Abstract
Background & Aims
The endocannabinoid and eicosanoid lipid signaling pathways have important roles in inflammatory syndromes. Monoacylglycerol lipase (MAGL) links these pathways, hydrolyzing the endocannabinoid 2-arachidonoylglycerol to generate the arachidonic acid precursor pool for prostaglandin production. We investigated whether blocking MAGL protects against inflammation and damage from hepatic ischemia/reperfusion (I/R) and other insults.
Methods
We analyzed the effects of hepatic I/R in mice given the selective MAGL inhibitor JZL184, in Mgll−/− mice, FAAH−/− mice, and in Cnr1−/− and Cnr2−/− mice, which have disruptions in the cannabinoid receptors 1 and 2 (CB1/2). Liver tissues were collected and analyzed, along with cultured hepatocytes and Kupffer cells. We measured endocannabinoids, eicosanoids, and markers of inflammation, oxidative stress, and cell death using molecular biology, biochemistry, and mass spectrometry analyses.
Results
Wild-type mice given JZL184 and Mgll−/− mice were protected from hepatic I/R injury by a mechanism that involved increased endocannabinoid signaling via CB2 and reduced production of eicosanoids in the liver. JZL184 suppressed the inflammation and oxidative stress that mediate hepatic I/R injury. Hepatocytes were the major source of hepatic MAGL activity and endocannabinoid and eicosanoid production. JZL184 also protected from induction of liver injury by D-(+)-galactosamine and lipopolysaccharides or CCl4.
Conclusions
MAGL promotes hepatic injury via endocannabinoid and eicosanoid signaling; blockade of this pathway protects mice from liver injury. MAGL inhibitors might be developed to treat for conditions that expose the liver to oxidative stress and inflammatory damage.
Keywords: mouse model, endocannabinoid signaling, eicosanoid production, surgery
Introduction
Hepatic ischemia reperfusion (I/R) injury is a major cause of morbidity and mortality in patients undergoing liver surgery/resection and transplantation1, 2. Inflammation and generation of reactive oxygen and nitrogen stress in the liver underlie the hepatic cell death, dysfunction, and ultimate organ failure arising from hepatic I/R. Anti-inflammatory agents have thus been proposed as one treatment strategy for improving the clinical outcome of surgical procedures involving liver resections or transplant2. Anti-inflammatory drugs that block cyclooxygenases (COX1 and COX2) and reduce pro-inflammatory eicosanoids, as well as cannabinoid agonists that stimulate the anti-inflammatory cannabinoid receptor type 2 (CB2 or Cnr2), exert significant hepatoprotective effects in liver of rodents exposed to I/R3–5. Both COX1 and COX2-selective and dual COX1/COX2 inhibition or genetic ablation of COX2 have been shown to confer protection against hepatic damage caused by I/R by attenuating neutrophil recruitment and cell death in the liver 4, 6. Studies have also shown that vasoconstrictive eicosanoids such as thromboxane A2 (TXA2) induce hepatic damage through platelet aggregation, induction of leukocyte adhesion, and elevations in pro-inflammatory cytokines7. Previous studies have shown that hepatic I/R leads to significant elevations in endogenous ligands for the cannabinoid receptors (“endocannabinoids”) 2-arachidonoylglycerol (2-AG) and anandamide1. Anandamide is considered to be a partial or full agonist of cannabinoid 1 (CB1) receptors, depending on the tissue and biological response measured, and it has very low efficacy at CB2 receptors. In contrast, 2-AG is considered to be the natural ligand for CB2 receptors8. Consistent with an important role of endocannabinoids and CB2 signaling in protecting liver against ischemic injury, Cnr2−/− mice develop increased I/R-induced inflammation and liver damage, and CB2 agonists suppress hepatic pro-inflammatory cytokine and chemokine production, inflammatory cell infiltration, oxidative and nitrative stress8. In contrast, inhibition of CB1 is protective, suggesting an opposing regulatory role of this signaling in mediating liver injury9–13. Similar opposing regulatory roles of CB1/2 signaling have been recently described in models of atherosclerosis, kidney and brain injury, and hepatic fibrosis8.
We recently discovered that the endocannabinoid and eicosanoid systems are metabolically coupled through the action of monoacylglycerol lipase (MAGL or Mgll), which hydrolyzes 2-AG to produce the arachidonic acid (AA) precursor pools for eicosanoid biosynthesis 14. Blocking MAGL reduces eicosanoids and neuroinflammatory responses in the brain and protects against neurodegeneration14. It remains unknown whether MAGL also plays a protective role in peripheral organs and, if so, by what mechanism. Here, we hypothesized that MAGL blockade might provide protection against inflammation and damage inflicted by hepatic I/R through either enhancing endocannabinoid signaling or suppressing eicosanoid production, or a combination of both pathways.
