampal and lateral ventricle structural differences and neurodegenerative CSF biomarkers in PD. Our results support an association between CSF Aβ measures and structural brain changes in newly diagnosed drug-naive PD patients. The observed associations in the cognitively normal PD group agree with other reports 10, 45 and suggests that both the CSF- and structural brain changes develop prior to manifestation of cognitive decline. In the PDMCI group we found a trend level significant negative association with right occipital horn enlargement and Aβ40. Yet the PDMCI subjects were on average older than the PDCN subjects and although we adjusted for age one can not fully exclude some residual effect. Thus one must consider the possibility that the observed ventricular changes are also related to aging. Future studies and our longitudinal analyses of the ParkWest data will be well positioned to address this issue.
In this study we did not find a significant correlation between WMH volumes and CSF Aβ markers in PD. This is in contrast to e.g. findings of a reported inverse relationship between the volume of chronic white matter change and CSF Aβ markers in a study of subjects with subjective cognitive impairment and MCI 20.
We failed to find an association between brain white matter change and cognition in this baseline ParkWest cohort, who had generally little WMH load 19. Others have found a possible association between white matter change and PDD 17, 18, and an independent relation between WMH and cognitive impairment in PD 17. White matter change has also been associated with cognitive impairment in patients with stroke 46. A recent study showed that increased WMH was associated with worsened motor performance 47, and may contribute to cognitive deficits in PD 16. It remains to be seen if WMH will have more impact on cognition in the longitudinal follow up of our cohort, as has been suggested 16.
A strong association between presence of ApoE4 and AD exists48, and ApoE4 was higher in subjects with severe WMH after a lacunar infarct 49. We did not find significant differences between groups with or without cognitive impairment regarding presence of the ApoE4 allele in the Parkwest study 15 or in a 10-year longitudinal study50. This could be partly related to the low WMH burden found in our cohort and the fact that they had no infarcts. Another possible explanation is that ApoE4 does not act via vascular pathology in the PD population. In this study we found a lower percentage of ApoE4carriers in the cognitively impaired subjects (22%) than in the cognitively normal group (38%)(Table 1).
Thus it is possible that factors other than ApoE4 might drive neurodegeneration, leading to atrophy in early PD.
Our study has both strengths and limitations. A major strength is its longitudinal population-based prospective cohort design, and its focus on newly diagnosed PD. Our research subjects are well characterized. The use of state-of-the-art imaging analysis techniques to identify regionally specific disease associated changes, which are difficult to detect with more conventional analyses, is another strength of this study.
One limitation of our analyses is the small sample size of the PDMCI group with available CSF (N=18), which restricts our power to detect statistical significant correlations in this group. Without post-mortem brain examination the absence or concomitant AD pathology in our subjects cannot be ascertained. However, many consider low CSF Aβ42, a widely accepted amyloid marker in AD, suggestive of concomitant AD pathology. Post mortem pathologic examinations of the ParkWest cohort are planned.
Our data show that CSF amyloid pathology correlates with ventricular expansion in PD, even in the earliest disease stages. The significance of the structural and CSF findings in respect to PD progression and cognitive decline will be ascertained as we proceed with the longitudinal analyses of the rich ParkWest dataset. The time course and sequence of the development of clinical, biochemical, structural and neuropathologic abnormalities in PD warrants further investigation.
Supplementary Material
Acknowledgments
Study funding
Supported by NIH (AG16570), the National Institute of Biomedical Imaging and Bioengineering (EB 01651), the National Library of Medicine (LM05639) and the National Center for Research Resources (RR019771).
This work was supported by the Research Council of Norway [grant number 177966], the Western Norway Regional Health Authority [grant number 911218], and by the Norwegian Parkinson’s disease Association. This project was also supported by Research grants from Stavanger University Hospital and the Western Norway Regional Health Authority [grant number 911464].
