Abstract
Microvascular disease, a characteristic of acute and chronic kidney diseases, leads to rarefaction of peritubular capillaries (PTCs), promoting secondary ischemic injury, which may be central to disease progression. Bidirectional signaling by EphB4 receptor and ephrinB2 ligand is critical for angiogenesis during murine development, suggesting that ephrinB2 reverse signaling may have a role in renal angiogenesis induced by injury or fibrosis. Here, we found that ephrinB2 reverse signaling is activated in the kidney only after injury. In mice lacking the PDZ intracellular signaling domain of ephrinB2 (ephrinB2 ΔV), angiogenesis was impaired and kidney injury led to increased PTC rarefaction and fibrosis. EphrinB2 ΔV primary kidney pericytes migrated more than wild-type pericytes and were less able to stabilize capillary tubes in three-dimensional culture and less able to stimulate synthesis of capillary basement membrane. EphrinB2 ΔV primary kidney microvascular endothelial cells migrated and proliferated less than wild-type microvascular endothelial cells in response to vascular endothelial growth factor A and showed less internalization and activation of vascular endothelial growth factor receptor-2. Taken together, these results suggest that PDZ domain-dependent ephrinB2 reverse signaling protects against PTC rarefaction by regulating angiogenesis and vascular stability during kidney injury. Furthermore, this signaling in kidney pericytes protects against pericyte-to-myofibroblast transition and myofibroblast activation, thereby limiting fibrogenesis.
Destruction of peritubular capillaries (PTCs), known as rarefaction, and the development of fibrosis are detected in all types of CKD, including allograft nephropathy in patients receiving kidney transplants.1–3 PTC rarefaction is believed to be a central driving force of CKD because capillary rarefaction may result in deficiency of oxygen/nutrient supply to cells and impaired tubular function, which in turn drives kidney injury. Interstitial fibrosis of the kidney exacerbates this problem and progressively replaces damaged parenchymal tissue with nonfunctioning scar tissue. Recent studies have identified a critical connection between PTC rarefaction and the development of interstitial fibrosis of the kidney.4,5 Kidney pericytes are an extensive population of mesenchyme-derived cells that are attached to endothelial cells (ECs) of PTCs where they perform vascular stabilizing and regulatory functions.4–9 However, in response to sustained injury, pericytes detach themselves from ECs and become scar-forming myofibroblasts, while simultaneously losing normal pericyte functions.4–6 The molecular mechanisms by which pericytes regulate microvascular function and the molecular mechanisms of PTC rarefaction after injury remain obscure.
Bidirectional signaling by the EphB4 receptor and the ephrinB2 ligand has been shown to be an essential angiogenesis cue during embryonic development of mice.10,11 Although ephrinB2 is a membrane-bound ligand, it has an intracellular domain that also possesses intrinsic signaling capacity called “reverse signaling.”12 Complete deletion of the intracellular domain of ephrinB2 resulted in a severe defect of angiogenesis and embryonic lethality, indicating a critical role for ephrinB2 reverse signaling in developmental blood vessel formation.13 Subsequently, ephrinB2 reverse signaling was shown to regulate both developmental and tumor angiogenesis by activating vascular endothelial growth factor receptor 2 (VEGFR-2).14,15 In separate studies, pericyte-specific ephrinB2 deficiency indicated that ephrinB2 is essential for normal coverage of the microvasculature by pericytes.16 In these mutant mice, pericytes did not bind to ECs, resulting in microvascular hemorrhage from unstable capillaries in multiple organs such as lung and skin, leading to perinatal lethality.16 In addition, these mutant mice exhibited aberrant fibrosis surrounding abnormally immature vessels in dermal tissue,16 suggesting a possible role of ephrinB2 reverse signaling in fibrogenesis. In adult mice, ephrinB has been reported to be phosphorylated and activated in ECs and pericytes in wounded skin of mice.17 However, there are no studies investigating the role of ephrinB2 reverse signaling in angiogenesis and fibrosis after tissue injury in adults. We therefore investigated the role of ephrinB2 signaling in the kidney after injury.
Results
Eph Receptors and Ephrin Ligands Are Expressed in Kidney Microvascular ECs, Pericytes, and Macrophages and Are Regulated in Kidney Injury
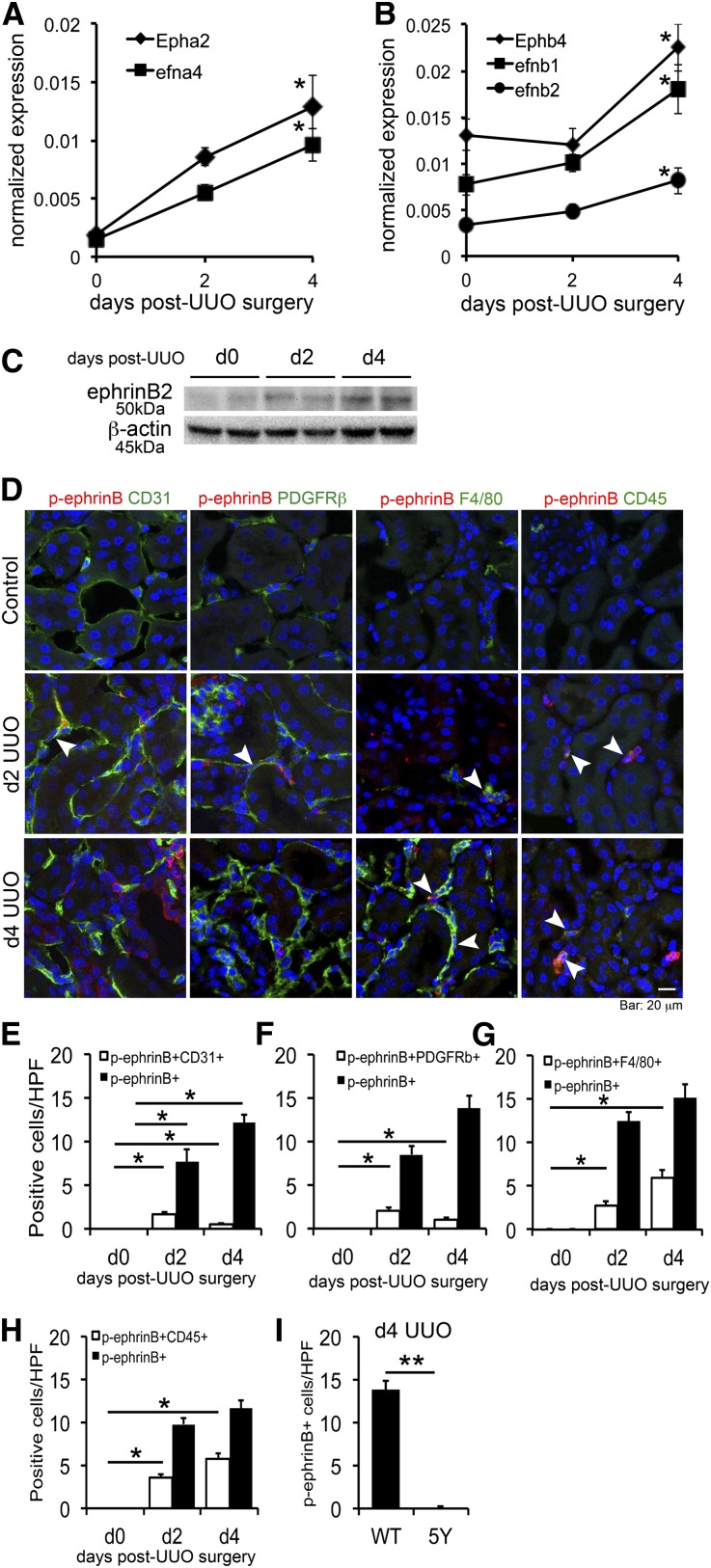
To identify Eph receptors and ephrin ligands expressed in the kidney and regulated during angiogenesis, we screened transcript levels for Eph/ephrin family genes in uninjured normal and injured kidneys 2 and 4 days after unilateral ureteral obstruction (UUO) (d2 and d4 UUO, respectively). The UUO model has a well defined phase of angiogenesis, which peaks at d4 followed by rarefaction of capillaries.4 Compared with normal kidney, Epha2, Ephb4 receptors, and efna4, efnb1, and efnb2 ligands were upregulated in UUO kidneys (Figure 1, A and B, and Supplemental Figure 1). Transcripts of other Eph receptors and ephrin ligands were not detectable or not regulated during kidney injury. To determine the distribution of this receptor/ligand family, we examined transcript levels in FACS-sorted kidney pericytes, microvascular endothelial cells (MVECs), and monocyte/macrophages, because these cells regulate angiogenesis in normal and injured kidneys.4,5 Ephb4, efnb1, and efnb2 were highly expressed in kidney pericytes, MVECs, and macrophages of the normal kidney (Table 1). However, all of these transcripts were modestly downregulated in nonepithelial kidney cells 4 days after UUO, except Ephb4 and efnb2 in pericytes, which were essentially unchanged. Because the whole kidney analysis indicated that these genes were upregulated in disease, we interrogated transcripts from primary tubular epithelial cells with and without TGF-β stimulation. Ephb4, efnb1, and efnb2 were upregulated in epithelial cells after TGF-β stimulation (Supplemental Figure 2), explaining the incremental rise of these transcripts in whole sample of UUO kidneys. In whole kidney, protein levels of ephrinB2 were increased in d4 UUO kidneys compared with control, indicating transcriptional levels reflect protein expression levels (Figure 1C).