Results
Hepatic I/R results in dysregulated endocannabinoid and eicosanoid metabolism
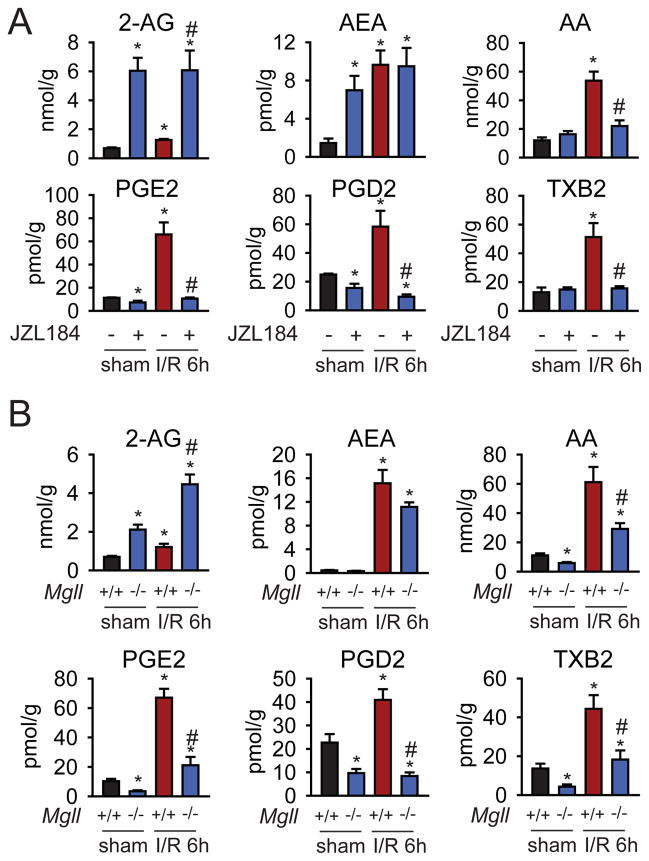
Consistent with our previous studies1, liver 2-AG and anandamide levels are substantially elevated 6 h post-I/R in mice, concomitant with higher levels of both AA and eicosanoids prostaglandin E2 (PGE2), PGD2, and thromboxane B2 (TXB2) (Fig. 1A). We find that pharmacological (selective MAGL inhibitor JZL184, 40 mg/kg, i.p.) or genetic (Mgll−/− mice) inactivation of MAGL further enhanced 2-AG levels and lowered the levels of AA and eicosanoids below basal levels in the liver 2 and 6 h post-I/R (Fig. 1A, B, Fig. S1). Inactivation of MAGL had no effect on early I/R-induced (I/R 2h) increased COX-2 mRNA expression, despite reductions in eicosanoids, suggesting that during early I/R MAGL blockade lowers eicosanoids likely by directly controlling the AA pool that generates eicosanoids in the liver (Fig. S2). However, hepatic COX-2 mRNA and protein expression were attenuated by MAGL blockade at 24 h of reperfusion (I/R 24h) (Fig. S2), suggesting that during later I/R MAGL blockade suppresses COX-2 expression which might also contribute to the reduced eicosanoids in the liver. The reductions in AA and eicosanoids were not blocked by treatment with CB1 or CB2 antagonists (Fig. S1), excluding cannabinoid-mediated mechanisms for suppressing eicosanoid synthesis. Although JZL184 raised basal anandamide (AEA) levels in mice with sham surgery, likely due to a partial blockade of the anandamide hydrolyzing enzyme, fatty acid amide hydrolase (FAAH)15, neither pharmacological nor genetic ablation of MAGL altered AEA levels in the liver 6 h post-I/R (Fig. 1A, B). These data thus indicate that the heightened levels of eicosanoids observed in hepatic I/R are largely derived from AA released by MAGL hydrolysis of 2-AG.
Figure 1. MAGL inactivation exerts bidirection al control over endocannabinoid and eicosanoid levels during hepatic I/R.
(A, B) Pharmacological (A) and genetic (B) inactivation of MAGL enhances 2-AG levels but does not affect the levels of the other endocannabinoid anandamide in the liver. MAGL blockade also lowers AA and eicosanoids in the I/R livers (A, B), measured after 6 h of reperfusion (peak injury). The potent and selective MAGL inhibitor JZL184 (40 mg/kg, i.p.) was given 1 h prior to inducing ischemia in the liver and endocannabinoid and eicosanoid levels were measured after 6 h of reperfusion after ischemia by SRM-based LC-MS/MS analysis. Data represent mean±sem of n=4–5 mice/group. Significance is represented as *p<0.05 between all groups versus sham vehicle-treated groups or sham Mgll+/+ groups; #p<0.05 between JZL184-treated or Mgll−/− I/R groups and the I/R vehicle-treated or Mgll+/+ groups.
MAGL inactivation attenuates hepatic I/R-induced tissue injury
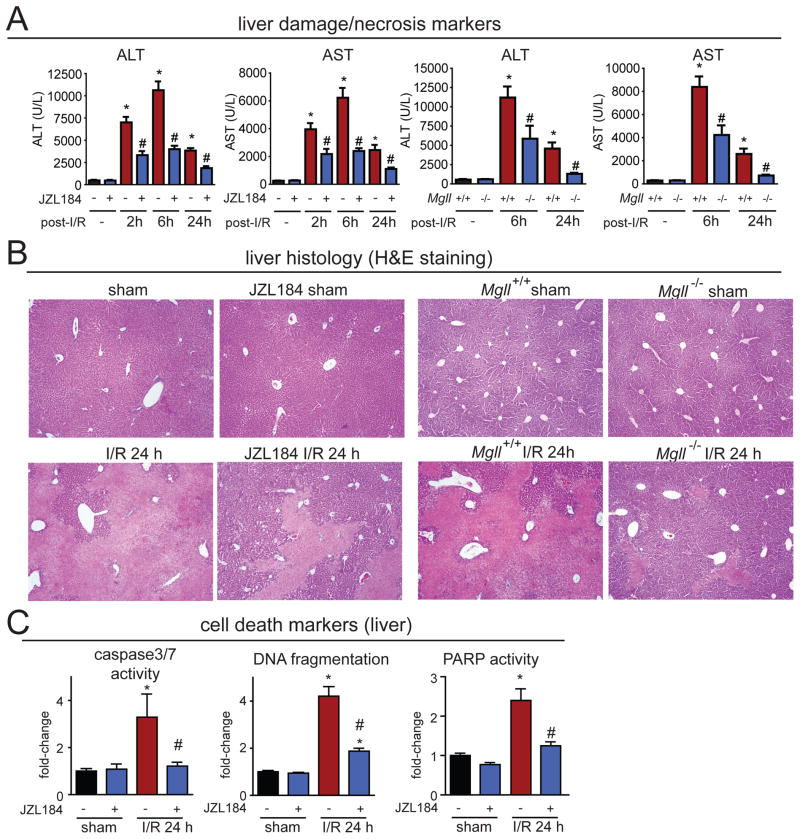
We next asked whether blocking MAGL protects the liver against hepatic I/R-induced cell death and damage. Both genetic and preventative pharmacological blockade (1 h prior to ischemia) of MAGL provided substantial hepatoprotection against I/R-induced liver injury, evidenced by attenuated serum levels of the acute liver damage/necrosis markers alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Figs. 2A, S3A), decreased coagulation necrosis seen in histological sections (Figs. 2B, S3B), as well as a decrease in delayed markers of apoptotic/necrotic cell demise (Figs. 2C, S4). These protective effects were not observed upon genetic or pharmacological inactivation of FAAH (Fig. S5).
Figure 2. MAGL inactivation attenuates hepatic I/R-induced tissue injury.
