Abstract
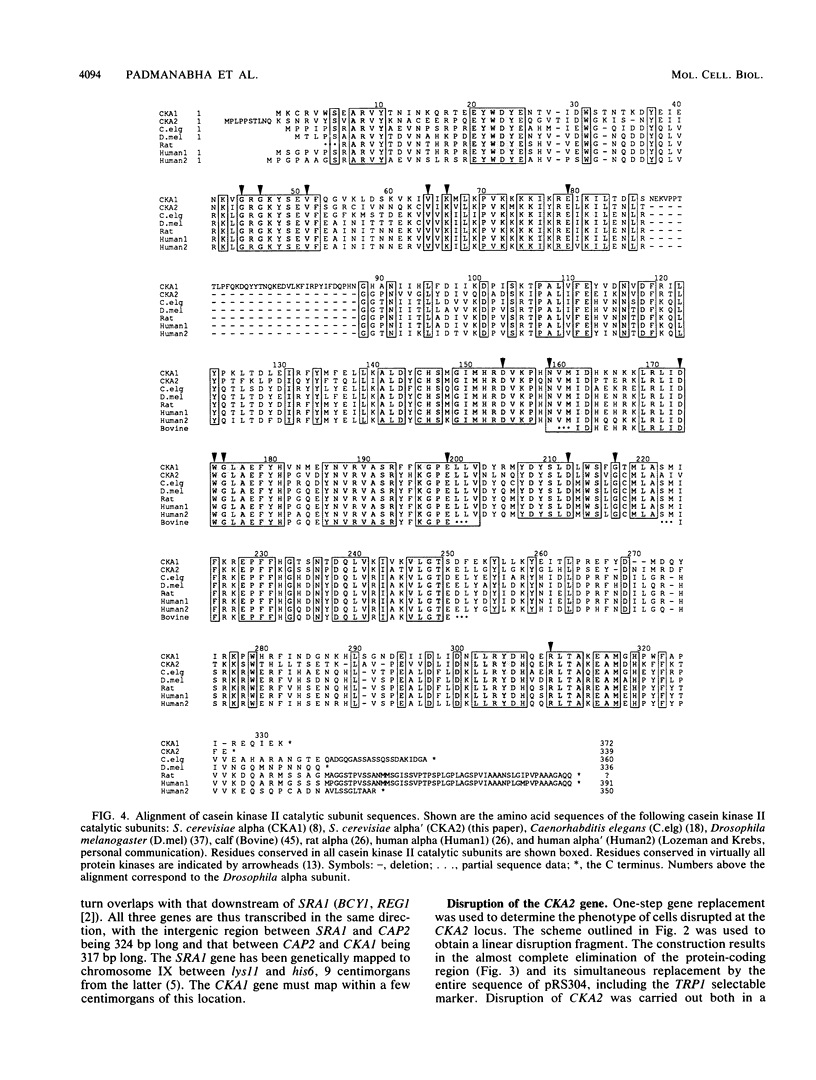
Casein kinase II of Saccharomyces cerevisiae contains two distinct catalytic subunits, alpha and alpha', which are encoded by the CKA1 and CKA2 genes, respectively. Null mutations in the CKA1 gene do not confer a detectable phenotype (J. L.-P. Chen-Wu, R. Padmanabha, and C. V. C. Glover, Mol. Cell. Biol. 8:4981-4990, 1988), presumably because of the presence of the CKA2 gene. We report here the cloning, sequencing, and disruption of the CKA2 gene. The alpha' subunit encoded by the CKA2 gene is 60% identical to the CKA1-encoded alpha subunit and 55% identical to the Drosophila alpha subunit (A. Saxena, R. Padmanabha, and C. V. C. Glover, Mol. Cell. Biol. 7:3409-3417, 1987). Deletions of the CKA2 gene were constructed by gene replacement techniques. Haploid cells in which the CKA2 gene alone is disrupted show no detectable phenotype, but haploid cells carrying disruptions in both the CKA1 and CKA2 genes are inviable. Cells in which casein kinase II activity is depleted increase substantially in size prior to growth arrest, and a significant fraction of the arrested cells exhibit a pseudomycelial morphology. Disruption of the activity also results in flocculation. Yeast strains lacking both endogenous catalytic subunit genes can be rescued by expression of the alpha and beta subunits of Drosophila casein kinase II or by expression of the Drosophila alpha subunit alone, suggesting that casein kinase II function has been conserved through evolution.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Osheroff N. Regulation of casein kinase II activity by epidermal growth factor in human A-431 carcinoma cells. J Biol Chem. 1989 Jul 15;264(20):11958–11965. [PubMed] [Google Scholar]
- Amatruda J. F., Cannon J. F., Tatchell K., Hug C., Cooper J. A. Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature. 1990 Mar 22;344(6264):352–354. doi: 10.1038/344352a0. [DOI] [PubMed] [Google Scholar]
- Cameron S., Levin L., Zoller M., Wigler M. cAMP-independent control of sporulation, glycogen metabolism, and heat shock resistance in S. cerevisiae. Cell. 1988 May 20;53(4):555–566. doi: 10.1016/0092-8674(88)90572-7. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Gibbs J. B., Tatchell K. Suppressors of the ras2 mutation of Saccharomyces cerevisiae. Genetics. 1986 Jun;113(2):247–264. doi: 10.1093/genetics/113.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Marshak D. R. Serum-stimulated cell growth causes oscillations in casein kinase II activity. J Biol Chem. 1989 May 5;264(13):7345–7348. [PubMed] [Google Scholar]