Data analyses were supported by the U.S. National Institutes of Health [AG16570 to L.G.A.], the Easton Consortium for Alzheimer’s Drug Discovery and Biomarker Development [to L.G.A.], the National Institute of Biomedical Imaging and Bioengineering [EB01651 to P.M.T], the National Library of Medicine [LM05639 to P.M.T] and the National Center for Research Resources [RR019771 to P.M.T].
List of Abbreviations
- AD
Alzheimer’s disease
- Aβ38
Amyloid beta 1-38
- Aβ40
Amyloid beta 1-40
- Aβ42
Amyloid beta 1-42
- APOE
Apolipoprotein E
- CSF
cerebrospinal fluid
- MCI
mild cognitive impairment
- MMSE
mini mental state examination
- MRI
magnetic resonance imaging
- PD
Parkinson’s disease
- PDD
Parkinson’s disease and dementia
- PDMCI
Parkinson’s disease and mild cognitive impairment
- PDCN
Cognitively normal PD subjects
- p-tau
phosphorylated Tau
- t-tau
total Tau
- WMH
White matter hyperintensities
- DLB
Dementia with Lewy bodies
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
Documentation of author roles:
1. Research project
Conception: JP Larsen, OB Tysnes, G Alves, K Bronnick, MK Beyer, LG Apostolova
Organization: JP Larsen, OB Tysnes, G Alves, K Bronnick, MK Beyer, LG Apostolova
Execution: JP Larsen, OB Tysnes, G Alves, K Bronnick, MK Beyer, LG Apostolova, M Kurz
2. Statistical analyses:
Design: MK Beyer, LG Apostolova, KS Bronnick
Execution: MK Beyer, LG Apostolova, KS Bronnick, KS Hwang, S Babakchanian
Review and critique: all authors
Methods development: Yi-Yu Chou, Paul M. Thompson
Analyses: MK Beyer, LG Apostolova, KS Bronnick, KS Hwang, M Kurz, S Babakchanian, J Somme, TO Dalaker.
3. Manuscript preparation:
Writing of first draft: MK Beyer and LG Apostolova
Review and critique: all authors
Disclosure statement
Mona K. Beyer reports no conflicts of interest.
Guido Alves has received research support from GSK and the Norwegian Parkinson’s disease Association, and honoraria for presentations from H. Lundbeck A/S and Orion Pharma.
Kristy S. Hwang reports no conflicts of interest.
Sona Babakchanian reports no conflicts of interest.
Yi-Yu Chou reports no conflicts of interest.
Martin W. Kurz has received honoraria for presentations from Pfizer.
Jan Petter Larsen has served on scientific advisory boards for H. Lundbeck A/S and GSK.
Paul M. Thompson reports no conflicts of interest.
Kolbjorn Bronnick holds stocks in Dual Attention and has received honoraria for presentations from H. Lundbeck A/S and Solvay Pharma.
Johanne Somme reports no conflicts of interest.
Ole Bjorn Tysnes has received honoraria for lectures and support to participate in scientific meetings from several companies engaged in Parkinson’s disease.
Liana Apostolova reports no conflicts of interest.
Turi Olene Dalaker reports no conflicts of interest.