(A) Liver damage/necrosis markers ALT and AST are significantly elevated in mouse plasma upon I/R induction 2, 6, and 24 h after reperfusion, and both pharmacological (JZL184, 40 mg/kg, i.p.) and genetic (Mgll−/−) inactivation of MAGL substantially reduces these elevated levels. (B) Liver histology (H&E staining) of mice subjected to sham surgery or 1 h ischemia followed by 24 h of reperfusion (I/R 24h) in vehicle versus JZL184-treated (40 mg/kg, i.p., given prior to induction of ischemia) or in Mgll+/+ versus Mgll−/− mice, showing representative images of coagulation necrosis (lighter areas) subjected to I/R that is significantly attenuated upon pharmacological or genetic ablation of MAGL. (C) MAGL inhibition attenuates I/R-induced delayed cell death markers (caspase 3/7 activity, DNA fragmentation, PARP activity) after 24 h of reperfusion (I/R 24h). Data represent mean±sem of n=6–9 mice/group for (A) and 5–11 for (C). Significance is represented as *p<0.05 between vehicle-treated I/R groups and sham vehicle-treated groups, and #p<0.05 between JZL184-treated or Mgll−/− I/R groups and the corresponding I/R vehicle-treated or Mgll+/+ groups in (A); *p<0.05 between vehicle-treated I/R group and the sham groups, and #p<0.05 between JZL184 treated I/R groups and vehicle-treated I/R groups in (C).
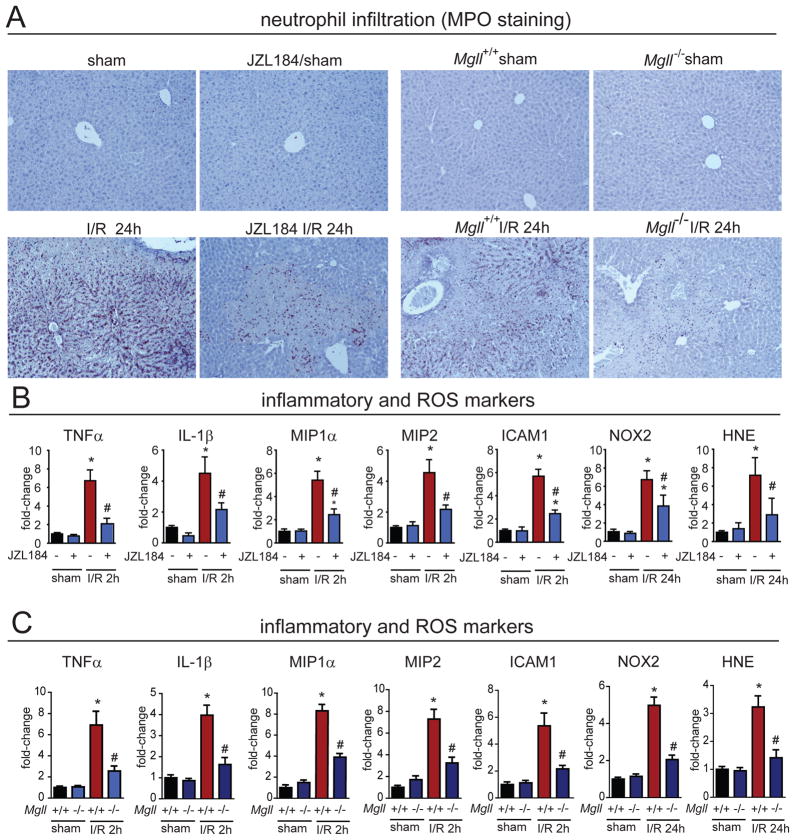
MAGL inactivation attenuates hepatic I/R-induced inflammation and oxidative stress
We next sought to investigate the pathophysiological mechanisms behind the hepatoprotective effect of MAGL inhibitors on I/R-induced liver injury. We found that MAGL inactivation significantly reduced inflammation, oxidative stress, and late apoptotic cell death (Figs. 2C, 3B, 3C, S4). Specifically, genetic and pharmacological inactivation of MAGL markedly attenuated the infiltration of neutrophils evidenced by substantially lower myeloperoxidase staining (MPO) (Figs. 3A, S4A). Pharmacological or genetic inactivation of MAGL also blocked I/R-induced acute early pro-inflammatory responses in cytokines tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β), chemokines macrophage inflammatory protein 1α and 2 (MIP-1α/CCL3 and MIP-2/CXCL2), and in hepatic expression of intercellular adhesion molecule 1 (ICAM-1) (Figs. 3B, 3C, S4). The delayed oxidative stress induced by I/R, as measured by the lipid peroxidation marker 4-hydroxynonenal (HNE) and reactive oxygen species generating NADPH oxidase isoform 2 (NOX2) expression, were also reduced in MAGL-inactivated mice (Figs. 3B, 3C, S4). Consistent with the hepatoprotection observed with both histological evaluation and biochemistry (serum ALT/AST levels), we found that MAGL inactivation reduced both apoptotic (caspase 3 and 7 activity and DNA fragmentation) and necrotic (poly(ADP-ribose) polymerase (PARP) activity) cell death markers (Figs. 2C, S4).
Figure 3. MAGL inactivation attenuates hepatic I/R-induced inflammation and oxidative stress.
(A) Both pharmacological and genetic blockade of MAGL causes massive delayed infiltration of neutrophils as assessed by MPO staining (brown staining) of livers after 24 h of reperfusion following induction of 1 h hepatic ischemia (I/R 24h). Representative images are shown. This neutrophil infiltration is significantly attenuated upon JZL184-treatment (40 mg/kg, i.p.) prior to ischemia or in Mgll−/− mice. (B) The I/R-induced early elevations in pro-inflammatory cytokines, chemokines TNF-α, IL-1β, MIP1-α, and MIP-2, and adhesion molecule ICAM-1 assessed after 2 h of reperfusion (I/R 2h) as well as the late oxidative stress markers NOX2 and HNE assessed after 24 h of reperfusion (I/R 24h), are significantly attenuated upon JZL184 treatment (40 mg/kg, i.p., prior to induction of ischemia) as well as in Mgll−/− I/R groups (C). Data represent mean±SEM of n=6–12 mice/group. Significance is represented as *p<0.05 between the indicated groups and the sham surgery vehicle-treated groups or sham Mgll+/+ groups; #p<0.05 versus the corresponding I/R vehicle-treated groups or Mgll+/+ groups.