Figure 1.
Eph receptors and ephrin ligands are expressed in kidney pericytes, MVECs, and macrophages and are regulated in kidney injury. (A and B) qPCR showing time course of transcript levels of Eph/ephrin family genes that are highly expressed and regulated in whole kidney after UUO kidney injury. Expression levels are normalized to GAPDH. n=3 per time point. *P<0.05 versus d0 UUO kidney. (C) Western blot for ephrinB2 and β-actin (loading control) using whole kidney lysates from normal and UUO kidneys. Note that ephrinB2 expression is increased after UUO. (D) Double-immunofluorescence staining for p-ephrinB (red) and each cell marker (green) in control, d2, and d4 UUO kidneys. Note that control kidneys do not have any p-ephrinB+ cells. In d2 UUO kidneys, some of the CD31+ MVECs or PDGFRβ+ pericytes express p-ephrinB. In d4 UUO kidneys, the major population of p-ephrinB+ cells is composed of F4/80+ macrophages and CD45+ leukocytes. Arrowheads indicate double-positive cells. (E–H) Quantification of p-ephrinB+ cell number in specific cell populations. (E) Quantification of p-ephrinB+ CD31+ MVECs. The number of double-positive cells peaks in d2 UUO. (F) Quantification of p-ephrinB+ PDGFRβ+ pericytes. The number of double-positive cells peaks in d2 UUO. (G) Quantification of p-ephrinB+ F4/80+ macrophages. The number of double-positive cells progressively increases after UUO kidney injury. (H) Quantification of p-ephrinB+ CD45+ leukocytes. The number of double-positive cells progressively increases after UUO kidney injury. (I) The number of p-ephrinB+ cells in d4 UUO kidneys of WT and ephrinB2 5Y (5Y) mice. Note that no p-ephrinB+ cells are detected in 5Y mice. *P<0.05; **P<0.01. Scale bar, 20 μm.
Table 1.
Transcript levels of Eph/ephrin family gene in pericytes, MVECs, and monocytes/macrophages
| Pericyte | |||||
| Gene | Epha2 | efna4 | Ephb4 | efnb1 | efnb2 |
| d0 UUO | 0.024 (0.001) | 0.007 (0.001) | 0.037 (0.003) | 0.091 (0.027) | 0.025 (0.001) |
| d4 UUO | 0.010 (0.002) | 0.009 (0.002) | 0.048 (0.012) | 0.030 (0.003) | 0.041 (0.010) |
| MVEC | |||||
| Gene | Epha2 | efna4 | Ephb4 | efnb1 | efnb2 |
| d0 UUO | 0.024 (0.006) | 0.002 (0.000) | 0.098 (0.006) | 0.036 (0.006) | 0.326 (0.056) |
| d4 UUO | 0.011 (0.004) | 0.005 (0.000) | 0.041 (0.008) | 0.019 (0.004) | 0.091 (0.025) |
| Monocyte/macrophage | |||||
| Gene | Epha2 | efna4 | Ephb4 | efnb1 | efnb2 |
| d0 UUO | 0.002 (0.001) | 0.001 (0.000) | 0.008 (0.002) | 0.006 (0.002) | 0.001 (0.000) |
| d4 UUO | 0.001 (0.000) | 0.000 (0.000) | 0.002 (0.000) | 0.004 (0.001) | 0.000 (0.000) |
Each cell population is sorted by FACS from normal (d0 UUO) and injured d4 post-UUO (d4 UUO) kidneys of WT mice. Epha1, Epha3–8, Epha10, efna1–3, efna5, Ephb1–3, Ephb6, and efnb3 were not detected. n=3 per time point. Normalized values are shown as mean (±SEM).
EphrinB2, but not EphrinB1, Reverse Signaling is Specifically Activated after Kidney Injury
Activated ephrinB signaling can be identified using anti-phospho-ephrinB (p-ephrinB) antibody, which specifically detects phosphorylated tyrosine residues at the intracellular domain of ephrinB.18 Coimmunostaining of kidney sections with anti-p-ephrinB antibodies and cell-specific markers demonstrated no detectable active ephrinB in normal kidney (Figure 1D). In d2 UUO kidneys, CD31+ MVECs and platelet-derived growth factor receptor β+ (PDGFRβ+) pericytes constituted approximately 20% of p-ephrinB+ cells, respectively. By d4 after UUO, the number of p-ephrinB+CD31+ or p-ephrinB+PDGFRβ+ cells decreased but they were still readily detectable (Figure 1, E and F). The cell type with the greatest p-ephrinB activity was F4/80+ macrophages or CD45+ leukocytes and the number of these cells increased from d2 to d4 after UUO (Figure 1, G and H). Thus, the major population of p-ephrinB+ cells is leukocytes, predominantly F4/80+ macrophages, whereas smaller populations are MVECs and pericytes. To test the relative contribution of ephrinB2 or ephrinB1 to total p-ephrinB signaling, we stained kidneys from ephrinB2 5Y mice (all five conserved tyrosine residues in the cytoplasmic domain of ephrinB2 are mutated so phosphorylation cannot occur).19 At d4 after UUO in ephrinB2 5Y mice, p-ephrinB was not detectable, indicating high specificity of p-ephrinB antibody and an apparent lack of ephrinB1 signaling via tyrosine phosphorylation (Figure 1I and Supplemental Figure 3).
