Abstract
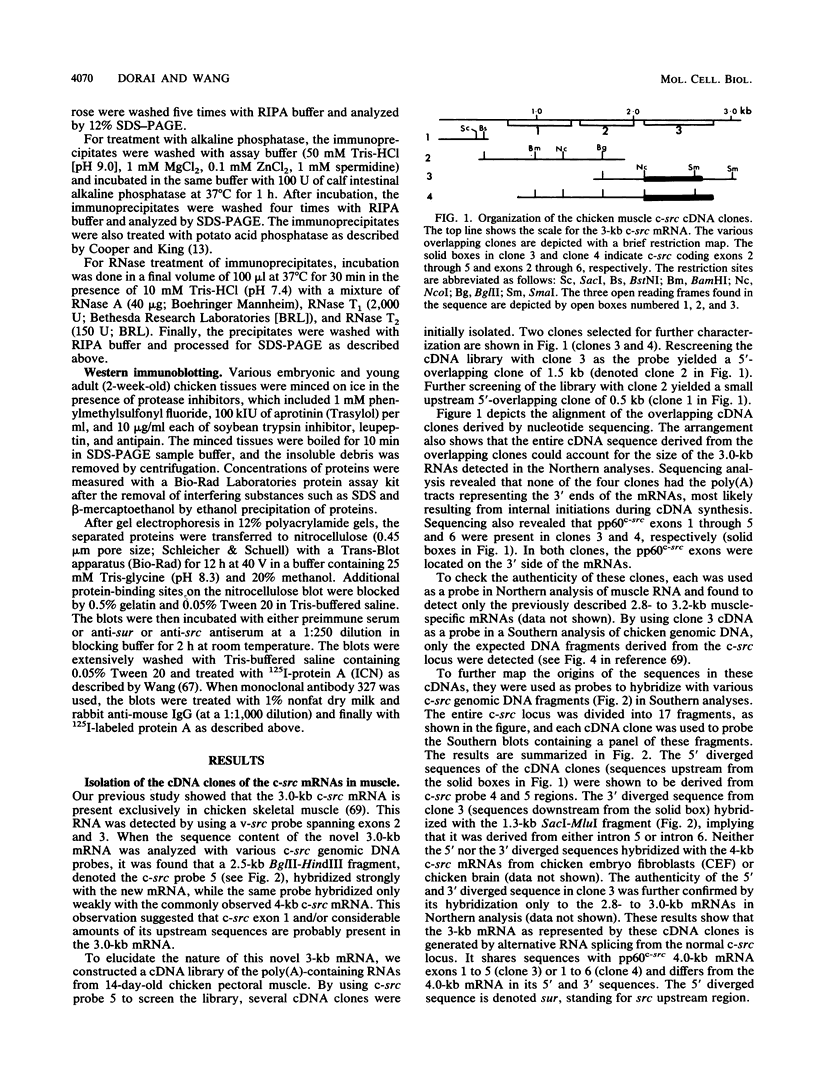
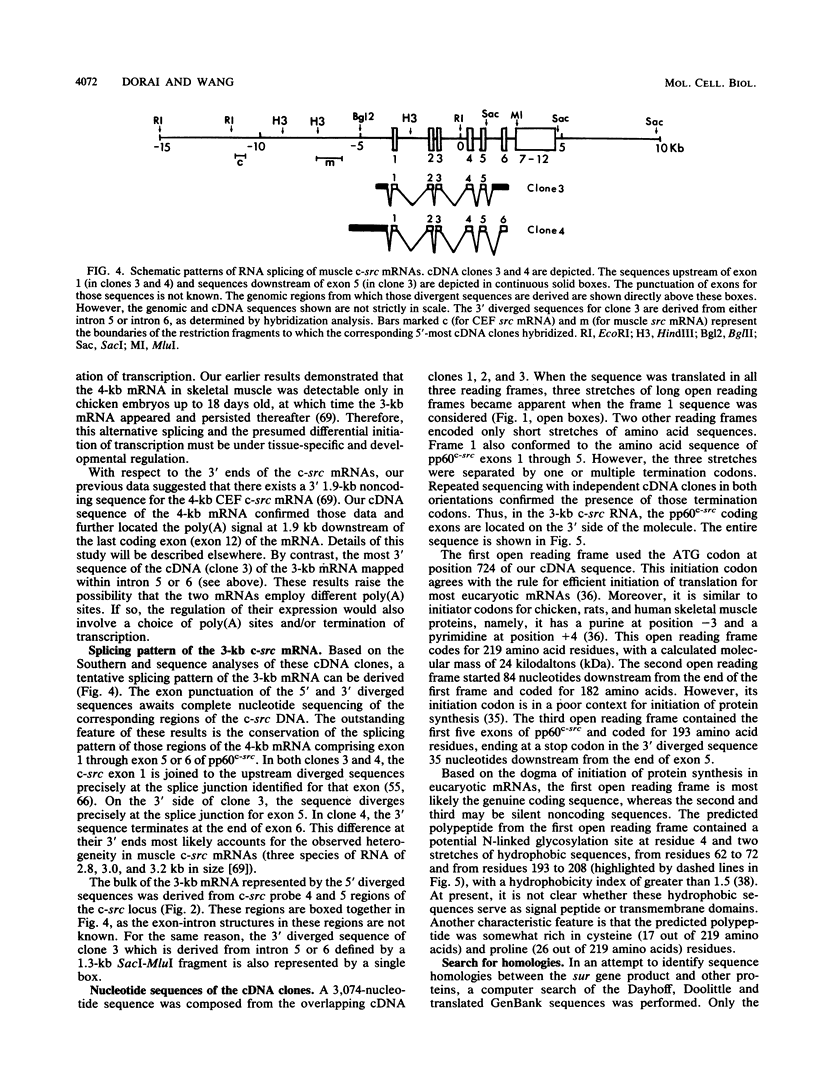
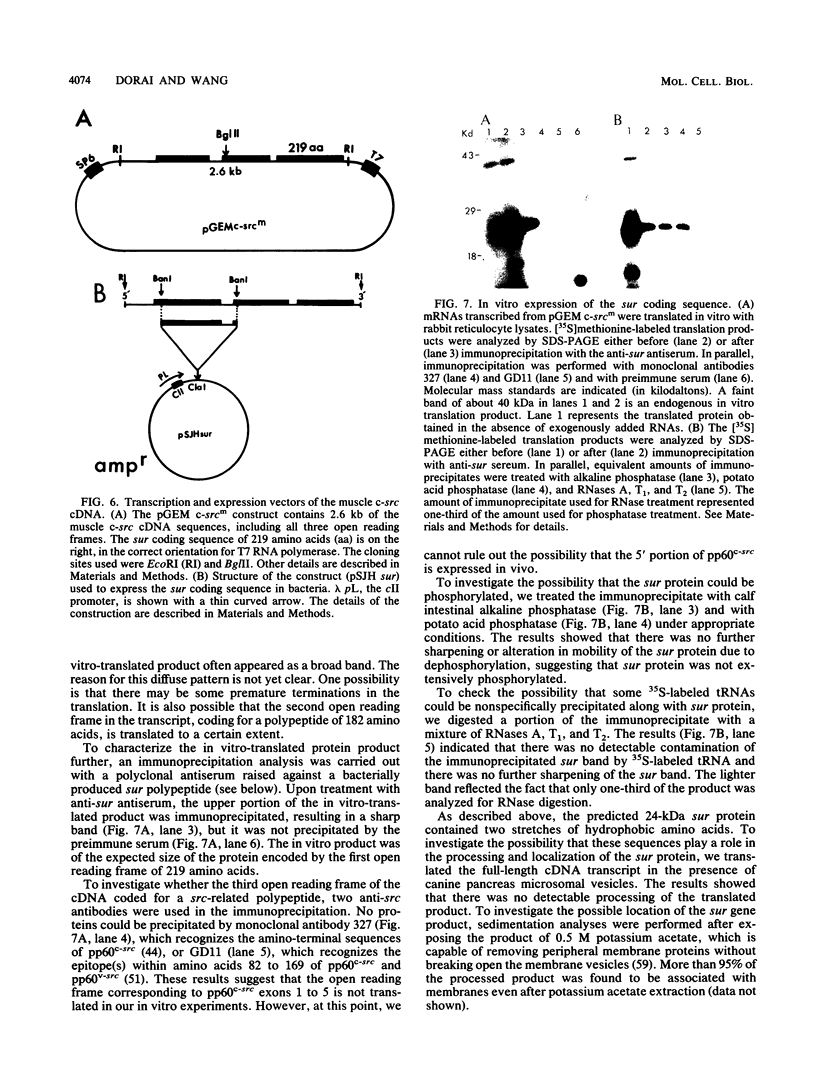
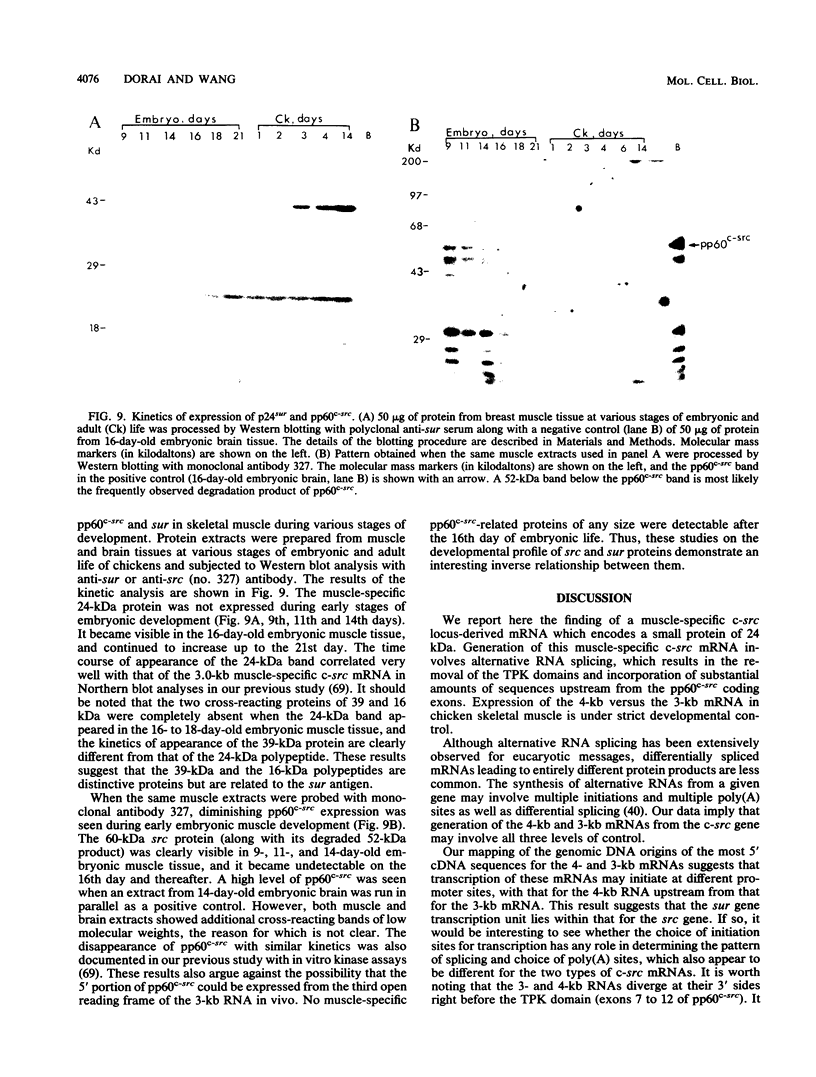
While the c-src locus is expressed as a 4.0-kilobase (kb) mRNA coding for pp60c-src in various chicken tissues, including embryonic muscle, it is expressed as a novel 3.0-kb mRNA in adult skeletal muscle. We have analyzed the primary structure of this alternatively transcribed and spliced c-src mRNA. The sequence revealed three open reading frames, with the previously defined c-src exons 1 through 5 or 6 comprising the third, on the 3' untranslated region of this 3-kb mRNA. The exons coding for the tyrosine kinase domain of pp60c-src were excluded. On the 5' side, 2 kb of sequence upstream from the previously defined exon 1 of the c-src gene was included in this mRNA. The start site for the 3-kb mRNA probably lies downstream of that for the 4-kb mRNA. The first reading frame of the 3.0-kb mRNA, called sur (for src upstream region), encoded a 24-kilodalton (kDa) protein product rich in cysteine and proline residues. In vitro analysis indicated that the 24-kDa sur protein was membrane associated. Antibodies to sur protein detected in vivo a 24-kDa muscle-specific protein which was developmentally regulated and corresponded to the switch from the 4-kb to the 3-kb c-src mRNA. A striking kinetic pattern of appearance of sur protein and disappearance of pp60c-src suggests that the expression of these two proteins is inversely related.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison R. Secretory protein translocation in a neurospora crassa in vitro system. Hydrolysis of a nucleoside triphosphate is required for posttranslational translocation. J Biol Chem. 1987 Dec 15;262(35):17031–17037. [PubMed] [Google Scholar]