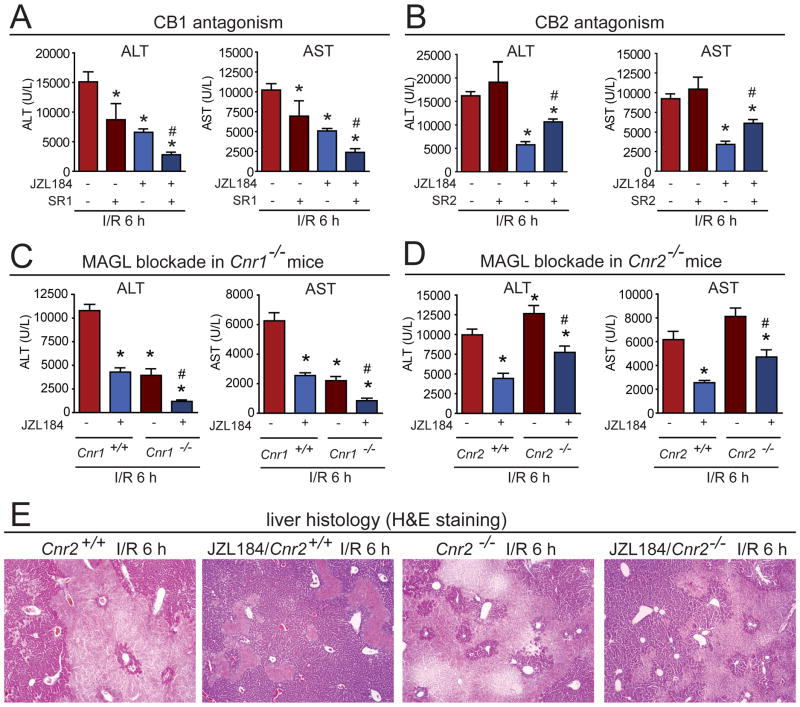
Hepatoprotective effects conferred by MAGL blockade are mediated partially by cannabinoid receptor type 2 (CB2R) but not receptor type 1 (CB1R)
We next tested whether the hepatoprotective effect induced by MAGL inactivation was due to heightened cannabinoid signaling, suppressed eicosanoid production, or a mixture of both mechanisms. Consistent with a partial contribution by endocannabinoids, we found that the decreased levels of ALT and AST in JZL184-treated mice subjected to I/R were significantly, but not completely reversed by the CB2R antagonist SR144528 (termed SR2), and were not attenuated by the cannabinoid receptor type 1 (CB1R or Cnr1) antagonist SR141716 (termed SR1) (Fig. 4A, B). As has been shown previously, SR1 treatment reduced ALT and AST levels when given alone16 and exerted additive hepatoprotective effects when administered with JZL184 (Fig. 4A). We observed similar results with JZL184 in Cnr1−/− and Cnr2−/− mice (Fig. 4C–E). Our results thus show that the protective effects of MAGL blockade in I/R-induced liver injury are partly, but not completely mediated by heightened endocannabinoid signaling acting on CB2 receptors. Considering the observed reductions in liver eicosanoids in MAGL-disrupted mice, combined with past evidence supporting a protective effect of COX inhibitors in liver injury models, we put forth that MAGL blockade likely reduces I/R-induced liver damage by a dual mechanism involving both heightened endocannabinoid signaling and reduced eicosanoid production.
Figure 4. Hepatoprotective effects conferred by MAGL are partially due to CB2, but not CB1 signaling.
(A, B) The effect of the CB1 and CB2 receptor antagonists SR1 (A) and SR2 (B) on ALT and AST levels after 6h of reperfusion (I/R 6h), respectively. SR1 (3 mg/kg, i.p.) reduces ALT and AST levels in I/R mice and this effect is additive when given in combination with JZL184 mice. SR2 (3 mg/kg, i.p.) partially reverses the JZL184-induced reductions in ALT and AST levels. (C, D) Pharmacological blockade of MAGL (JZL184, 40 mg/kg, i.p.) after 6 h of reperfusion (I/R 6h) exerts further decreases or attenuation of ALT and ASTlevels in Cnr1−/− (C) and Cnr2−/−(D) mice, respectively. (E) Liver histology (H&E staining) of Cnr2+/+or Cnr2−/− mice subjected to 1 h ischemia followed by 6 h of reperfusion (I/R 6h) or sham surgery in vehicle versus JZL184-treated (40 mg/kg, i.p., given prior to induction of ischemia) mice, showing an attenuated hepatoprotective response to JZL184 in Cnr2−/− I/R mice compared to JZL184-treated Cnr2+/+ mice. Data represent mean±sem of n=6–12 mice/group. Significance is represented as *p<0.05 between the indicated groups and vehicle-treated I/R group (A and B) or vehicle-treated Cnr1+/+or Cnr2+/+ I/R groups (C and D), and #p<0.05 between SR1 or SR2-treated JZL184-treated I/R groups (A and B) or JZL184-treated Cnr1−/−or Cnr2−/− I/R groups (C and D) versus the corresponding JZL184-treated I/R groups or vehicle-treated Cnr1−/−or Cnr2−/− I/R groups, respectively.
Cell type specificity of endocannabinoid and eicosanoid generation and signaling
We next wanted to delve deeper into the specific cell types responsible for generating the endocannabinoids and eicosanoids, and to identify the target cells of these lipid signals. We first found that MAGL activity was substantially higher in isolated hepatocytes compared to non-parenchymal cells (NPCs, Fig. S6A). I/R-induced liver injury significantly elevated the levels of 2-AG, AA, and eicosanoids in hepatocytes but not in NPCs, demonstrating that hepatic I/R promotes dysregulated endocannabinoid-eicosanoid metabolism primarily in hepatocytes (Fig. S6B). While blocking MAGL in vivo raised 2-AG levels in both hepatocytes and NPCs, reductions in AA and eicosanoids only occurred in hepatocytes (Fig. S6B–D).
To investigate which cell types 2-AG signals upon, we used flow cytometry and qPCR to demonstrate that CB2 receptors are expressed primarily on Kupffer cells, endothelial cells and neutrophils, but not on hepatocytes (Fig. S7). Consistent with this premise, we showed that MAGL blockade by JZL184, but not 2-AG, in isolated hepatocytes exposed to hypoxia-reoxygenation attenuated hepatocyte cell death as determined by reduced lactate dehydrogenase (LDH) and ALT release in vitro (Fig. S8). However, 2-AG treatment of isolated Kupffer cells caused a partially CB2-dependent reduction in TNFα levels in response to LPS stimulation (Fig. S9). In contrast, MAGL inhibition had no effect on LPS-induced TNFα release (data not shown). Collectively, our results indicate that both hepatocytes and non-parenchymal cells produce 2-AG that signals onto CB2 receptors on Kupffer cells, neutrophils, and endothelial cells, while eicosanoids are primarily generated by hepatocytes during hepatic I/R.