Loss of PDZ-Dependent EphrinB2 Signaling, but not Phosphotyrosine-Dependent Signaling, Impairs Angiogenesis after Kidney Injury
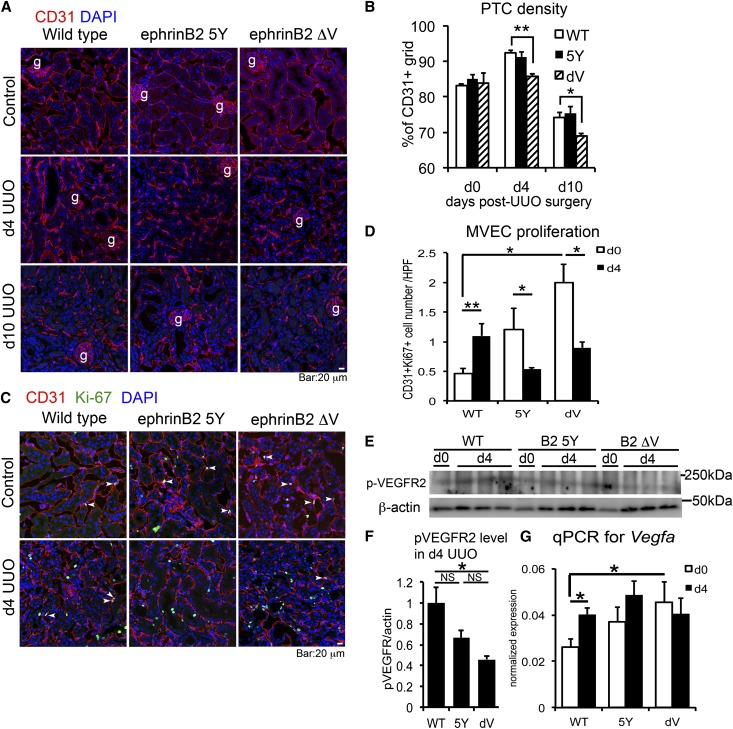
EphrinB ligands, upon activation, can signal by tyrosine phosphorylation of their intracellular domain (phosphotyrosine-dependent signaling), or by binding of effector proteins containing a PDZ domain to the PDZ binding motif of the ephrinB intracellular domain (PDZ-dependent signaling).18 The former signaling has been proposed to be linked to the latter signaling after EphB-ephrinB binding.18 We reasoned that both signaling modalities would likely be activated during kidney injury, although there is no simple assay for PDZ-dependent signaling. We therefore examined whether impaired ephrinB2 signaling affects PTC rarefaction in UUO kidneys by studying mutant mice that lack phosphotyrosine-dependent ephrinB2 signaling (ephrinB2 5Y) or PDZ-dependent ephrinB2 signaling (ephrinB2 ΔV). EphrinB2 ΔV mice lack a single valine residue at the end of ephrinB2 cytoplasmic domain,19 which is essential for PDZ-dependent ephrinB2 signaling. In 8- to 9-week-old mutant mice, the microvasculature had normal density and architecture in the cortex (Figure 2, A and B). However, PTCs of normal ephrinB2 ΔV kidneys showed a significantly higher number of Ki-67+ proliferative MVECs, assessed by CD31 co-immunostaining (Figure 2, C and D). In response to UUO injury, the number of proliferative PTC MVECs decreased in ephrinB2 ΔV kidneys, whereas wild-type (WT) kidneys displayed a marked increase in MVEC proliferation (Figure 2, C and D).4 Following injury, ephrinB2 ΔV MVECs in PTCs also showed increased apoptosis compared with WT MVECs (Supplemental Figure 4A). These abnormal proliferative and cell survival responses were associated with a lack of angiogenesis seen 4 days after UUO and associated with acceleration in subsequent PTC rarefaction (Figure 2, A and B). The effect of ephrinB2 ΔV mutation was also studied after unilateral ischemia reperfusion injury (IRI). Similarly to the UUO models, IRI in mutant kidneys resulted in significantly greater PTC rarefaction than in WT kidneys, indicating that ephrinB2 signaling regulates endothelial integrity in response to multiple injuries (Supplemental Figure 5, A and B).
Figure 2.
Defective PDZ-dependent ephrinB2 signaling promotes progressive capillary rarefaction and impairs angiogenesis after UUO kidney injury. (A) Immunofluorescence images of CD31+ MVECs in control, d4, and d10 UUO kidneys of WT, ephrinB2 5Y (5Y), and ephrinB2 ΔV (dV) mice. (B) Quantification of PTC density. n=3–4 per group per time point. (C) Immunofluorescence images of CD31+ Ki-67+ proliferative MVECs in control and d4 UUO kidneys of WT, ephrinB2 5Y, and ephrinB2 ΔV mice. Arrowheads indicate double-positive cells. (D) Quantification of CD31+ Ki-67+ proliferative MVECs. n=3 per group per time point. (E) Western blot for p-VEGFR2, β-actin in d0 (normal) and d4 UUO kidneys of WT, ephrinB2 5Y, and ephrinB2 ΔV mice. (F) The protein band intensity of p-VEGFR2 in d4 UUO kidneys assessed by densitometry. Intensity of p-VEGFR2 band is normalized by β-actin band intensity. Note that p-VEGFR2 expression is reduced in ephrinB2 ΔV compared with WT. n=3 per group. (G) qPCR showing VEGFA transcriptional level in d0 and d4 UUO kidneys of WT, ephrinB2 5Y, and ephrinB2 ΔV mice. n=3 per group. *P<0.05; **P<0.01. g, glomerulus; NS, no significant difference. Scale bar, 20 μm.
Kidneys from ephrinB2 5Y mice also showed impaired MVEC proliferative responses after UUO injury but these defects were milder and did not result in increased PTC rarefaction in UUO kidneys (Figure 2, A–D). We evaluated whether ephrinB signaling was affecting endothelial progenitor cell (EPC) recruitment as an explanation for these observations. Fewer than 0.5% of CD31+ cells were leukocytes and when quantified their numbers did not differ between mutant and WT kidneys. In addition, factors associated with EPC recruitment, such as transcript levels for Cxcl12 (stromal cell–derived factor-1 [SDF-1]) and its receptor Cxcr4, were also no different between mutant and WT kidneys, which collectively suggest that EPCs were not significant factors in the impaired angiogenesis and pronounced rarefaction of ephrinB2 mutant kidneys (Supplemental Figure 4, B and C).
EphrinB2 signaling has been reported to regulate VEGFR2 function,14 which is restricted to kidney ECs. We assessed VEGFR2 activity in whole kidneys by quantifying phosphorylated VEGFR2 (p-VEGFR2) expression. The level of p-VEGFR2 was significantly lower in diseased ephrinB2 ΔV kidneys than WT kidneys (Figure 2, E and F), which was associated with abnormally high levels of Vegfa in nondiseased ephrinB2 ΔV kidneys (Figure 2G).
PDZ-Dependent EphrinB2 Signaling-Deficient Mice Have Enhanced Fibrosis after Kidney Injury
Abnormal ephrinB2 signaling has been shown to profoundly affect pericyte function in the embryonic skin leading to unstable capillaries and fibrosis.16 Because ephrinB2 signaling is active in kidney pericytes (Figure 1F), we hypothesized that perturbed ephrinB2 signaling would compromise pericyte functions after injury. Overactivated pericytes may be less able to stabilize PTCs and more likely to become scar-forming myofibroblasts.5 In d10 UUO kidneys, ephrinB2 ΔV mice exhibited more collagen deposition assessed by picrosirius red staining and higher numbers of α-smooth muscle actin (αSMA)–expressing myofibroblasts than WT mice. Increased numbers of myofibroblasts were also detected at d4 after UUO (Figure 3, A–D). EphrinB2 5Y mice showed similar myofibroblast numbers and fibrotic responses to WT mice after UUO (Figure 3, C and D) but ephrinB2 ΔV kidney myofibroblasts were more proliferative than WT myofibroblasts (Figure 3, E and F), indicating that phosphotyrosine-dependent signaling plays no role in fibrogenesis, whereas PDZ-dependent signaling does play a role. Consistent with these findings, in d10 UUO kidneys, transcripts of αSMA (Acta2), Col1a1, and Col3a1 significantly increased in ephrinB2 ΔV mice compared with WT or ephrinB2 5Y mice (Figure 3G). In the unilateral IRI model of kidney disease, ephrinB2 ΔV kidneys had more collagen deposition than WT kidneys (Supplemental Figure 5, C and D), consistent with a generalized role for PDZ-dependent signaling pathway in fibrogenesis. Because inflammatory macrophages responding to injury can promote fibrosis in the kidney,20 we evaluated macrophage numbers and genes associated with macrophage activation and differentiation, but did not detect any differences in overall numbers or M1/M2 macrophage marker expression in any of mutant kidneys compared with WT kidneys (Supplemental Figure 6).
Figure 3.
Kidney fibrosis is enhanced due to lack of PDZ-dependent ephrinB2 signaling. (A) Images of picrosirius red-stained control and d10 UUO kidney sections for fibrillar collagen deposition (red). (B) Morphometric quantification of fibrillar collagen in d0 (normal) and d10 UUO kidneys of WT, ephrinB2 5Y (5Y), and ephrinB2 ΔV (dV) mice. n=3–4 per group. (C) Immunofluorescence images of αSMA+ myofibroblasts in control, d4, and d10 UUO kidneys of WT, ephrinB2 5Y, and ephrinB2 ΔV mice. (D) Number of αSMA+ myofibroblasts in control and UUO kidneys of WT, ephrinB2 5Y, and ephrinB2 ΔV mice. n=3–4 per group. (E) Immunofluorescence images of αSMA+ Ki-67+ proliferative myofibroblasts in control and d10 UUO kidneys. Arrowheads indicate double-positive cells. (F) Quantification of αSMA+ Ki-67+ proliferative myofibroblasts. n=3 per group per time point. (G) qPCR of αSMA (Acta2) and collagen I, α1 (Col1a1) and collagen III, α1 (Col3a1) transcripts in control and d10 UUO kidneys of WT, ephrinB2 5Y, and ephrinB2 ΔV mice. n=3–4 per group per time point. *P<0.05; **P<0.01. NS, no significant difference; g, glomerulus; a, arteriole. Scale bar, 20 μm.