- Anthony D. T., Schuetze S. M., Rubin L. L. Transformation by Rous sarcoma virus prevents acetylcholine receptor clustering on cultured chicken muscle fibers. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2265–2269. doi: 10.1073/pnas.81.7.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnekow A., Schartl M. Cellular src gene product detected in the freshwater sponge Spongilla lacustris. Mol Cell Biol. 1984 Jun;4(6):1179–1181. doi: 10.1128/mcb.4.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Nguyen H. T., Medford R. M., Destree A. T., Mahdavi V., Nadal-Ginard B. Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell. 1985 May;41(1):67–82. doi: 10.1016/0092-8674(85)90062-5. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Cotton P., Lustig A., Yonemoto W., Lipsich L., Coussens P., Barrett J. N., Nonner D., Keane R. W. Characterization of the altered form of the c-src gene product in neuronal cells. Genes Dev. 1987 May;1(3):287–296. doi: 10.1101/gad.1.3.287. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Cowan W. M., Hunter T., Eckhart W. pp60c-src expression in the developing rat brain. Proc Natl Acad Sci U S A. 1988 May;85(10):3348–3352. doi: 10.1073/pnas.85.10.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Kaplan P. L., Hunter T., Eckhart W. Alterations in pp60c-src accompany differentiation of neurons from rat embryo striatum. Mol Cell Biol. 1987 May;7(5):1830–1840. doi: 10.1128/mcb.7.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. The proto-oncogene c-ets is preferentially expressed in lymphoid cells. Mol Cell Biol. 1985 Nov;5(11):2993–3000. doi: 10.1128/mcb.5.11.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol Cell Biol. 1985 Jan;5(1):27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee C. E., Griffin J., Sastre L., Miller L. J., Springer T. A., Piwnica-Worms H., Roberts T. M. Differentiation of myeloid cells is accompanied by increased levels of pp60c-src protein and kinase activity. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5131–5135. doi: 10.1073/pnas.83.14.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler M., Barnekow A. Differential expression of the cellular oncogenes c-src and c-yes in embryonal and adult chicken tissues. Biosci Rep. 1984 Sep;4(9):757–770. doi: 10.1007/BF01128817. [DOI] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hoffmann F. M., Fresco L. D., Hoffman-Falk H., Shilo B. Z. Nucleotide sequences of the Drosophila src and abl homologs: conservation and variability in the src family oncogenes. Cell. 1983 Dec;35(2 Pt 1):393–401. doi: 10.1016/0092-8674(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C., Rübsamen H. Expression of pp60c-src protein kinase in adult and fetal human tissue: high activities in some sarcomas and mammary carcinomas. Cancer Res. 1983 Apr;43(4):1696–1702. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Neither arginine nor histidine can carry out the function of lysine-295 in the ATP-binding site of p60src. Mol Cell Biol. 1986 Mar;6(3):751–757. doi: 10.1128/mcb.6.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlik C. C., Mahaffey J. W., Coutu M. D., Fyrberg E. A. Organization of contractile protein genes within the 88F subdivision of the D. melanogaster third chromosome. Cell. 1984 Jun;37(2):469–481. doi: 10.1016/0092-8674(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R. Subcellular localization of pp60src in RSV-transformed cells. Curr Top Microbiol Immunol. 1983;107:51–124. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Court D., Papas T. S. High-level expression in Escherichia coli of the carboxy-terminal sequences of the avian myelocytomatosis virus (MC29) v-myc protein. Gene. 1983 Jul;23(1):75–84. doi: 10.1016/0378-1119(83)90218-4. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Levy B. T., Sorge L. K., Meymandi A., Maness P. F. pp60c-src Kinase is in chick and human embryonic tissues. Dev Biol. 1984 Jul;104(1):9–17. doi: 10.1016/0012-1606(84)90031-9. [DOI] [PubMed] [Google Scholar]