Inactivation of MAGL exerts hepatoprotective effects even when administered after reperfusion
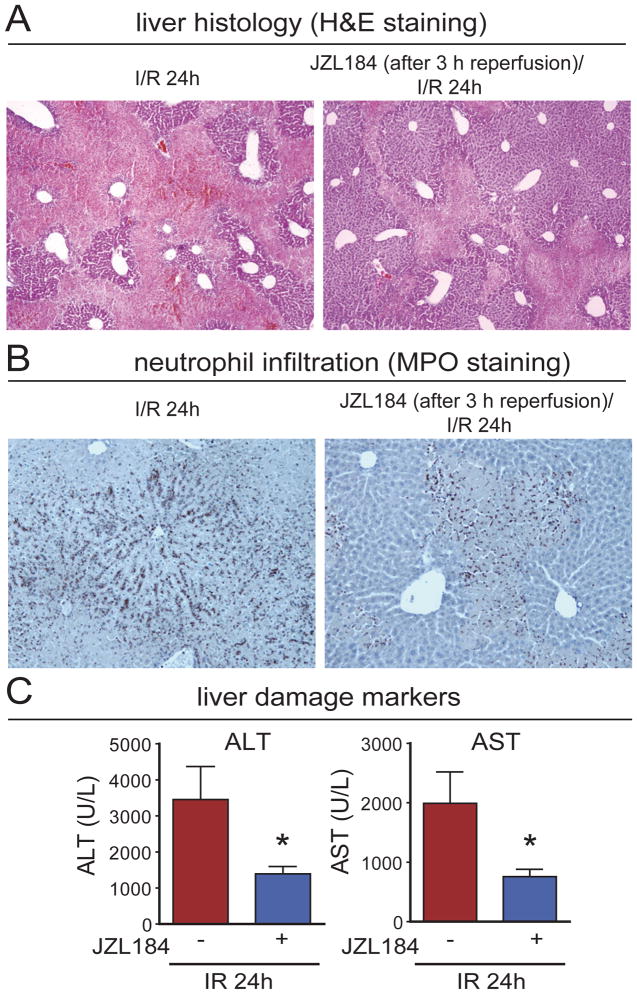
We further asked if pharmacological inhibition of MAGL is also protective when initiated after the induction of hepatic ischemia. Strikingly, we found that treatment with JZL184 (40 mg/kg, i.p.) even during the reperfusion period resulted in significant hepatoprotection when administered 1 and 3 h after induction of hepatic reperfusion (Figs. 5, S10, S11). These provocative results suggest that MAGL inhibitors can protect against hepatic I/R injury when administered not only before, but also after the liver is exposed to ischemic or hypoxic conditions.
Figure 5. MAGL inhibition exerts hepatoprotection even when given after reperfusion.
Coagulation necrosis (A), neutrophil infiltration (B), and liver damage markers ALT and AST (C) are reduced in hepatic I/R even when JZL184 is administered at 3 h of reperfusion. All parameters were measured at 24 h of reperfusion (24h I/R). Data represent mean±SEM of n=6 mice/group. *p<0.05 versus vehicle-treated I/R group.
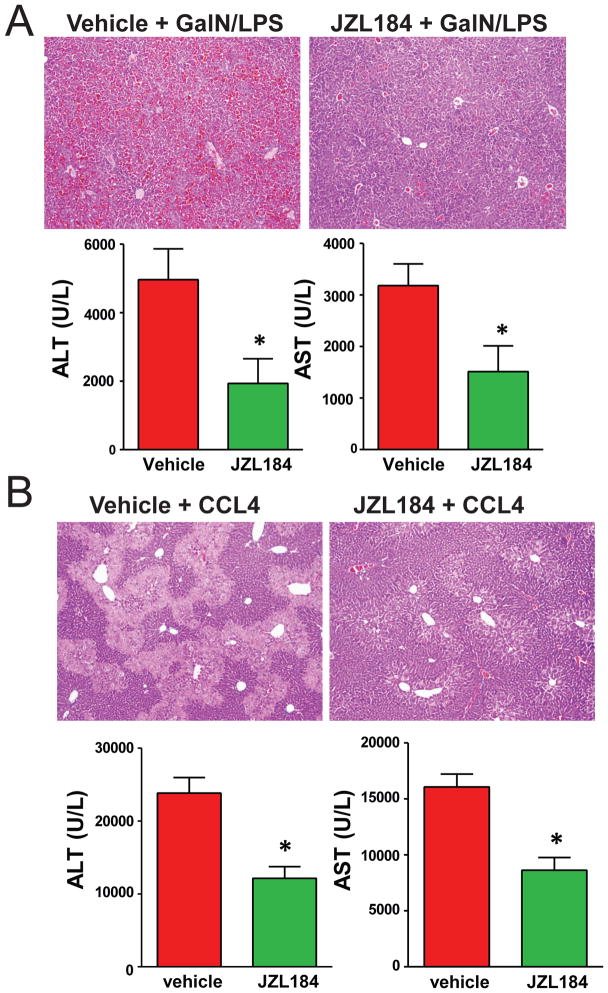
Inactivation of MAGL also protects liver damage in murine hepatitis models induced by GalN/LPS or CCl4
Finally, we tested whether inactivation of MAGL is also hepatoprotective in liver injury models caused by insults other that hepatic I/R. We found that MAGL blockade with JZL184 significantly protected mice against lethality caused by liver failure in the galactosamine (GalN) and LPS (GalN/LPS) model (83 % lethality in vehicle-treated compared to 42 % in JZL184-treated mice (p=0.04 by Fisher’s exact test)). In non-lethal experiments, JZL184 pretreatment significantly reduces GalN/LPS-induced liver damage (Fig. 6A). JZL184 pretreatment also effectively suppressed CCl4-induced hepatic injury (Fig. 6B). Consistent with liver I/R model, MAGL inactivation by JZL184 also heightened 2-AG signaling and attenuated the GalN/LPS and CCl4-induced enhanced hepatic eicosanoid levels and liver injury (Fig. S12). MAGL inactivation by JZL184 significantly attenuated hepatic COX-2 mRNA, but not protein levels induced by GalN/LPS or CCl4 (Fig. S13).
Figure 6. MAGL inactivation by JZL184 attenuates liver damage in acute murine liver injury models induced by GalN/LPS or CCl4.
Liver damage/necrosis markers ALT and AST are significantly elevated in mouse serum upon treatment with GalN/LPS for 7 h (A, lower panels) or CCl4 for 24 h (B, lower panels), and JZL184 (20 mg/kg, i.p.) pretreatment substantially reduces these elevated enzyme levels. Liver histology (H&E staining, original magnification 40×) showing representative images of liver tissue damage induced by GalN/LPS (A, upper panels) or CCl4 (B, upper panels), which is significantly attenuated upon MAGL inhibition by JZL184. *p<0.05 versus vehicle-treated control groups.