EphA/EphrinA Signaling Plays No Role in Kidney Microvascular Responses to Injury
EphA2 and ephrinA4 are expressed and regulated in the kidney in response to disease (Figure 1A). EphA2 has been reported to regulate interactions between ECs and pericytes.21 We therefore studied the kidney microvascular responses in EphA2-deficient mice. No differences were found in PTC density and number of myofibroblasts that appear in control and d4 UUO kidneys of WT and EphA2-deficient mice, suggesting minor roles of EphA2 in kidney injury (Supplemental Figure 7).
EphrinB2 PDZ Binding Motif-Mutant Kidney Pericytes Are Overactivated and Exhibit Impaired Capacity to Stabilize Microvasculature In Vitro
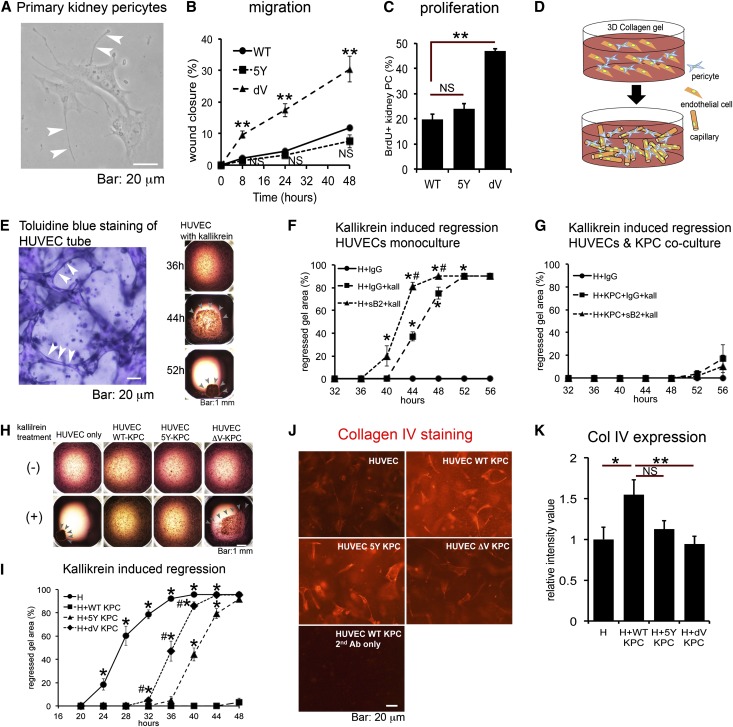
Because our studies pointed to mutation of the ephrinB2 PDZ domain in MVECs and pericytes, rather than macrophages, as the dominant cellular explanation for the kidney phenotype detected, we dissected the cellular mechanisms in these cells. First we generated primary kidney pericytes from WT and mutant kidneys. In response to TGF-β, ephrinB2 ΔV pericytes were more migratory than WT or ephrinB2 5Y pericytes (Figure 4, A and B, and Supplemental Figure 8A). Proliferation was assessed by incorporation of 5-bromo-2-deoxyuridine (BrdU). After 6 hours of TGF-β stimulation, there was almost no proliferation (Supplemental Figure 8B), but 24 hours after TGF-β stimulation, pericytes proliferated and ephrinB2 ΔV pericytes were significantly more proliferative than WT or ephrinB2 5Y pericytes (Figure 4C). Because ephrinB2 and its cognate receptor, EphB4, are both expressed by MVECs and pericytes, we assessed whether EphB4-ephrinB2 interaction is important in capillary stability. For this, we blocked EphB4-ephrinB2 interaction using a soluble form of ephrinB2 (ephrinB2-Fc). Human umbilical vein endothelial cells (HUVECs), seeded in a three-dimensional (3D) collagen gel, spontaneously form a capillary network but this network is unstable in the presence of serine proteases such as kallikrein (Figure 4, D and E).22 When the network is destabilized, it collapses, resulting in retraction and then collapse of the gel (Figure 4E). Compared with control, ephrinB2-Fc promoted microvascular instability after kallikrein stimulation (Figure 4F) but ephrinB2-Fc of itself did not cause collapse without kallikrein (data not shown), suggesting that EphB4-ephrinB2 bidirectional signaling contributes to normal microvascular integrity through EC/EC interactions. Using this assay, we previously showed that kidney pericytes, if added to the gel, migrate to and bind to the microvascular network, where they stabilize the capillary network by multiple mechanisms.5 In the presence of 30% kidney pericytes (3 kidney pericytes for every 10 HUVECs), the capillary tubes were almost completely stabilized even after kallikrein stimulation (Figure 4G) indicative of a vascular stabilization effect by kidney pericytes. This stabilization was not disrupted by adding ephrinB2-Fc (Figure 4G), suggesting that EphB4-ephrinB2 bidirectional signaling is not necessary for microvascular stabilization mediated by pericyte-endothelial interactions. Next we assessed whether ephrinB2 mutant pericytes stabilized capillaries normally in this 3D assay. Whereas WT pericytes stabilized capillaries, ephrinB2 ΔV pericytes were much less capable of stabilizing the capillaries (Figure 4, H and I) and this was associated with a decreased capacity of ephrinB2 ΔV pericytes to stimulate synthesis of a major component of vascular basement membrane, type IV collagen, by HUVECs in the gel (Figure 4, J and K). These results indicate that ephrinB2 signaling plays an important role in pericyte function to regulate microvascular stability, but this may be independent of the receptor, EphB4.
Figure 4.
Defective PDZ-dependent ephrinB2 signaling induces more migratory and proliferative characteristics in kidney pericytes and compromises the pericyte mediated-vascular stabilizing functions. (A) Phase contrast image of primary kidney pericytes. Arrowheads indicate extended processes. (B) Migration assay under TGF-β stimulation (10 ng/ml) using kidney pericytes isolated from WT, ephrinB2 5Y (5Y), and ephrinB2 ΔV (dV) mice. Migration of kidney pericytes was assessed by the percentage of scratch wound closure. Creation of wound in monolayer of primary kidney pericytes denotes time zero. n=6/group. **P<0.01 versus WT. (C) Proliferation assay under TGF-β stimulation (10 ng/ml) using kidney pericytes assessed by BrdU incorporation for 24 hours. n=3 per group. **P<0.01. (D) Schematic of coculture of ECs (orange) with pericytes (blue) in 3D collagen gel in wells where ECs spontaneously form endothelial capillary tubes. Addition of pericytes to this assay permits migration and binding of pericytes to capillary tubes. (E) The left panel is a toluidine blue-stained gel showing capillary tubes (arrowheads) within the gel. The right panel shows representative images of gel regression in monoculture of HUVECs stimulated with kallikrein. Indicated time shows hours after kallikrein treatment. Note that, under kallikrein stimulation, HUVEC tubes start to regress, leading to progressive gel collapse. Arrowheads indicate the remaining gel. (F) Blocking ephrinB2 reverse signaling in monoculture of HUVEC (H) in 3D gel using soluble ephrinB2-Fc. Addition of ephrinB2-Fc (sB2, 10 μg/ml) to kallikrein (kall, 1 μg/ml) accelerates gel collapse compared with kallikrein plus control IgG. n=10 per condition. *P<0.05 versus H+IgG. #P<0.05 versus H+IgG+kall. (G) Blocking ephrinB2 reverse signaling in coculture of HUVEC with WT kidney pericytes (KPCs) in 3D gel using soluble ephrinB2-Fc (sB2). Kallikrein (kall) concentration is 1 μg/ml, n=10 per condition. Note that pericytes nearly completely inhibit kallikrein induced-gel collapse even in the presence of ephrinB2-Fc. Experiments in F and G were performed simultaneously. (H) Representative images of gels under indicated culture conditions at the 36-hour time point. Note that under kallikrein stimulation, gel collapse starts at the 36-hour point in wells of HUVEC with ephrinB2 ΔV kidney pericytes (ΔV-KPC), but not in the wells of HUVEC with WT kidney pericytes (WT-KPC) or HUVEC with ephrinB2 5Y kidney pericytes (5Y-KPC). Arrowheads indicate remaining gel. Bar: 1 mm. (I) Time course of gel collapse in indicated culture conditions. Note ephrinB2 ΔV kidney pericytes (dV-KPC) demonstrate remarkably impaired vascular stabilizing functions compared with WT kidney pericytes (WT-KPC). Kallikrein concentration is 2 μg/ml, n≥20 per condition. *P<0.05 versus HUVEC+WT-KPC; #P<0.05 versus HUVEC+5Y-KPC. (J) Representative images of 3D gel stained for human type IV collagen (red). (K) Quantification of type IV collagen expression by measurement of fluorescence intensity. n=10 per condition. *P<0.05; **P<0.01. NS, no significant difference. Scale bar, 20 μm in A, E (left panel), and J; 1 mm in E (right panel) and H.