- Levy J. B., Dorai T., Wang L. H., Brugge J. S. The structurally distinct form of pp60c-src detected in neuronal cells is encoded by a unique c-src mRNA. Mol Cell Biol. 1987 Nov;7(11):4142–4145. doi: 10.1128/mcb.7.11.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. A., Brugge J. S., Levine J. M. Induction of altered c-src product during neural differentiation of embryonal carcinoma cells. Science. 1986 Nov 14;234(4778):873–876. doi: 10.1126/science.3095923. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Aubry M., Shores C. G., Frame L., Pfenninger K. H. c-src gene product in developing rat brain is enriched in nerve growth cone membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5001–5005. doi: 10.1073/pnas.85.14.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F., Sorge L. K., Fults D. W. An early developmental phase of pp60c-src expression in the neural ectoderm. Dev Biol. 1986 Sep;117(1):83–89. doi: 10.1016/0012-1606(86)90350-7. [DOI] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., Shibuya M., Hsu M. T., Wang L. H. Proto-oncogene c-ros codes for a molecule with structural features common to those of growth factor receptors and displays tissue specific and developmentally regulated expression. Mol Cell Biol. 1986 May;6(5):1478–1486. doi: 10.1128/mcb.6.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Schartl M., Barnekow A. Differential expression of the cellular src gene during vertebrate development. Dev Biol. 1984 Oct;105(2):415–422. doi: 10.1016/0012-1606(84)90298-7. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Coussens P. M., Yaciuk P. Overexpression of the c-src protein does not induce transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Zelenetz A. D., Cooper G. M. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981 May;24(2):531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Drees B., Kornberg T., Bishop J. M. The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell. 1985 Oct;42(3):831–840. doi: 10.1016/0092-8674(85)90279-x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., McGrath J. P., Levinson A. D. A mutation at the ATP-binding site of pp60v-src abolishes kinase activity, transformation, and tumorigenicity. Mol Cell Biol. 1985 Jul;5(7):1772–1779. doi: 10.1128/mcb.5.7.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baker B., Varmus H. E., Bishop J. M. Characteristics of cellular RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):381–386. doi: 10.1016/0092-8674(78)90206-4. [DOI] [PubMed] [Google Scholar]
- Sugano S., Stoeckle M. Y., Hanafusa H. Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell. 1987 May 8;49(3):321–328. doi: 10.1016/0092-8674(87)90284-4. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H., Junghans R. P., Ju G., Skalka A. M. Comparison between the viral transforming gene (src) of recovered avian sarcoma virus and its cellular homolog. Mol Cell Biol. 1981 Nov;1(11):1024–1037. doi: 10.1128/mcb.1.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Iijima S., Dorai T., Lin B. Regulation of the expression of proto-oncogene c-src by alternative RNA splicing in chicken skeletal muscle. Oncogene Res. 1987 Jun;1(1):43–59. [PubMed] [Google Scholar]
- Wang S. Y., Hayward W. S., Hanafusa H. Genetic variation in the RNA transcripts of endogenous virus genes in uninfected chicken cells. J Virol. 1977 Oct;24(1):64–73. doi: 10.1128/jvi.24.1.64-73.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]