Discussion
There is increasing evidence suggesting that CB2 stimulation by pharmacological ligands may represent a promising treatment strategy for various liver diseases, as well as other disorders ranging from gastrointestinal, kidney, neurodegenerative, autoimmune diseases to pain and cancer8. Activation of CB2 signaling affords protection in hepatic I/R by attenuation of acute pro-inflammatory responses orchestrated by activated endothelial and Kupffer cells, as well as by inhibition of delayed neutrophil infiltration and neutrophil-mediated liver injury8. Pharmacological or genetic inactivation of COX2 has also been shown to protect the liver against injury by suppressing inflammation and hepatocyte cell death17,18. We show here that MAGL blockade exerts hepatoprotective effects in multiple liver injury models through coordinately enhancing endocannabinoid CB2 and lowering eicosanoid pathways thereby limiting neutrophil infiltration and neutrophil-mediated liver damage. MAGL thus serves as a critical metabolic node that simultaneously controls two important lipid signaling pathways that limit liver injury.
We also provide compelling evidence for the intricate cell-to-cell communications of endocannabinoid and eicosanoid signals that contribute to the hepatoprotective effects conferred by MAGL inactivation. We show that endocannabinoids are generated by both hepatocytes and NPCs whereas eicosanoids primarily arise from hepatocytes. Consistent with previous reports, we also show that CB2 receptors are not expressed on hepatocytes, but instead localized to NPCs including Kupffer cells8. These results are further corroborated by our in vitro experiments showing that, in contrast to the inability to protect hepatocytes from hypoxia-induced cell death, 2-AG pre-treatment significantly inhibits LPS-induced TNFα release from Kupffer cells. Our data are in agreement with a recently published study focusing on CB2-mediated Kupffer cell polarization in alcohol-induced liver injury, which showed that Kupffer cells from CB2−/− mice have enhanced LPS-stimulated TNFα induction whereas activation of CB2 by JWH-133 inhibited TNFα production in LPS-treated RAW264.7 cells19. We also find that MAGL blockade reduces hepatic eicosanoid levels as early as 2 h after reperfusion, before the infiltration of inflammatory cells into the liver, without any concordant changes in COX2 expression. These results show that the eicosanoid lowering effects of MAGL blockade are likely due to reductions in the AA pool that generates eicosanoids rather than an indirect effect on COX2 expression. Secondly, these results suggest that the initial hepatoprotective effect is likely through reducing eicosanoids rather than enhancing endocannabinoids since the inflammatory immune cells that express CB2 are not yet present 2 h after reperfusion. These results are consistent with literature precedence showing anti-inflammatory effect of CB2 signaling in various immune and activated endothelial cells during I/R8. The above mentioned is also consistent with our results (not shown) that the high-mobility group proteinB1, released upon early hepatocellular necrosis during I/R to activate Kupffer and other inflammatory cells (e.g. neutrophils) through toll-like receptors, was also substantially reduced during I/R upon JZL184 treatment at an early time (2h) of reperfusion (before neutrophil infiltration) coinciding with reduction of hepatic eicosanoid levels. However, we acknowledge that the interpretation derived from our cell-type specificity studies may be confounded by the procedures involved in isolating the individual cell-types.
While previous reports have demonstrated that some eicosanoids such as prostaglandin E2 and prostacyclin analogs may be anti-inflammatory during liver inflammation given exogenously, lowering eicosanoids broadly by either genetic or pharmacological blockade of COX2 have also been shown to be hepatoprotective in multiple other studies. We cannot rule out the possibility of other mechanisms involved in our hepatoprotective effects observed, such as contribution of prostaglandin glyceryl esters or other potential biological active monoacylglycerols20, which may also modulate hepatic COX2 expression induced by various insults20. However, we believe that broadly lowering eicosanoids by MAGL inactivation causes an overall net anti-inflammatory eicosanoid environment, much like that with COX inhibition, that is responsible, at least in part, for our hepatoprotective phenotypes in addition to heightened endocannabinoid signaling. We also cannot exclude the possibility that these eicosanoids which have been shown to be both anti and pro-inflammatory exert their biological effects in cell-type, tissue-type, and context-dependent manners.
One potential advantage of MAGL inhibitors over dual COX1/COX2 inhibitors and COX2-selective inhibitors is that MAGL only controls eicosanoid metabolism in specific tissues such as the brain, liver, and lung, but not, for example, in the gut14. Thus, MAGL blockade could avoid some of the mechanism-based gastrointestinal and cardiovascular side-effects21 associated with COX inhibition, and may even protect against COX inhibitor-induced gastrointestinal injury through endocannabinoid-dependent mechanisms14, 22. Furthermore, because MAGL controls the AA precursor pools for general eicosanoid biosynthesis, MAGL inhibitors may have broader effects that extend beyond COX-mediated pathways (e.g. CYP450-generated 5,6-EET). While previous studies have shown that chronic and complete inhibition of MAGL results in desensitization of CB1 signaling in the nervous system23, 24, we show here that CB2-mediated hepatoprotective effects are still maintained in MAGL−/− mice, indicating that immune cell CB2 function does not become desensitized under chronic MAGL ablation. While the studies described here all employ acute liver injury models, it will be important to consider any potential adverse effects that may arise from brain CB1 desensitization in any future therapies that require chronic MAGL inhibitor dosing.
In contrast to CB2 signaling, which attenuates hepatic injury, fibrosis and promotes regeneration5, 19, 25, CB1 signaling contributes to increased damage and fibrosis in multiple liver pathologies where CB1 inhibition is protective 11, 13. Previous studies have also demonstrated hepatoprotective effects of CB1 antagonists in I/R9, 16. In this study, we show that MAGL inhibitors, similarly to direct CB2 agonists, can be efficiently combined with CB1 antagonists to achieve even greater benefits. This also indicates that the enhanced 2-AG signaling conferred by MAGL blockade is selectively stimulating CB2 but not CB1 to exert hepatoprotective effects, and forecasts a potential therapeutic utility of the combination of MAGL inhibitors with peripherally restricted CB1 antagonists that would avoid the central anxiety and depression effects that halted the clinical development of global CB1 antagonists12.