Kidney MVECs Lacking PDZ-Dependent EphrinB2 Signaling Show Impaired Internalization and Activation of VEGFR2
To dissect the contribution of ephrinB2 signaling in kidney MVECs to aberrant angiogenesis and enhanced PTC rarefaction after kidney injury, we generated primary kidney MVECs from PTCs. A CD31+CD11b− cell population was sorted by FACS from kidney single cell suspension excluding glomeruli and arterioles (Supplemental Figure 9, A–D), was cultured in hypoxic conditions, and showed no epithelial or myeloid contamination (Figure 5A).23,24 ECs migrate into the injured area of vessels to cover the area with newly populating ECs, leading to new vessel formation.25 Enhanced migration of ECs is associated with greater angiogenic capacity.25 In response to VEGFA, ephrinB2 ΔV MVECs were significantly less migratory than WT MVECs (Figure 5B), and ephrinB2 5Y MVECs showed intermediate migratory responses. EphrinB2 ΔV MVECs also proliferated less in response to VEGFA (Figure 5C). Recent studies in lung, retina, and skin14,15 suggest that ephrinB2 is necessary for VEGFR signaling, by stimulating receptor internalization to endosomes where active signaling occurs.26 To investigate VEGFR2 internalization in ephrinB2 ΔV MVECs, an “antibody feeding” assay was performed.14 In steady state, VEGFR2 was predominantly on the cell surface in WT and mutant MVECs. In response to VEGF stimulation, most VEGFR2 was found in intracellular compartments in WT MVECs. However, VEGFR2 was not internalized after VEGFA stimulation in ephrinB2 ΔV MVECs (Figure 5D). In whole kidney lysates, ephrinB2 ΔV kidney had reduced p-VEGFR2 expression (Figure 2E). To test whether failed internalization of VEGFR2 results in impaired activation of VEGFR2, we labeled MVECs for p-VEGFR2. Consistent with our in vivo findings, ephrinB2 ΔV MVECs showed little phosphorylation of VEGFR2 in response to VEGFA stimulation (Figure 5E).
Figure 5.
Defective PDZ-dependent signaling of ephrinB2 results in impaired migration and proliferation of kidney MVECs and compromises internalization and phosphorylation of VEGFR2. (A) Phase contrast and immunofluorescence images of primary kidney MVECs. Sorted and cultured MVECs express CD31 (marker for ECs) but not E-cadherin (marker for epithelial cell) and CD11b (marker for monocyte/macrophage). (B) Migration assay in response to mouse VEGF stimulation (10 ng/ml) using kidney MVECs isolated from WT, ephrinB2 5Y (5Y), and ephrinB2 ΔV (dV) mice. Migration is assessed by the percentage of wound closure. Creation of wound in monolayer of cells denotes time zero. n=3 per group. *P<0.05 versus WT; **P<0.01 versus WT; #P<0.05 versus 5Y. (C) Proliferation assay in response to mouse VEGF stimulation (10 ng/ml) using kidney MVECs assessed by BrdU incorporation for 24 h. n=3 per group. **P<0.01. (D) The left panel shows representative fluorescence images of individual kidney MVECs which indicate the distribution of VEGFR2 in response to VEGFA (100 ng/ml) for 30 minutes. The right panel indicates the proportion of internalized (red) compared with surface (green) VEGFR2 based on fluorescence intensities. n≥20 per condition. **P<0.01. (E) The left panel shows representative kidney MVECs stained for p-VEGFR2 at tyrosine 1175 (red) in control conditions or with VEGF (100 ng/ml) stimulation for 30 minutes. The right panel indicates the proportion of phosphorylated compared with total VEGFR2 based on fluorescence intensities. The ratio of p-VEGFR2 to total VEGFR2 is normalized to the values of WT group. n=20 per condition. ** P<0.01. NS, no significant difference. Scale bar, 20 μm in A; 10 μm in D and E.
Discussion
Using two different models of kidney injury with fibrosis, these studies show that activated ephrinB2 reverse signaling counteracts both capillary rarefaction and fibrosis. Although the EphA/ephrinA pathway appears to play little functional role in kidney disease, we provide clear evidence for the importance of ephrinB2 as an endogenous inhibitor of kidney disease progression. EphrinB2 signals both via tyrosine phosphorylation and via its PDZ binding motif, but our studies suggest that PDZ-dependent signaling is distinct and critical for regenerative responses to renal interstitial injury.
To further dissect the function of ephrinB2, we studied its role in pericytes and ECs in vitro, using primary cell cultures. EphrinB2 ΔV kidney MVECs proliferate slowly compared with WT MVECs (data not shown), and have impaired migratory and proliferative responses to VEGFA. Moreover, in ephrinB2 ΔV mice, similarly to lung MVECs, kidney MVECs exhibit markedly impaired VEGFR2 internalization and activation in response to VEGFA.14 In keeping with these observations, ephrinB2 ΔV kidney MVECs show impaired proliferation and enhanced apoptosis in response to injury in vivo. Therefore, ephrinB2 regulates a number of important functions in MVECs, including VEGFR2 signaling. Surprisingly, control ephrinB2 ΔV kidneys appropriately constitute microvasculature, but ephrinB2 ΔV MVECs proliferate excessively in healthy kidney, suggesting that they are either activated or unstable. Whether ephrinB1 can replace ephrinB2 functionally or whether complete deletion of the ephrinB2 gene in adults results in a more severe phenotype of MVECs remains to be determined.
EphrinB2 signaling deficiency in kidney pericytes results in quite different biologic changes. Pericytes lacking PDZ-dependent signaling show enhanced proliferation and enhanced migration with an overactivated phenotype. These mutant pericytes are less able to stabilize capillaries and less able to stimulate capillary basement membrane synthesis, potentially explaining why MVECs are more activated in ephrinB2 ΔV normal kidneys. These findings are consistent with previous observations in embryonic development where complete deletion of ephrinB2 in perivascular cells results in overactivation of pericytes with impaired vascular stabilizing capacity.16 Surprisingly, ephrinB2 ΔV pericytes in healthy kidneys do not express the activation marker αSMA, and pericyte abnormalities manifest mainly in disease circumstances.
These findings support and build on recent studies of ephrinB2 function in perivascular cells of the skin as well as MVECs of the lung.14,16 In mice with pericyte-specific ephrinB2 deletion, microvasculature is insufficiently covered with pericytes, and capillaries in multiple organs are immature,16 indicating that ephrinB2 is essential for pericytes to stabilize microvasculature. Furthermore, aberrant collagen was deposited around immature capillaries of skin in these mutant neonates.16 Recently, pulmonary ephrinB2 ΔV MVECs were shown to fail to internalize and phosphorylate VEGFR2 after VEGFA stimulation.14 This study also showed significantly impaired vascular density in retina of ephrinB2 ΔV neonates as well as in tumor transplanted into ephrinB2 ΔV mice compared with WT controls.14 Another study demonstrated that HUVECs ectopically expressing ephrinB2 ΔV were less migratory than those expressing WT ephrinB2 independently of EphB receptor binding.27
On the basis of previous findings, a model was proposed for ephrinB signaling, where upon binding of EphB receptor to ephrinB-ligand, phosphotyrosine-dependent signaling occurs first, followed by PDZ-dependent signaling.18 In the kidney, however, ephrinB2 ΔV UUO kidneys show p-ephrinB+ cells equal to WT UUO kidneys, suggesting that phosphotyrosine-dependent signaling is activated regardless of PDZ-dependent signaling. Because ephrinB2 5Y UUO kidneys showed a similar degree of PTC rarefaction and fibrosis to WT UUO kidneys, we speculate that PDZ-dependent signaling is activated independently of phosphotyrosine signaling.