- Chen-Wu J. L., Padmanabha R., Glover C. V. Isolation, sequencing, and disruption of the CKA1 gene encoding the alpha subunit of yeast casein kinase II. Mol Cell Biol. 1988 Nov;8(11):4981–4990. doi: 10.1128/mcb.8.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Cochet C., Chambaz E. M. Oligomeric structure and catalytic activity of G type casein kinase. Isolation of the two subunits and renaturation experiments. J Biol Chem. 1983 Feb 10;258(3):1403–1406. [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Davis T. N., Urdea M. S., Masiarz F. R., Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986 Nov 7;47(3):423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Holm C., Meeks-Wagner D. W., Fangman W. L., Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42(2):169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Hu E., Rubin C. S. Casein kinase II from Caenorhabditis elegans. Properties and developmental regulation of the enzyme; cloning and sequence analyses of cDNA and the gene for the catalytic subunit. J Biol Chem. 1990 Mar 25;265(9):5072–5080. [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund J. K., Czech M. P. Insulin-like growth factor I and insulin rapidly increase casein kinase II activity in BALB/c 3T3 fibroblasts. J Biol Chem. 1988 Nov 5;263(31):15872–15875. [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- Lipke P. N., Hull-Pillsbury C. Flocculation of Saccharomyces cerevisiae tup1 mutants. J Bacteriol. 1984 Aug;159(2):797–799. doi: 10.1128/jb.159.2.797-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Kuenzel E. A., Krebs E. G., Eisenman R. N. Myc oncoproteins are phosphorylated by casein kinase II. EMBO J. 1989 Apr;8(4):1111–1119. doi: 10.1002/j.1460-2075.1989.tb03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiori F., Meggio F., Marin O., Borin G., Calderan A., Ruzza P., Pinna L. A. Synthetic peptide substrates for casein kinase 2. Assessment of minimum structural requirements for phosphorylation. Biochim Biophys Acta. 1988 Oct 7;971(3):332–338. doi: 10.1016/0167-4889(88)90149-8. [DOI] [PubMed] [Google Scholar]
- Meisner H., Heller-Harrison R., Buxton J., Czech M. P. Molecular cloning of the human casein kinase II alpha subunit. Biochemistry. 1989 May 2;28(9):4072–4076. doi: 10.1021/bi00435a066. [DOI] [PubMed] [Google Scholar]
- Mendenhall M. D., Richardson H. E., Reed S. I. Dominant negative protein kinase mutations that confer a G1 arrest phenotype. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4426–4430. doi: 10.1073/pnas.85.12.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mulner-Lorillon O., Marot J., Cayla X., Pouhle R., Belle R. Purification and characterization of a casein-kinase-II-type enzyme from Xenopus laevis ovary. Biological effects on the meiotic cell division of full-grown oocyte. Eur J Biochem. 1988 Jan 15;171(1-2):107–117. doi: 10.1111/j.1432-1033.1988.tb13765.x. [DOI] [PubMed] [Google Scholar]
- Padmanabha R., Glover C. V. Casein kinase II of yeast contains two distinct alpha polypeptides and an unusually large beta subunit. J Biol Chem. 1987 Feb 5;262(4):1829–1835. [PubMed] [Google Scholar]
- Piggott J. R., Rai R., Carter B. L. A bifunctional gene product involved in two phases of the yeast cell cycle. Nature. 1982 Jul 22;298(5872):391–393. doi: 10.1038/298391a0. [DOI] [PubMed] [Google Scholar]