Despite the fact that hepatic I/R, as well as I/R injury of other organs, is a common complication of many diseases and surgical procedures, there is currently a remarkable lack of pharmacological therapies to provide improved outcomes and avoid organ failure and death. In this study, we find that pharmacological inhibition of MAGL either before or after the initiation of hepatic I/R confers substantial protection against the inflicted injury. We therefore put forth MAGL and its inhibitors as a novel next-generation therapeutic strategy towards not only preventing, but also treating I/R injury, in hope of improving the outcome of diseases and surgeries that expose the liver to hypoxia, inflammation, and oxidative stress. Importantly, we also show that MAGL inactivation by JZL184 is similarly effective in protecting GalN/LPS- and CCl4-induced acute liver injury, indicating that MAGL inhibitors may have broader utility beyond conditions that cause hepatic I/R. Since the mechanisms underlying these tissue injury share lots of similarities across other organs26, generally involving damage through inflammation and oxidative stress, we anticipate that MAGL inhibitors may exert beneficial effects in other pathologies (e.g. myocardial infarction, stroke, whole body ischemia associated with various forms of shock, etc.) where either CB2 agonists or COX inhibitors have both shown efficacy27–31.
Materials and Methods
Mice and Chemicals
C57BL/6 mice of 6–8 week-old were purchased from Jackson Laboratory (Bar Harbor, ME). Mgll−/− mice were generated previously23. JZL184 was purchased from Tocris Bioscience. CB1 (SR141716A/rimonabant/SR1) and CB2 antagonists (SR144528/SR2) were obtained from NIDA Drug Supply Program, Research Triangle Park, NC, USA). All these pharmacological reagents were dissolved in 18:1:1 saline:Tween 80:DMSO and administered by intraperitoneal (i.p.) injection at 10 μL/g mouse body weight.
Induction of hepatic I/R
Partial hepatic I/R (1 h of ischemia followed by reperfusion for 2 h, 6 h or 24 h) was induced as previously described 3, 5, 16, 32 and detailed in Supplements. JZL184, CB1/2 antagonists were administered by i.p. injection at various time points (1 h before ischemia, 1 and 3 h after reperfusion) as indicated. This animal study was approved by the Institutional Animal Care and Use Committees of NIAAA, and has been carried out in line with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
Measurement of endocannabinoids and eicosanoids
Endocannabinoids and eicosanoids were measured using single reaction monitoring (SRM)-based liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, as previously described14 and detailed in Supplements.
Determination of liver damage and injury
The activities of aspartate amino-transferase (AST) and alanine amino-transferase (ALT) were measured in serum samples using a clinical chemistry analyzer system (VetTest 8008, IDEXX laboratories, Westbrook, ME). Histological analysis of liver tissue damage was assessed by standard hematoxylin and eosin (H&E) staining of the tissue sections (5 μm thickness). For immunohistochemical staining of hepatic neutrophils, a primary antibody against mouse myeloperoxidase (Biocare Medical, Concord, CA) was used.
Determination of inflammation, oxidative stress, and cell death
Inflammatory, oxidative stress, and cell death markers were quantified based on previously established procedures3, 16. Please see Supplemental Methods for more details.
Isolation of hepatocytes (HCs), nonparenchymal cells (NPCs), and Kupffer cells (KCs) from mouse liver
Cell isolation from mouse liver was performed as described previously33, 34 and detailed in Supplements. Briefly, mouse liver was perfused in situ with a solution containing 0.075% type IV collagenase (Sigma, St. Louis, MO). After mechanical dissociation and further digestion in 0.009% collagenase, HCs and NPCs were isolated by a series of gradient centrifugation using Percoll (GE Healthcare Bio-Sciences Uppsala, Sweden). KCs were purified using the MACS system (Miltenyi Biotec, Auburn, CA) after immunostaining with a monoclonal anti-F4/80 antibody and subsequent magnetic labeling.
Hypoxia-Reoxygenation (H/R) treatment of isolated mouse hepatocytes
The cultured mouse HCs were treated with 2-AG or JZL184 for 4 h, and were then subjected to hypoxia (1% O2) for 12 h followed by reoxygenation for additional 12 h. Hepatocellular death induced by H/R was estimated by measuring LDH and ALT levels of the culture medium as described in Supplements.
Mouse KCs culture and treatments
Purified KCs were seeded into 96-well plates in RPMI supplemented with 10% bovine serum albumin, and were allowed to adhere to the plates for 12 h. The cells were pretreated with AM630 and were then incubated with various concentrations of 2-AG for 4 hours at 37°C, followed by stimulation with LPS. After 90 min, culture media were collected and assayed for TNFα using an ELISA kit (R&D).
Murine hepatitis models induced by GalN/LPS or CCl4
C57BL/6 mice were treated by i.p. with 800 mg/kg of D-(+)-Galactosamine (GalN, Sigma, St. Louis, MO) together with 1 μg/kg of LPS (from Escherichia coli 0127:B8, Sigma, St. Louis, MO). The mice were euthanized 7–8 h after GalN/LPS challenge, and blood and liver tissues were collected. For lethality study, LPS was used at a dose of 1.5 μg/kg, and mortality was assessed up to 8 hours after GalN/LPS challenge. JZL184-treated mice received i.p. injection of 20 mg/kg of JZL184 30 min before GalN/LPS treatment. For CCl4-induced liver injury, mice were injected i.p. with 2 ml/kg of 10% CCl4 (Sigma, St. Louis, MO) diluted in olive oil. The mice were sacrificed 24 h after CCl4 injection, and the blood and livers were collected to assess liver injury.
Statistical analysis
The results were expressed as mean±SEM. Differences among experimental groups were evaluated by Student’s t-test or ANOVA whenever is appropriate, and the significance of differences between groups was assessed by Newman-Keuls post-hoc test. The analysis was performed using a statistical software package (GraphPad- Prism 5; GraphPad, La Jolla, CA, USA). Significance was defined as p<0.05.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Program of the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (NIAAA) (PP), University of California, Berkeley/Department of Nutritional Sciences and Toxicology startup funds (DKN, MMM), National Institute on Drug Abuse (R00DA030908 (DKN, MMM) and DA017259 (BFC)), and the Skaggs Institute for Chemical Biology (BFC). Authors are indebted to Dr. George Kunos, the Scientific Director of NIAAA, for continuous support.