In the presented studies, we did not focus in detail on the EphB4 receptor, which binds to ephrinB2 ligand, but was expressed by kidney pericytes and MVECs.11 EphB4 deficiency in mice also induces an embryonic lethality due to defects in developmental angiogenesis and phenocopies ephrinB2 deficiency,11 suggesting that EphB4 is a major activator of ephrinB2. We directly assessed the importance of EphB4-ephrinB2 bidirectional signaling for capillary integrity and stability by blocking this bidirectional signaling using soluble ephrinB2-Fc in 3D coculture systems. Our studies suggested that EphB4-ephrinB2 signaling contributes to microvascular integrity through EC/EC interaction but not to microvascular stability via pericyte-endothelial crosstalk.
Currently, we do not know the downstream effector proteins of phosphotyrosine-dependent or PDZ-dependent ephrinB2 reverse signaling. Mäkinen et al. reported that among PDZ proteins described to bind ephrinB ligands, PDZ-RGS3 and Dishevelled-2 (Dvl2) localization patterns were altered in ephrinB2 ΔV ECs.19 Dvl2 is a candidate downstream mediator of PDZ-dependent ephrinB2 signaling, because silencing of Dvl2 inhibits endothelial proliferation.28
The predominant population of cells that showed activated ephrinB2 assessed by tyrosine phosphorylation was F4/80+ macrophages. We did not find differences in macrophage number, or markers of activation or polarization between WT and mutant UUO kidneys, suggesting that PDZ-dependent ephrinB2 signaling in macrophages does not contribute to macrophage function; however, further studies such as cell type–specific deletion of ephrinB2 will be required to confirm these results.
Finally, mice without phosphotyrosine-dependent signaling (ephrinB2 5Y mice) showed a minor abnormality regarding kidney injury compared with WT mice. However, ephrinB2 5Y MVECs showed aberrant proliferation in normal kidney and ephrinB2 5Y pericytes stabilized capillaries significantly less than WT pericytes in 3D coculture. These abnormal characteristics of ephrinB2 5Y mice were milder than those of ephrinB2 ΔV mice. This may explain the similar degree of PTC rarefaction and fibrosis between WT and ephrinB2 5Y mice. Because the terminal mutated two tyrosine residues are in PDZ binding motif,29 it is possible that PDZ-dependent signaling is weakly impaired in ephrinB2 5Y mice.
In conclusion, after kidney injury, loss of PDZ-dependent ephrinB2 signaling impairs vascular stabilization functions of kidney pericytes and angiogenesis in MVECs, resulting in PTC rarefaction, and enhances kidney pericyte migration and proliferation and probably promotes pericyte detachment from vasculature as well as pericyte differentiation into scar-forming myofibroblasts, leading to fibrosis.
Concise Methods
Materials
All reagents were purchased from Sigma-Aldrich and plasticware from BD Falcon and all media are from Mediatech unless otherwise mentioned.
Experimental Animals
WT C57BL/6 mice were purchased from Charles River Laboratories. Collagen type I, α1 (coll1a1) reporter mice (Coll-GFPTr mice) were generated and validated as previously described on C57BL/6 background (>10 backcross).30,31 EphrinB25Y/+ and ephrinB2ΔV/+ knock-in mice were on CD1 background (5 backcross) as described,19 and provided by Dr. Amparo Acker-Palmer (Goethe University, Frankfurt, Germany) and Dr. Rüdiger Klein (Max Planck Institute of Neurobiology, Martinsried, Germany). Littermates were selected for homozygosity (EphrinB25Y/5Y and ephrinB2ΔV/ΔV) for use as experimental animals, and littermates that were homozygous for the WT allele (ephrinB2+/+) were used as controls. Genotyping by PCR of genomic DNA from ephrinB2 5Y and ΔV mice was performed using the following primers and conditions: (1) WT allele (450 bp): forward, 5′-ctctgtgtggaagtactgttg-3′; reverse, 5′-ccgccaatgtgtgtctgtagc-3′; and genotyping protocol of 95°C for 45 seconds, 62°C for 45 seconds, 72°C for 45 seconds, ×35 cycles; and (2) knock-in alleles (650 bp): forward, 5′-cggagtgcgcagaactgg-3′; reverse, 5′-gatttgcgttcacaaagggac-3′; and genotyping protocol of 95°C for 45 seconds, 60°C for 45 seconds, 72°C for 45 seconds, ×35 cycles. EphA2−/− mice (B6;129S6-Epha2tm1Jrui/J, stock number: 006028) and control B6129SF1 mice were from Jackson Laboratories. Genotyping was performed according to the supplier’s instructions.
Mouse Models of Kidney Injury
The surgical procedures were performed as previously described.4,6,7 In brief, mice aged 8–12 weeks were anesthetized with ketamine hydrochloride (80 mg/kg body weight, intraperitoneally) and xylazine (20 mg/kg body weight, intraperitoneally) before surgery. To create kidney injury, UUO or unilateral IRI was performed.4 To perform UUO surgery, the left kidney was exposed through a flank incision and the left ureter tied off at the level of the lower pole of the kidney with two 4.0 silk ties. Mice were euthanized 4 or 10 days after obstruction. IRI to the left kidney was performed through a similar incision under anesthesia at core body temperature of 36.7°C–37.2°C. A surgical clamp was placed over the artery and vein for 28 minutes and then removed. Return of blood flow was confirmed visually. All animal studies were performed under protocols approved by Department of Comparative Medicine, University of Washington.
Tissue Preparation and Histology
Mouse tissues were prepared and stained as previously described.4,6,7 Briefly, kidneys were either snap-frozen or fixed with PLP fixative (4% paraformaldehyde [PFA]/75 mM l-lysine/10 mM sodium periodate) (4°C, 2 hours), cryopreserved in 18% sucrose in PBS (4°C, overnight), and frozen. OCT-embedded (Sakura Finetek) frozen sections (7-μm) for immunofluorescence studies were blocked in 10% normal serum (Jackson ImmunoResearch) followed by incubation with primary antibody in blocking serum (4°C, overnight). Primary antibodies used in this study included rat anti-CD31 (1:200; BD Pharmingen), rat anti-PDGFRβ (1:200; eBioscience), rat anti-F4/80 (1:200; Invitrogen), FITC-conjugated anti-CD45 (1:200; eBioscience,), rabbit anti-p-ephrinB (1:200; Cell Signaling), FITC-conjugated anti-CD206 (1:100; BioLegend), rabbit anti-cleaved caspase-3 (1:200; Cell Signaling), Cy3-conjugated-αSMA (1:200; Sigma-Aldrich), and rabbit anti-Ki67 (1:200; Vector Laboratories). Sections were incubated with either FITC- or Cy3-conjugated secondary antibody (1:200 for FITC-conjugated antibody, 1:400 for Cy3-conjugated antibody; Jackson ImmunoResearch) (room temperature, 1 hour) and postfixed with 1% PFA/PBS (room temperature, 5 minutes) followed by mounting in Vectashield plus 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Images were captured by confocal or standard fluorescence microscopy (Nikon Eclipse). PTC density was analyzed as described with some modifications.32 In brief, images (×200) were captured from CD31-stained sections (20 images per mouse). Each image was divided into 266 squares by a grid. To calculate PTC density, each square containing CD31+ region resulted in a positive score. The final PTC density index was represented as a percentage of positive score. Specific cells were quantified as described.4,6 In brief, digitally captured images (×400) were analyzed (20 images per mouse). Positive cells were defined as cells in which >75% of the cell area, immediately surrounding a nucleus (detected by DAPI), was positive for FITC or Cy3 fluorescence indicative of the antigen expression. Ki-67+ cells were identified by positive nuclear staining for FITC fluorescence. Interstitial fibrosis was assessed using picrosirius red-stained paraffin sections as described.4,20 Picrosirius red+ area was morphometrically defined and quantified.