References
- 1.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 2.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. Epub 2006/05/25. [DOI] [PubMed] [Google Scholar]
- 3.Hurtig HI, Trojanowski JQ, Galvin J, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54:1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 4.Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 74:77–84. doi: 10.1212/WNL.0b013e3181c7da8e. Epub 2009/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliday GM, McCann H. The progression of pathology in Parkinson’s disease. Ann N Y Acad Sci. 2010;1184:188–195. doi: 10.1111/j.1749-6632.2009.05118.x. [DOI] [PubMed] [Google Scholar]
- 6.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. Jama. 2009;302:385–393. doi: 10.1001/jama.2009.1064. Epub 2009/07/23. [DOI] [PubMed] [Google Scholar]
- 7.Holmberg B, Johnels B, Blennow K, Rosengren L. Cerebrospinal fluid Abeta42 is reduced in multiple system atrophy but normal in Parkinson’s disease and progressive supranuclear palsy. Mov Disord. 2003;18:186–190. doi: 10.1002/mds.10321. Epub 2003/01/23. [DOI] [PubMed] [Google Scholar]
- 8.Mollenhauer B, Trenkwalder C. Neurochemical biomarkers in the differential diagnosis of movement disorders. Mov Disord. 2009;24:1411–1426. doi: 10.1002/mds.22510. [DOI] [PubMed] [Google Scholar]
- 9.Compta Y, Marti MJ, Ibarretxe-Bilbao N, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord. 2009;24:2203–2210. doi: 10.1002/mds.22594. Epub 2009/10/02. [DOI] [PubMed] [Google Scholar]
- 10.Alves G, Bronnick K, Aarsland D, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080–1086. doi: 10.1136/jnnp.2009.199950. Epub 2010/06/16. [DOI] [PubMed] [Google Scholar]
- 11.Montine TJ, Shi M, Quinn JF, et al. CSF Abeta(42) and tau in Parkinson’s disease with cognitive impairment. Mov Disord. 2010;25:2682–2685. doi: 10.1002/mds.23287. Epub 2010/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolova LG, Hwang KS, Andrawis JP, et al. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging. 2010;31:1284–1303. doi: 10.1016/j.neurobiolaging.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou YY, Lepore N, Saharan P, et al. Ventricular maps in 804 ADNI subjects: correlations with CSF biomarkers and clinical decline. Neurobiol Aging. 2010;31:1386–1400. doi: 10.1016/j.neurobiolaging.2010.05.001. Epub 2010/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. Epub 2009/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolova L, Alves G, Hwang KS, et al. Hippocampal and ventricular changes in Parkinson’s disease mild cognitive impairment. Neurobiol Aging. 2012;33:2113–2124. doi: 10.1016/j.neurobiolaging.2011.06.014. Epub 2011/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol. 2011;7:229–236. doi: 10.1038/nrneurol.2011.21. Epub 2011/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SJ, Kim JS, Yoo JY, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord. 2010;24:227–233. doi: 10.1097/WAD.0b013e3181d71a13. Epub 2010/05/18. [DOI] [PubMed] [Google Scholar]
- 18.Beyer MK, Aarsland D, Greve OJ, Larsen JP. Visual rating of white matter hyperintensities in Parkinson’s disease. Mov Disord. 2006;21:223–229. doi: 10.1002/mds.20704. Epub 2005/09/15. [DOI] [PubMed] [Google Scholar]
- 19.Dalaker TO, Larsen JP, Dwyer MG, et al. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson’s disease. NeuroImage. 2009;47:2083–2089. doi: 10.1016/j.neuroimage.2009.06.020. Epub 2009/06/23. [DOI] [PubMed] [Google Scholar]
- 20.Selnes P, Blennow K, Zetterberg H, et al. Effects of cerebrovascular disease on amyloid precursor protein metabolites in cerebrospinal fluid. Cerebrospinal Fluid Res. 2010;7:10. doi: 10.1186/1743-8454-7-10. Epub 2010/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. Epub 2008/11/21. [DOI] [PubMed] [Google Scholar]
- 22.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 23.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. Epub 2005/10/21. [DOI] [PubMed] [Google Scholar]
- 24.Alves G, Muller B, Herlofson K, et al. Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2009;80:851–857. doi: 10.1136/jnnp.2008.168211. Epub 2009/02/28. [DOI] [PubMed] [Google Scholar]