- Prowald K., Fischer H., Issinger O. G. Enhanced casein kinase II activity in human tumour cell cultures. FEBS Lett. 1984 Oct 29;176(2):479–483. doi: 10.1016/0014-5793(84)81222-3. [DOI] [PubMed] [Google Scholar]
- Riles L., Olson M. V. Nonsense mutations in essential genes of Saccharomyces cerevisiae. Genetics. 1988 Apr;118(4):601–607. doi: 10.1093/genetics/118.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. M., Bell L. E., Siefken D. A., Jacob S. T. A heparin-sensitive nuclear protein kinase. Purification, properties, and increased activity in rat hepatoma relative to liver. J Biol Chem. 1981 Jul 25;256(14):7468–7477. [PubMed] [Google Scholar]
- Russell P., Moreno S., Reed S. I. Conservation of mitotic controls in fission and budding yeasts. Cell. 1989 Apr 21;57(2):295–303. doi: 10.1016/0092-8674(89)90967-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A., Padmanabha R., Glover C. V. Isolation and sequencing of cDNA clones encoding alpha and beta subunits of Drosophila melanogaster casein kinase II. Mol Cell Biol. 1987 Oct;7(10):3409–3417. doi: 10.1128/mcb.7.10.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H. R., Reichert G. H., Issinger O. G. Enhanced casein kinase II activity during mouse embryogenesis. Identification of a 110-kDa phosphoprotein as the major phosphorylation product in mouse embryos and Krebs II mouse ascites tumor cells. Eur J Biochem. 1986 Dec 15;161(3):733–738. doi: 10.1111/j.1432-1033.1986.tb10501.x. [DOI] [PubMed] [Google Scholar]
- Schultz J., Carlson M. Molecular analysis of SSN6, a gene functionally related to the SNF1 protein kinase of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Oct;7(10):3637–3645. doi: 10.1128/mcb.7.10.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Tuohy T. M., Mosurski K. R. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986 Jul 11;14(13):5125–5143. doi: 10.1093/nar/14.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommercorn J., Krebs E. G. Induction of casein kinase II during differentiation of 3T3-L1 cells. J Biol Chem. 1987 Mar 15;262(8):3839–3843. [PubMed] [Google Scholar]
- Sommercorn J., Mulligan J. A., Lozeman F. J., Krebs E. G. Activation of casein kinase II in response to insulin and to epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K., Kuenzel E. A., Walsh K. A., Krebs E. G. Amino acid sequence of the beta subunit of bovine lung casein kinase II. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4851–4855. doi: 10.1073/pnas.84.14.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987 Jul 17;50(2):277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Weiss W. A., Friedberg E. C. Normal yeast tRNA(CAGGln) can suppress amber codons and is encoded by an essential gene. J Mol Biol. 1986 Dec 20;192(4):725–735. doi: 10.1016/0022-2836(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