Isolation and Culture of Cells from Kidney
Primary pericytes, MVECs, and monocyte/macrophages were isolated from adult mouse kidneys using FACS or magnetic-activated cell sorting (MACS) as described.5,24,33–35 Separately primary proximal tubular epithelial cells were selectively cultured as described.33,35
Isolation and Culture of Primary Kidney Pericyte
For preparation of kidney single cell suspension, the kidney was decapsulated, diced, and then incubated (37°C, 30 minutes) with Liberase (0.2 mg/ml; Roche) and DNase (100 U/ml; Roche) in DMEM/F12. Digestion was curtailed by addition of DMEM/F12 including 10% FBS. The suspension was then filtered through a cell strainer (40 μm; Fisher Scientific) to remove glomeruli, centrifuged, and re-suspended in cold MACS buffer (1× PBS, 0.5% BSA, 2 mM EDTA, pH 7.2). Cells were incubated with rabbit anti-PDGFRβ polyclonal antibodies.5,36 After washing, cells were incubated with goat anti-rabbit IgG microbeads (Miltenyi) (4°C, 15 minutes), re-suspended, and positive selection was performed by MACS magnetic bead separation (Miltenyi). Selected cells were cultured in DMEM/F12 with 10% FBS, 1% penicillin/streptomycin, and 1% insulin-transferrin-selenite (ITS), in 6-well plates precoated with 0.2% gelatin. Purity of primary cultures was confirmed as described.5 Cells were used between passages 2 and 5. To directly isolate total RNA from pericytes, Coll-GFPTr kidneys with or without UUO treatment were digested and Green Fluorescent Protein (GFP)+ cells from kidney were sorted by FACSAria II (BD Biosciences) as previously described.5
Isolation and Culture of Primary Kidney MVECs
After kidney single cell suspension was prepared as described above, cell pellet was re-suspended in cold FACS buffer (1× PBS, 0.1% BSA, 2 mM EDTA, pH 7.4). Kidney single cell suspension was sorted using FACS Aria II immediately after phycoerythrin-conjugated anti-CD31 and FITC-conjugated anti-CD11b (eBioscience) antibodies were applied to the sample. CD31+ CD11b− cell population was sorted and cultured in endothelial medium (DMEM, 10% FBS, 1% penicillin/streptomycin, 10 ng/ml recombinant mouse VEGF; PeproTech) and 20 ng/ml recombinant mouse basic fibroblast growth factor (PeproTech)24 in 6-well plates precoated with 0.2% gelatin. Cells were cultured in CO2 incubator with 5% O2.24 Primary cultured cells used in all experiments were between passages 2 and 5.
Isolation of Primary Monocyte/Macrophages
During sorting of kidney MVECs, CD11b+ CD31− cell population was isolated and collected into RLT buffer (Qiagen) in order to extract RNA for quantitative RT-PCR (qPCR) assay.24
Isolation and Culture of Proximal Tubular Epithelial Cells
Primary cultures were generated using established methods as previously described.33,35 The cells used in this study were passage 0, and have been characterized previously.33,35
qPCR
Total RNA was extracted using TRIzol (Invitrogen). Purity of RNA was determined by A260 to A280 ratio. cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad). qPCR assay was performed using an ABI7900HT (Life Technology) and iTaq SYBR Green supermix with ROX (Bio-Rad) as described.5,35 Specificity of each primer pair was confirmed by identification of single PCR product with expected size in agarose gel electrophoresis. Primer sequences are listed in Supplemental Table 1.
Western Blot Analyses
The procedure was performed as described5,35 with some modifications. In brief, tissue samples were homogenized with 1× cell lysis buffer (Cell Signaling) including 1 mM PMSF, 1 mM sodium orthovanadate (MP Biomedicals), and Complete Mini proteinase inhibitor cocktail (Roche). Homogenized samples were centrifuged at 12,000×g (4°C, 15 minutes). Tissue extracts containing 50 μg of protein were prepared in Laemmli buffer (Bio-Rad)/β-mercaptoethanol (MP Biomedicals), separated in SDS-PAGE, transferred onto polyvinylidene difluoride membrane (Millipore) and blocked. The membrane was incubated with goat anti-ephrinB2 (1:100; R&D Systems), rabbit anti-p-VEGFR2 (Tyr1175) (1:1000; Cell Signaling), or rabbit anti-actin (1:1000; Santa Cruz) antibodies (4°C, overnight). Primary antibodies were detected by peroxidase-conjugated anti-goat or rabbit IgG (1:10,000; Jackson ImmunoResearch) and visualized with SuperSignal West Femto Substrate (Thermo Scientific).
Migration Assays
Migration of kidney pericytes and kidney MVECs was assessed using a scratch wound assay as described5,37 with some modifications.
Migration Assay of Primary Kidney Pericyte
Cells were seeded in gelatin-coated 24-well plates and cultured until 100% confluent. The monolayer of cells was wounded using a 200-μl micropipette tip. After wells were washed with PBS three times, serum-free DMEM/F12 including TGF-β (10 ng/ml; PeproTech) or vehicle was applied. Prefixed points along wounds were photographed at 0, 8, 24, and 48 hours after wound creation. Cell free areas were measured using ImageJ software. A percentage of wound closure was calculated by the following formula: [(wound area during healing − original wound area)/original wound area] × 100.
Migration Assay of Primary Kidney MVECs
In gelatin-coated 24-well plates, cells were cultured in endothelial medium until 100% confluent. Cells were maintained in CO2 incubator with 5% O2 and were placed in DMEM including 0.1% FBS and 10 ng/ml VEGF (serum-deprived medium) for 24 hours before scratching. After wounding, medium was replaced with fresh serum-deprived medium. Percentage wound closure was assessed as described above.
Proliferation Assays
Proliferation of kidney pericytes and kidney MVECs was analyzed using BrdU incorporation assay as described5 with some modifications.
Proliferation of Primary Kidney Pericytes
3.0×104 cells were seeded to gelatin-coated 24-well plates, cultured in DMEM/F12 with 10% FBS and 1% ITS for 2 days, followed by serum-free DMEM/F12 for 1 day. Cells were then stimulated with DMEM/F12 including 3% FBS, 10 ng/ml TGF-β, and 10 μM BrdU for 24 hours, fixed in acid ethanol denatured, permeabilized, and incubated with FITC-conjugated anti-BrdU antibody (1:200; eBioscience) and mounted (Vectashield with DAPI).
Proliferation of Primary Kidney MVECs
The same assay was performed as detailed for pericytes unless otherwise mentioned. Cells were cultured in endothelial medium for 2 days after seeding, and then cultured for 2 days in serum-free DMEM including VEGF (10 ng/ml) and BrdU (10 μM) and cells were maintained for 24 hours.
Three-Dimensional Vascular Tube Regression Assay
The assay was performed as described5,22,38 with some modifications. In brief, HUVECs (ATCC, P3–6), were grown in Vascular Cell Basal Medium (ATCC) containing Endothelial Cell Growth Kit-BBE (ATCC). HUVECs (3.0×106 cells/ml) were suspended in 3.75 mg/ml of rat tail collagen type I (Invitrogen) on ice, transferred to CO2 incubator (37°C, 30 minutes) to induce polymerization of collagen, and were allowed to form 3D networks in the presence of feeding medium (1× M199 medium [Sigma-Aldrich], 40 ng/ml human VEGF165 [PeproTech], 40 ng/ml human basic fibroblast growth factor [PeproTech], 50 μg/ml ascorbic acid, and 50 ng/ml phorbol ester) in 384-well plates. For quantitative analysis of gel contraction, 15 μl of HUVECs alone or HUVECs with 30% of primary kidney pericytes (3 kidney pericytes for every 10 HUVECs) were placed and cultured using 60 μl of feeding medium in 384-well plates (n≥20 per condition). Addition of medium with or without human plasma kallikrein (2 μg/ml final) (Enzyme Research Laboratories) denoted time zero. In some experiments, soluble ephrin-B2 Fc or control (10 μg/ml final; R&D Systems) was applied to feeding medium at time zero. Cultures were monitored every 4 hours for gel contraction. Upon initiation of gel contraction, each gel area (×20) was photographed and measured using ImageJ software. Percentage of regressed gel area was calculated as follows: [(original gel area − current gel area)/original gel area] × 100.
Staining of Sorted MVECs
Cells were fixed with 4% PFA (30 minutes, 4°C) and blocked with 10% donkey serum/PBS (room temperature, 30 minutes). Primary antibodies (rat anti-CD31 [eBioscience 1:200], goat anti-E-cadherin [R&D systems, 1:200], rat anti-CD11b [eBioscience, 1:200]) were incubated with cells (4°C, overnight). Primary antibodies were detected with Cy3-conjugated secondary antibodies, postfixed, and then mounted (Vectashield with DAPI).