- 25.Fahn S, Elton RL. Unified Parkinson’s disease Rating Scale. In: Fahn S, Marsden CD, Calne DM, Lieberman A, editors. Recent development in Parkinson’s disease. Florham Park, NJ: MacMillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 26.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, Mc Hugh PR. “ Mini - mental State.” A practical method for grading the mental state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Delis DC, Kramer JHEK, Ober BA. Adult version. 2. San Antonio: The Psychological Corporation: Harcourt Assessment Inc; 2000. CVLT II. California Verbal Learning Test. [Google Scholar]
- 29.Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- 30.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test. Arch Neurol. 1978;35:364–367. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 31.Stroop JR. Studies in interference of serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- 32.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 33.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 34.Morra JH, Tu Z, Apostolova LG, Green AE, Toga AW, Thompson PM. Automatic subcortical segmentation using a contextual model. Med Image Comput Comput Assist Interv. 2008;11:194–201. doi: 10.1007/978-3-540-85988-8_24. Epub 2008/11/05. [DOI] [PubMed] [Google Scholar]
- 35.Chou YY, Lepore N, de Zubicaray GI, et al. Automated ventricular mapping with multi-atlas fluid image alignment reveals genetic effects in Alzheimer’s disease. Neuroimage. 2008;40:615–630. doi: 10.1016/j.neuroimage.2007.11.047. Epub 2008/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. Epub 2002/12/17. [DOI] [PubMed] [Google Scholar]
- 38.Belsey DA, Kuh E, Welsch R. Regression Diagnostics: Identifying Influential Data and Sources of Cillinearity. New York: Wiley; 1980. [Google Scholar]
- 39.Gabelle A, Roche S, Geny C, et al. Correlations between soluble alpha/beta forms of amyloid precursor protein and Abeta38, 40, and 42 in human cerebrospinal fluid. Brain Res. 1357:175–183. doi: 10.1016/j.brainres.2010.08.022. Epub 2010/08/18. [DOI] [PubMed] [Google Scholar]
- 40.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. Epub 2006/08/01. [DOI] [PubMed] [Google Scholar]
- 41.Mulugeta E, Londos E, Ballard C, et al. CSF amyloid beta38 as a novel diagnostic marker for dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2011;82:160–164. doi: 10.1136/jnnp.2009.199398. Epub 2010/11/05. [DOI] [PubMed] [Google Scholar]
- 42.Carlson NE, Moore MM, Dame A, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008;70:828–833. doi: 10.1212/01.wnl.0000280577.43413.d9. Epub 2007/11/30. [DOI] [PubMed] [Google Scholar]
- 43.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. Brain Atrophy in Healthy Aging Is Related to CSF Levels of A{beta}1-42. Cereb Cortex. 2010 doi: 10.1093/cercor/bhp279. Epub 2010/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tosun D, Schuff N, Truran-Sacrey D, et al. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiology of Aging. 2010;31:1340–1354. doi: 10.1016/j.neurobiolaging.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. Epub 2010/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong Y, Mok V, Wong A, et al. The age-related white matter changes scale correlates with cognitive impairment. Eur J Neurol. 2010;17:1451–1456. doi: 10.1111/j.1468-1331.2010.03078.x. Epub 2010/05/25. [DOI] [PubMed] [Google Scholar]
- 47.Bohnen NI, Muller ML, Zarzhevsky N, et al. Leucoaraiosis, nigrostriatal denervation and motor symptoms in Parkinson’s disease. Brain. 2011;134:2358–2365. doi: 10.1093/brain/awr139. Epub 2011/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wider C, Ross OA, Nishioka K, et al. An evaluation of the impact of MAPT, SNCA and APOE on the burden of Alzheimer’s and Lewy body pathology. J Neurol Neurosurg Psychiatry. 2012;83:424–429. doi: 10.1136/jnnp-2011-301413. Epub 2012/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen HM, Baum L, Cheung WS, et al. Apolipoprotein E epsilon4 allele is associated with the volume of white matter changes in patients with lacunar infarcts. Eur J Neurol. 2006;13:1216–1220. doi: 10.1111/j.1468-1331.2006.01436.x. Epub 2006/10/14. [DOI] [PubMed] [Google Scholar]
- 50.Kurz MW, Dekomien G, Nilsen OB, Larsen JP, Aarsland D, Alves G. APOE alleles in Parkinson disease and their relationship to cognitive decline: a population-based, longitudinal study. J Geriatr Psychiatry Neurol. 2009;22:166–170. doi: 10.1177/0891988709332945. Epub 2009/03/27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.