Abbreviations
- MAGL or Mgll
Monoacylglycerol lipase
- 2-AG
arachidonoylglycerol
- CB2R or Cnr2
cannabinoid receptor type 2
- AA
arachidonic acid
- I/R
ischemia/reperfusion
- FAAH
fatty acid amide hydrolase
- COX
cyclooxygenases
- TXA2
thromboxane A2
- PGE2
prostaglandin E2
- AEA
anandamide
- i.p
intraperitoneal
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- MPO
myeloperoxidase staining
- IL-1β
interleukin 1β
- MIP-1α and MIP-2
chemokines macrophage inflammatory protein 1α and 2
- ICAM-1
intercellular adhesion molecule 1
- HNE
4-hydroxynonenal
- NOX2
NADPH oxidase isoform 2
- PARP
poly(ADP-ribose) polymerase
- GalN
D-(+)-Galactosamine
- CCl4
carbon tetrachloride
Footnotes
Disclosures: DKN and BFC have filed a patent (US Patent Application Serial No. 12/998,642) “Methods and compositions related to targeting monoacylglycerol lipase” which relates to inhibitors of monoacylglycerol lipase and associated methods, compositions, and potential uses for treating human disorders that are associated with endocannabinoid signaling. ZC, MMM, PM, HX, KE, EH, EH, GH, PP have no conflict of interest to declare.
Author contributions: ZC, MMM, PM, HX, KE, EH, EH, GH, DKN: performing experiments, acquisition of data; analysis and interpretation of data; statistical analysis; discussions and revisions of manuscript. DKN, BFC, ZC and PP: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. PP: obtained funding; study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pacher P, Hasko G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2008;153:252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh KB, Toledo AH, Rivera-Chavez FA, et al. Inflammatory mediators of liver ischemia-reperfusion injury. Exp Clin Transplant. 2009;7:78–93. [PubMed] [Google Scholar]
- 3.Horvath B, Magid L, Mukhopadhyay P, et al. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br J Pharmacol. 2012;165:2462–2478. doi: 10.1111/j.1476-5381.2011.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi M, Takeyoshi I, Kurabayashi M, et al. The effects of a cyclooxygenase-2 inhibitor, FK3311, on total hepatic ischemia-reperfusion injury of the rat. Hepatogastroenterology. 2007;54:522–526. [PubMed] [Google Scholar]
- 5.Batkai S, Osei-Hyiaman D, Pan H, et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamada T, Tsuchihashi S, Avanesyan A, et al. Cyclooxygenase-2 deficiency enhances Th2 immune responses and impairs neutrophil recruitment in hepatic ischemia/reperfusion injury. J Immunol. 2008;180:1843–1853. doi: 10.4049/jimmunol.180.3.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoyama Y, Nimura Y, Nagino M, et al. Role of thromboxane in producing hepatic injury during hepatic stress. Arch Surg. 2005;140:801–807. doi: 10.1001/archsurg.140.8.801. [DOI] [PubMed] [Google Scholar]
- 8.Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caraceni P, Pertosa AM, Giannone F, et al. Antagonism of the cannabinoid CB-1 receptor protects rat liver against ischaemia-reperfusion injury complicated by endotoxaemia. Gut. 2009;58:1135–1143. doi: 10.1136/gut.2007.147652. [DOI] [PubMed] [Google Scholar]
- 10.Jeong WI, Osei-Hyiaman D, Park O, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira-Clerc F, Julien B, Grenard P, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 12.Kunos G, Tam J. The case for peripheral CB(1) receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol. 2011;163:1423–1431. doi: 10.1111/j.1476-5381.2011.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam J, Liu J, Mukhopadhyay B, et al. Endocannabinoids in liver disease. Hepatology. 2011;53:346–355. doi: 10.1002/hep.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomura DK, Morrison BE, Blankman JL, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long JZ, Li W, Booker L, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batkai S, Mukhopadhyay P, Horvath B, et al. Delta8-Tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors. Br J Pharmacol. 2012;165:2450–2461. doi: 10.1111/j.1476-5381.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begay CK, Gandolfi AJ. Late administration of COX-2 inhibitors minimize hepatic necrosis in chloroform induced liver injury. Toxicology. 2003;185:79–87. doi: 10.1016/s0300-483x(02)00594-2. [DOI] [PubMed] [Google Scholar]
- 18.Han C, Li G, Lim K, et al. Transgenic expression of cyclooxygenase-2 in hepatocytes accelerates endotoxin-induced acute liver failure. J Immunol. 2008;181:8027–8035. doi: 10.4049/jimmunol.181.11.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louvet A, Teixeira-Clerc F, Chobert MN, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54:1217–1226. doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 20.Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chemical reviews. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dajani EZ, Islam K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. 2008;59 (Suppl 2):117–133. [PubMed] [Google Scholar]
- 22.Kinsey SG, Nomura DK, O’Neal ST, et al. Inhibition of monoacylglycerol lipase attenuates nonsteroidal anti-inflammatory drug-induced gastric hemorrhages in mice. J Pharmacol Exp Ther. 2011;338:795–802. doi: 10.1124/jpet.110.175778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlosburg JE, Blankman JL, Long JZ, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chanda PK, Gao Y, Mark L, et al. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- 25.Julien B, Grenard P, Teixeira-Clerc F, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 26.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maheshwari A, Badgujar L, Phukan B, et al. Protective effect of Etoricoxib against middle cerebral artery occlusion induced transient focal cerebral ischemia in rats. Eur J Pharmacol. 2011;667:230–237. doi: 10.1016/j.ejphar.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- 29.Defer N, Wan J, Souktani R, et al. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J. 2009;23:2120–2130. doi: 10.1096/fj.09-129478. [DOI] [PubMed] [Google Scholar]
- 30.Tuma RF, Steffens S. Targeting the endocannabinod system to limit myocardial and cerebral ischemic and reperfusion injury. Curr Pharm Biotechnol. 2012;13:46–58. doi: 10.2174/138920112798868665. [DOI] [PubMed] [Google Scholar]
- 31.Montecucco F, Lenglet S, Braunersreuther V, et al. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:612–620. doi: 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Cao Z, Yuan Y, Jeyabalan G, et al. Preactivation of NKT cells with alpha-GalCer protects against hepatic ischemia-reperfusion injury in mouse by a mechanism involving IL-13 and adenosine A2A receptor. Am J Physiol Gastrointest Liver Physiol. 2009;297:G249–258. doi: 10.1152/ajpgi.00041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Z, Dhupar R, Cai C, et al. A critical role for IFN regulatory factor 1 in NKT cell-mediated liver injury induced by alpha-galactosylceramide. J Immunol. 2010;185:2536–2543. doi: 10.4049/jimmunol.1000092. [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Hou Y, Chen H, et al. Sample preparation method for isolation of single-cell types from mouse liver for proteomic studies. Proteomics. 2011;11:3556–3564. doi: 10.1002/pmic.201100157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.