Assessment of Endothelial Basement Membrane Synthesis
This assay was performed as described39 with some modifications. Briefly, HUVECs alone or HUVECs with 30% of kidney pericytes were embedded in 3D collagen gel and cultured with feeding medium for 4 days without kallikrein stimulation. Staining procedure was performed at 4°C unless mentioned. Gels were fixed (2% PFA/PBS [1 hour]), blocked (100 mM Tris-HCl [pH 7.4], 1% BSA, and 1% donkey serum [overnight]), and then incubated with goat anti-human collagen type IV antibody (SouthernBiotech, 1:200) in PBS with 10% donkey serum, followed by washing with PBS. Cy3-conjugated secondary antibody (1:400) was incubated with gels (room temperature, 2 hours). Gels were washed with PBS (overnight) and postfixed with 1% PFA/PBS. Fluorescence intensities of gels were measured by a fluorescence microplate reader (BioTek).
Antibody Feeding Assay
Receptor internalization was assessed using a method as described14 with some modifications. In brief, primary MVECs were seeded on gelatin-coated glass 1.5 cm coverslips in 12-well plates with endothelial medium until 30% confluent. Next, MVECs were cultured in serum-free DMEM without growth factors for 4 hours, and then blocked (37°C, 10 minutes) in blocking solution (DMEM, 2% BSA, 4% donkey serum). Rat anti-VEGFR2 antibody (1:40; eBioscience) in blocking solution was incubated with MVECs (37°C, 20 minutes). After washing with warm PBS twice, cells were stimulated with 100 ng/ml human VEGF165 (37°C, 30 minutes) and fixed with 4% PFA/PBS (4°C, 30 minutes). Surface receptors (green) were detected by incubation with FITC-conjugated secondary antibody (1:200) without permeabilization (room temperature, 2 hours). After washing with PBS three times, cells were permeabilized with ice-cold PBS containing 0.2% Triton X, 2% BSA, and 4% donkey serum (30 minutes). Cy3-conjugated secondary antibody (1:200) was incubated with cells (room temperature, 1 hour) to visualize internalized receptors (red), followed by washing with PBS. Coverslips were mounted in Vectashield with DAPI, images captured by confocal microscopy. Green or red intensities were defined and quantified. Percentage of internalized receptors was calculated as follows: [total red intensities/(total red intensities + total green intensities)] × 100.
Phosphorylation of VEGFR2
This was performed as described14 with some modifications. Primary MVECs on coverslips were placed in serum-free DMEM without growth factors for 4 hours, stimulated with 100 ng/ml human VEGF165 (37°C, 30 minutes), and fixed with 4% PFA/PBS (4°C, 30 minutes), then blocked (PBS, 10% donkey serum, and 0.2% Triton X) (room temperature, 30 minutes), and incubated with rabbit anti-p-VEGFR2 (Tyr1175) antibody (1:200) in blocking solution (4°C, overnight) followed by Cy3-conjugated secondary antibody (1:200) (room temperature, 2 hours) to visualize phosphorylated receptors (red). After washing and blocking, cells were incubated with rat anti-VEGFR2 antibody (1:200) in blocking solution (4°C, overnight), followed by incubation with FITC-conjugated secondary antibody (1:200) to label total VEGFR2 (green). Finally, cells were postfixed with 1% PFA/PBS, mounted (Vectashield with DAPI), and images acquired by confocal microscopy. Percentage of phosphorylated receptors was calculated as follows: [total red intensities/(total red intensities + total green intensities)] × 100. Results were shown relative to WT MVECs.
Statistical Analyses
Results are presented as mean ± SEM. To analyze the difference between two groups, the 2-tailed t test was used. When >2 groups were present, we used one-way ANOVA followed by Tukey’s honestly significant difference post hoc test. A P value <0.05 was considered significant.
Disclosures
J.S.D. is on the scientific advisory board for Promedior Inc. and Regulus Therapeutics, has stock options with Promedior Inc., is the co-founder of Muregen LLC, and has recently consulted for Pharmaceuticals: Gilead, Abbott, Takeda, Bristol-Myers Squibb, GlaxoSmithKline, Biogen Idec, and Boehringer Ingelheim.
Acknowledgments
The authors thank Dr. Ioanna Bethani and Dr. Amparo Acker-Palmer (Goethe University, Frankfurt, Germany) for ephrinB2 5Y and ΔV mice and advice, Dr. William Stallcup (Burnham Institute, La Jolla, CA) for anti-PDGFRβ antibody, Dr. Christine Abrass (University of Washington, Seattle, WA), Dr. George Davis (University of Missouri, Columbia, MO) and Dr. M. Luisa Iruela-Arispe (University of California, Los Angeles, CA) for advice, and the Lynne and Mike Garvey Microscopy Suite at South Lake Union for microscopy support.
The Duffield laboratory is funded by grants from the National Institutes of Health (DK84077, DK87389, DK93493, DK94768, NCATS 1UH2 TR000504), American Heart Association (12040023), University of Washington Institute for Stem Cell and Regenerative Medicine, Genzyme (Research in Progress Grant), NephCure Foundation, and Regulus Therapeutics.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Getting the “Inside” Scoop on EphrinB2 Signaling in Pericytes and the Effect on Peritubular Capillary Stability,” on pages 521–523.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012080871/-/DCSupplemental.
References
- 1.Basile DP: Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P: The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74: 495–504, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Steegh FM, Gelens MA, Nieman FH, van Hooff JP, Cleutjens JP, van Suylen RJ, Daemen MJ, van Heurn EL, Christiaans MH, Peutz-Kootstra CJ: Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol 22: 1024–1029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS: Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178: 911–923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS: Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23: 868–883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kida Y, Duffield JS: Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol 38: 467–473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrimpf C, Duffield JS: Mechanisms of fibrosis: The role of the pericyte. Curr Opin Nephrol Hypertens 20: 297–305, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Wang HU, Chen ZF, Anderson DJ: Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93: 741–753, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Gerety SS, Wang HU, Chen ZF, Anderson DJ: Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell 4: 403–414, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Kuijper S, Turner CJ, Adams RH: Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med 17: 145–151, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R: The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell 104: 57–69, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A: Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465: 487–491, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, Barberis A, Benjamin LE, Mäkinen T, Nobes CD, Adams RH: Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465: 483–486, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH: Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A, Tosato G: EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood 114: 1707–1716, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R: EphrinB phosphorylation and reverse signaling: Regulation by Src kinases and PTP-BL phosphatase. Mol Cell 9: 725–737, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Mäkinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA: PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev 19: 397–410, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin SL, Castaño AP, Nowlin BT, Lupher ML, Jr, Duffield JS: Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Okazaki T, Ni A, Baluk P, Ayeni OA, Kearley J, Coyle AJ, Humbles A, McDonald DM: Capillary defects and exaggerated inflammatory response in the airways of EphA2-deficient mice. Am J Pathol 174: 2388–2399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders WB, Bayless KJ, Davis GE: MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci 118: 2325–2340, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Stockinger H, Gadd SJ, Eher R, Majdic O, Schreiber W, Kasinrerk W, Strass B, Schnabl E, Knapp W: Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol 145: 3889–3897, 1990 [PubMed] [Google Scholar]
- 24.Ieronimakis N, Balasundaram G, Reyes M: Direct isolation, culture and transplant of mouse skeletal muscle derived endothelial cells with angiogenic potential. PLoS ONE 3: e0001753, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhart-King CA: Endothelial cell adhesion and migration. Methods Enzymol 443: 45–64, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Eichmann A, Simons M: VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol 24: 188–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bochenek ML, Dickinson S, Astin JW, Adams RH, Nobes CD: Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J Cell Sci 123: 1235–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cirone P, Lin S, Griesbach HL, Zhang Y, Slusarski DC, Crews CM: A role for planar cell polarity signaling in angiogenesis. Angiogenesis 11: 347–360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin D, Gish GD, Songyang Z, Pawson T: The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem 274: 3726–3733, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Krempen K, Grotkopp D, Hall K, Bache A, Gillan A, Rippe RA, Brenner DA, Breindl M: Far upstream regulatory elements enhance position-independent and uterus-specific expression of the murine alpha1(I) collagen promoter in transgenic mice. Gene Expr 8: 151–163, 1999 [PMC free article] [PubMed] [Google Scholar]
- 31.Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA: DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology 37: 267–276, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS: Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 121: 2211–2220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV: Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 118: 1657–1668, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS: MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4: 121ra18, 2012 [DOI] [PMC free article] [PubMed]
- 36.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB: NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222: 218–227, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN: Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A 105: 7738–7743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE: Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175: 179–191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE: Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114: 5091–5101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]